Abstract
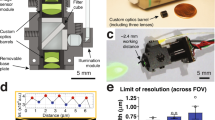
Spinal cord circuits play crucial roles in transmitting pain, but the underlying activity patterns within and across spinal segments in behaving mice have remained elusive. We developed a wearable widefield macroscope with a 7.9-mm2 field of view, ~3- to 4-μm lateral resolution, 2.7-mm working distance and <10-g overall weight and show that highly localized painful mechanical stimuli evoke widespread, coordinated astrocyte excitation across multiple spinal segments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Additional (for example, raw image) data that support the findings of this study are available from the corresponding author. Because of their size, they can only be shared on reasonable request. Source data are provided with this paper.
Code availability
The custom software or code generated during the current study is available from the corresponding author. Because it requires expert guidance for proper use, it will be shared on reasonable request.
References
Koch, S. C., Acton, D. & Goulding, M. Spinal circuits for touch, pain, and itch. Annu. Rev. Physiol. 80, 189–217 (2018).
Nelson, N. A., Wang, X., Cook, D., Carey, E. M. & Nimmerjahn, A. Imaging spinal cord activity in behaving animals. Exp. Neurol. 320, 112974 (2019).
Abraira, V. E. & Ginty, D. D. The sensory neurons of touch. Neuron 79, 618–639 (2013).
Harrison, M. et al. Vertebral landmarks for the identification of spinal cord segments in the mouse. NeuroImage 68, 22–29 (2013).
Aharoni, D. & Hoogland, T. M. Circuit investigations with open-source miniaturized microscopes: past, present and future. Front. Cell. Neurosci. 13, 141 (2019).
Chen, S. et al. Miniature fluorescence microscopy for imaging brain activity in freely-behaving animals. Neurosci. Bull. 36, 1182–1190 (2020).
Scott, B. B. et al. Imaging cortical dynamics in GCaMP transgenