Abstract
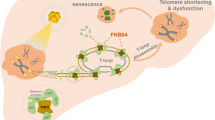
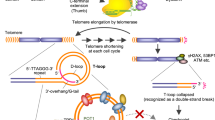
Telomere dysfunction is intricately linked to the aging process and stands out as a prominent cancer hallmark. Here we demonstrate that telomerase activity is differentially regulated in cancer and normal cells depending on the expression status of fructose-1,6-bisphosphatase 1 (FBP1). In FBP1-expressing cells, FBP1 directly interacts with and dephosphorylates telomerase reverse transcriptase (TERT) at Ser227. Dephosphorylated TERT fails to translocate into the nucleus, leading to the inhibition of telomerase activity, reduction in telomere lengths, enhanced senescence and suppressed tumor cell proliferation and growth in mice. Lipid nanoparticle-mediated delivery of FBP1 mRNA inhibits liver tumor growth. Additionally, FBP1 expression levels inversely correlate with TERT pSer227 levels in renal and hepatocellular carcinoma specimens and with poor prognosis of the patients. These findings demonstrate that FBP1 governs cell immortality through its protein phosphatase activity and uncover a unique telomerase regulation in tumor cells attributed to the downregulation or deficiency of FBP1 expression.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the main text, extended data figures and supplementary information file. Data are also available from the corresponding author upon reasonable request. The crystal structure of human liver FBP1 is available in the Protein Data Bank under accession code 5ZWK. The structure of TERT was predicted by AlphaFold (https://alphafold.ebi.ac.uk/entry/O14746). Mass spectrometry data have been deposited in the ProteomeXchange with accession code PXD050213. Source data are provided with this paper.
Change history
22 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41589-024-01623-3
References
Chakravarti, D., LaBella, K. A. & DePinho, R. A. Telomeres: history, health, and hallmarks of aging. Cell 184, 306–322 (2021).
Blackburn, E. H. & Gall, J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in tetrahymena. J. Mol. Biol. 120, 33–53 (1978).
Creighton, H. B. & McClintock, B. A correlation of cytological and genetical crossing-over in Zea mays. Proc. Natl Acad. Sci. USA 17, 492–497 (1931).
Sahin, E. & DePinho, R. A. Axis of ageing: telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 13, 397–404 (2012).
Nakamura, T. M. & Cech, T. R. Reversing time: origin of telomerase. Cell 92, 587–590 (1998).
Wang, J., Xie, L. Y., Allan, S., Beach, D. & Hannon, G. J. Myc activates telomerase. Genes Dev. 12, 1769–1774 (1998).
Li, X. et al. Programmable base editing of mutated TERT promoter inhibits brain tumour growth. Nat. Cell Biol. 22, 282–288 (2020).
Jeong, S. A. et al. Akt-mediated phosphorylation increases the binding affinity of hTERT for importin α to promote nuclear translocation. J. Cell Sci. 128, 2287–2301 (2015).
Tejwani, G. A. Regulation of fructose-bisphosphatase activity. Adv. Enzymol. Relat. Areas Mol. Biol. 54, 121–194 (1983).
Huangyang, P. et al. Fructose-1,6-bisphosphatase 2 inhibits sarcoma progression by restraining mitochondrial biogenesis. Cell Metab. 31, 174–188 (2020).
Li, B. et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 513, 251–255 (2014).
Li, F. et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat. Cell Biol. 22, 728–739 (2020).
Liao, K. et al. A feedback circuitry between polycomb signaling and fructose-1, 6-bisphosphatase enables hepatic and renal tumorigenesis. Cancer Res. 80, 675–688 (2020).
Wang, Z. et al. Fructose-1,6-bisphosphatase 1 functions as a protein phosphatase to dephosphorylate histone H3 and suppresses PPARα-regulated gene transcription and tumour growth. Nat. Cell Biol. 24, 1655–1665 (2022).
Li, H., Zhao, L. L., Funder, J. W. & Liu, J. P. Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J. Biol. Chem. 272, 16729–16732 (1997).
Denu, J. M., Stuckey, J. A., Saper, M. A. & Dixon, J. E. Form and function in protein dephosphorylation. Cell 87, 361–364 (1996).
Walton, K. M. & Dixon, J. E. Protein tyrosine phosphatases. Annu. Rev. Biochem. 62, 101–120 (1993).
Qian, X. et al. PTEN suppresses glycolysis by dephosphorylating and inhibiting autophosphorylated PGK1. Mol. Cell 76, 516–527 (2019).
Cesare, A. J. & Reddel, R. R. Alternative lengthening of telomeres: models, mechanisms and implications. Nat. Rev. Genet. 11, 319–330 (2010).
Heaphy, C. M. et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 333, 425 (2011).
Brosnan-Cashman, J. A. et al. ATRX loss induces multiple hallmarks of the alternative lengthening of telomeres (ALT) phenotype in human glioma cell lines in a cell line-specific manner. PLoS ONE 13, e0204159 (2018).
Tusell, L., Pampalona, J., Soler, D., Frias, C. & Genesca, A. Different outcomes of telomere-dependent anaphase bridges. Biochem. Soc. Trans. 38, 1698–1703 (2010).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Kon, E., Ad-El, N., Hazan-Halevy, I., Stotsky-Oterin, L. & Peer, D. Targeting cancer with mRNA–lipid nanoparticles: key considerations and future prospects. Nat. Rev. Clin. Oncol. 20, 739–754 (2023).
Kubiatowicz, L. J., Mohapatra, A., Krishnan, N., Fang, R. H. & Zhang, L. mRNA nanomedicine: design and recent applications. Exploration (Beijing) 2, 20210217 (2022).
Gu, L. et al. Fructose-1,6-bisphosphatase is a nonenzymatic safety valve that curtails AKT activation to prevent insulin hyperresponsiveness. Cell Metab. 35, 1009–1021 (2023).
Zhu, W. et al. Fructose-1,6-bisphosphatase 1 dephosphorylates IκBα and suppresses colorectal tumorigenesis. Cell Res. 33, 245–257 (2023).
Chen, M. J., Dixon, J. E. & Manning, G. Genomics and evolution of protein phosphatases. Sci. Signal. 10, eaag1796 (2017).
Romero, P. et al. Computational prediction of human metabolic pathways from the complete human genome. Genome Biol. 6, R2 (2005).
Xu, D. et al. The evolving landscape of noncanonical functions of metabolic enzymes in cancer and other pathologies. Cell Metab. 33, 33–50 (2021).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Bian, X. et al. Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends Cell Biol. 32, 786–799 (2022).
Lu, Z. & Hunter, T. Metabolic kinases moonlighting as protein kinases. Trends Biochem. Sci. 43, 301–310 (2018).
Dasgupta, S. et al. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature 556, 249–254 (2018).
Xu, D. et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature 580, 530–535 (2020).
Liu, R. et al. Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. Mol. Cell 81, 2722–2735 (2021).
Guo, D. et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IκBα. Cell Metab. 34, 1312–1324 (2022).
Liu, G. M. & Zhang, Y. M. Targeting FBPase is an emerging novel approach for cancer therapy. Cancer Cell Int. 18, 36 (2018).
Chen, H., Xia, Y., Fang, D., Hawke, D. & Lu, Z. Caspase-10-mediated heat shock protein 90β cleavage promotes UVB irradiation-induced cell apoptosis. Mol. Cell. Biol. 29, 3657–3664 (2009).
Tong, Y. et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol. Cell 81, 2303–2316 (2021).
Qian, X. et al. Phosphoglycerate kinase 1 phosphorylates Beclin1 to induce autophagy. Mol. Cell 65, 917–931 (2017).
Li, X. et al. Nuclear PGK1 alleviates ADP-dependent inhibition of CDC7 to promote DNA replication. Mol. Cell 72, 650–660(2018).
Xu, D. et al. The protein kinase activity of fructokinase A specifies the antioxidant responses of tumor cells by phosphorylating p62. Sci. Adv. 5, eaav4570 (2019).
Kimura, M. et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat. Protoc. 5, 1596–1607 (2010).
Zhang, J. M., Yadav, T., Ouyang, J., Lan, L. & Zou, L. Alternative lengthening of telomeres through two distinct break-induced replication pathways. Cell Rep. 26, 955–968 (2019).
Idilli, A. I., Segura-Bayona, S., Lippert, T. P. & Boulton, S. J. A C-circle assay for detection of alternative lengthening of telomere activity in FFPE tissue. STAR Protoc. 2, 100569 (2021).
Guan, K. L. & Dixon, J. E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J. Biol. Chem. 266, 17026–17030 (1991).
He, X. et al. Loss of hepatic aldolase B activates Akt and promotes hepatocellular carcinogenesis by destabilizing the Aldob/Akt/PP2A protein complex. PLoS Biol. 18, e3000803 (2020).
Mowry, E. M. & Corboy, J. R. Another sphingosine 1-phosphate receptor modulator for the treatment of patients with multiple sclerosis. Lancet Neurol. 18, 983–985 (2019).
Du, L. et al. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J. Exp. Med. 217, e20191115 (2020).
Yang, W. et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 480, 118–122 (2011).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (82188102 and 82030074, Z.L.; 82072630, D.X.; 82173114, Z.W.; 82103351, M.L.; 82103354, X.H.; 82372816, D.G.), the Ministry of Science and Technology of the People’s Republic of China (2020YFA0803300, Z.L.; 2021YFA0805600, D.X.), the China Postdoctoral Science Foundation (2021M692827, X.H.), the Zhejiang Natural Science Foundation Key Project (LD22H160002, D.X.) and the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (SN-ZJU-SIAS-006). Z.L. is the Kuancheng Wang Distinguished Chair.
Author information
Authors and Affiliations
Contributions
Z.L. conceptualized the study. Z.L., D.X. and M.L. designed the study. M.L., Z.W., J.T., H.H., X.H., S.L., D.G., X.J., L.Y., H.Y., L.X. and Z.M. performed the molecular and animal experiments and analyzed the data. H.J., H.Z., R.Y. and J.F. performed molecular docking. T.L. provided tissue microarray. Z.L. wrote the manuscript, with comments from all other authors.
Corresponding authors
Ethics declarations
Competing interests
Z.L. owns shares in Signalway Life Sciences, which supplied the rabbit antibodies that recognize TERT pS227. Z.L.’s interest in this company had no bearing on its being chosen to supply these reagents. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Yuanyu Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 FBP1 binds to TERT and acts as a protein phosphatase to dephosphorylate TERT.
b, c, g, i, j, l, m, Immunoprecipitation (IP) and immunoblotting analyses were performed with the indicated antibodies. The experiments were conducted at least three times with similar results. (a) FBP1 immunoprecipitants from Huh7 cells were analyzed by mass spectrometry. Selected peptide hits are shown. (b) 786-O cells were transfected with or without Flag-FBP1 (b, left) or FBP2 (b, right). Immunoprecipitation using the indicated antibody was performed. (c) 786-O cells were transfected with or without Flag-FBP1 as indicated. Cytosol and nuclear fractions were prepared. Immunoprecipitation was performed. (d, e) The metabolic activities (d), Km and Vmax (e) of WT His-FBP1 or N273A proteins purified from bacteria were determined. Data represent the mean ± s.d. Statistical significance was determined by two-way ANOVA. P values were shown. (f) Glycolytic flux in 786-O cells stably expressing WT Flag-FBP1 or N273A was determined. Data represent the mean ± s.d. (n = 3 biological independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test. P values were shown. (g) Immunoprecipitation analyses in 786-O cells expressing the indicated Flag-FBP1 proteins. (h) Specificity validation of the anti-TERT pS227 antibody by preincubation of the anti-TERT pS227 antibody with or without the TERT pS227 peptide before IHC analyses. Scale bars, 50 μm. (i) Immunoprecipitation was performed in 786-O cells with indicated antibodies. (j) Bacterially-purified WT His-FBP1 or S170D proteins were incubated with TERT pS227 peptide (j, top). FBP1 protein expressions were shown (j, bottom). The released phosphate was measured. Data represent the mean ± s.d. (n = 3 biological independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test. P values were shown. (k) Purified 293T-expressed PP2A proteins were incubated with the TERT pS227 peptide. The released phosphate was measured. Data represent the mean ± s.d. (n = 3 biological independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test. P values were shown. (l, m) Immunoblotting was performed in FBP1-deficient 786-O cells expressing PP2A WT or Y307F proteins (l), and cells expressing a scramble or PPP2CA shRNA (m).
Extended Data Fig. 2 FBP1 C129 is in a reduced state and forms a covalent phospho-C129 intermediate for TERT pS227 dephosphorylation.
b, f, g, Immunoprecipitation (IP) and immunoblotting analyses were performed with the indicated antibodies. The experiments were conducted at least three times with similar results. (a) Molecular docking analysis showing the structures of a TERT pS227 protein and FBP1 protein. (b) Immunoprecipitation was performed in 786-O cells expressing WT Flag-FBP1 or C129S. (c, d) The metabolic activities (c), Km and Vmax (d) of WT His-FBP1 or C129S proteins purified from bacteria were determined. Data represent the mean ± s.d. Statistical significance was determined by two-way ANOVA. P values were shown. (e) Glycolytic flux in 786-O cells stably expressing WT Flag-FBP1 or C129S was determined. Data represent the mean ± s.d. (n = 3 biological independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test. P values were shown. (f) Immunoblotting was performed in FBP1 shRNA expressing Huh7 cells with or without Flag-rFBP1 WT or C129S expression. (g) An in vitro kinase reaction was performed by incubating a TERT S227 peptide with purified active GST-AKT1 in the presence or absence of [γ-32P]-ATP. Autoradiography and immunoblot analyses were performed.
Extended Data Fig. 3 Depletion of FBP1 increases the nuclear accumulation of TERT, telomerase activity, and telomere lengths.
(a-d) Normal renal cells HK2 were stably transfected with a control (shNT) or FBP1 shRNA. Immunofluorescence analyses (a), telomerase activity (b) (n = 3 biological independent replicates), QFISH analyses (c) (n = 8 biological independent replicates) and southern blot analyses for telomere restriction fragment (TRF) (d) were determined. QFISH representative images were shown (c, left), and the immunofluorescence intensity was quantitated for eight cells using the ZEISS Zen Microscopy Software (c, right). Data are representative of three independent experiments. Scale bar, 10 μm. Tel, telomere. (e) Telomerase activity in 786-O cells with or without Flag-FBP2 expression was measured. Data represent the mean ± s.d. (n = 3 biological independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test.
Extended Data Fig. 4 FBP1 reduces the nuclear accumulation of TERT, telomerase activity, and telomere lengths and induces tumor cell senescence and inhibited proliferation.
(a) Immunofluorescence staining was performed with the 786-O cells expressing the indicated FBP1 proteins, scale bar, 10 μm. Data are representative of three independent experiments. (b) Cytosolic and nuclear fractions of the 786-O cells with or without expression of the indicated FBP1 protein were prepared. Immunoblotting analyses were performed with the indicated antibodies. The experiments were conducted at least three times with similar results. (c) Telomerase activity (n = 3 biological independent replicates) in FBP1 shRNA expressing Huh7 cells with or without the indicated Flag-rFBP1 protein overexpression was determined. Data represent the mean ± s.d. (n = 3 biological independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test. P values were shown. (d) Progressed QFISH analyses at the indicated time were performed (top) in 786-O cells, the immunofluorescence intensity was quantitated (bottom). (e) QFISH analyses in FBP1 shRNA expressing Huh7 cells with or without the indicated Flag-rFBP1 protein overexpression was determined. Representative images were shown (top), and the immunofluorescence intensity was quantitated (bottom). Immunofluorescence intensity (d, e) was quantitated for five cells using the ZEISS Zen Microscopy Software. Data represent the mean ± s.d. (n = 8 biological independent replicates in d, n = 5 biological independent replicates in e). Statistical significance was determined by two-tailed unpaired Student’s t test. P values were shown. Scale bar, 10 μm. Tel, telomere.
Extended Data Fig. 5 FBP1 does not alter ALT activity.
a, c, Immunoblotting analyses were performed with the indicated antibodies. The experiments were conducted at least three times with similar results. (a) Immunoblotting analyses of the indicated 786-O, Huh7 (left), and LN18 cells (right) were performed with the indicated antibodies. (b) C-circle in the 786-O cells expressing WT or the mutated FBP1 (80 days) was measured (n = 3 biological independent replicates). Data represent the mean ± s.d. Statistical significance was determined by two-tailed unpaired Student’s t test. (c) TERT shRNA expressing 786-O cells with or without Flag-FBP1 protein expression were stably transfected with TERT shRNA resistant V5-rTERT WT or S227D. Immunoblotting analyses were performed with the indicated antibodies.
Extended Data Fig. 6 FBP1 expression inhibits tumor growth by inhibiting TERT activity.
(a, b) Cell proliferation (a) (n = 3 biological independent replicates) and colony formation (b) were analyzed in FBP1 shRNA expressing Huh7 cells with or without the indicated Flag-rFBP1 protein overexpression. (c-e) Huh7 cells with or without depletion of endogenous FBP1 and with or without reconstituted overexpression of the indicated Flag-FBP1 proteins were subcutaneously injected into nude mice (n = 5 per group). Representative images are shown (c). Tumor weights (d) and tumor volumes (e) were measured. Data represent the mean ± s.d. Statistical significance was determined by two-tailed unpaired Student’s t test (a, d, e). P values were shown.
Extended Data Fig. 7 FBP1-mediated dephosphorylation of TERT disrupts telomere function, leading to senescence of tumor cells and tumor growth inhibition.
IHC analyses were performed with the indicated antibodies. (a) 786-O cells with or without stably expressing the indicated Flag-FBP1 protein were subcutaneously injected into nude mice. (b) FBP1 shRNA expressing Huh7 cells with or without the indicated Flag-rFBP1 protein overexpression were subcutaneously injected into nude mice. (c) TERT shRNA expressing 786-O cells with or without Flag-FBP1 protein expression were stably transfected with TERT shRNA resistant V5-rTERT WT, S227A or S227D. Cells were subcutaneously injected into nude mice. Representative images are shown (upper). The expression levels of the indicated proteins were quantified for ten microscopic fields of the tumor samples (lower). IHC Score=intensity×area. The intensity was graded as 0, 1, 2, 3. The area of positive staining was assessed by 0–25%, 26–50%, 51–75%, 76–100%, corresponding to 0, 1, 2, 3, 4 grades, respectively. Scale bars, 50 μm. Data represent the mean ± s.d. Statistical significance was determined by two-tailed unpaired Student’s t test. P values were shown.
Extended Data Fig. 8 FBP1 expression is inversely correlated with TERT S227 phosphorylation level and is negatively associated with the clinical aggressiveness of ccRCC and HCC.
(a) IHC analyses of human HCC specimens were performed with the indicated antibodies. Representative images are shown. Scale bars, 100 μm. (b) IHC staining of human HCC samples with the indicated antibodies was scored, and correlation analyses were performed. A Pearson correlation test was used (two-tailed) (n = 90). Some of the dots on the graphs represent more than one specimen (that is, some scores overlapped). P values were shown. (c) Kaplan-Meier plots of the overall survival rates of HCC patients (n = 90) in the groups with high (staining score, 4–8) and low (staining score, 0–3) expression of FBP1 and TERT pS227. The P values were calculated by the log-rank test. P values were shown.
Extended Data Fig. 9 LNP-mediated delivery of FBP mRNA was safe for mice.
(a) Schematic illustration of LNP preparation. A microfluidic-based mixing of lipids to construct LNPs encapsulating the indicated mRNA. (b-h) The MOCK LNPs or PBS (control group) were intravenously injected into 6-week-old nude mice every other day for a total of 2.5 weeks at a dose of 0.5 mg/kg body weight (n = 5 mice per group). Mice heart, liver, spleen, lung and kidney were harvested for H&E staining (b). Data are representative of six independent micrographs. Scale bars, 100 μm. Mice blood were collected for AST (c), ALT (d), CR (e), BUN (f), CK (g) and LDH (h) analysis (n = 5 mice per group). AST, aspartate transaminase; ALT, alanine aminotransferase; CR, creatinine; BUN, blood urea nitrogen; CK, creatine kinase; LDH, lactate dehydrogenase. (i-k) The 786-O cells were subcutaneously injected into 6-week-old nude mice. The LNPs with or without the indicated FBP1 mRNAs were intravenously injected every other day for a total of 2.5 weeks at a dose of 0.5 mg/kg body weight. [U-13C6]-glucose (1.5 mg/kg body weight) was injected into mouse tail veins before sacrificing the mice. Metabolic flux analyses of injected [U-13C6]-glucose in xenograft tumor (i), liver (j), and kidney (k) were measured (n = 3 mice per group). Data represent the mean ± s.d. Statistical significance was determined by two-tailed unpaired Student’s t test (c-k). P values were shown.
Extended Data Fig. 10 LNP-mediated delivery of FBP mRNA and effectively inhibits tumor growth.
(a-c) The 786-O cells were subcutaneously injected into 6-week-old nude mice (n = 5 per group). The LNPs with or without the indicated FBP1 mRNAs were intravenously injected every other day at a dose of 0.5 mg/kg body weight for a total of 2.5 weeks. Tumor volumes (a) and weights (b) were calculated. IHC analyses of mouse tumor tissues were performed with the indicated antibodies (c). Representative images are shown (c, upper). The expression levels of the indicated proteins were quantified for ten microscopic fields of the tumor samples (c, lower). Scale bars, 50 μm. Data represent the mean ± s.d. Statistical significance was determined by two-tailed unpaired Student’s t test. P values were shown.
Supplementary information
Supplementary Information
Supplementary Table 1
Source data
Source Data Fig. 1
Unprocessed western blots
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Unprocessed western blots
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 1
Unprocessed western blots
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Unprocessed western blots
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Unprocessed western blots
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Unprocessed western blots
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Source Data Extended Data Fig. 9
Statistical Source Data
Source Data Extended Data Fig. 10
Statistical Source Data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Wang, Z., Tao, J. et al. Fructose-1,6-bisphosphatase 1 dephosphorylates and inhibits TERT for tumor suppression. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01597-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01597-2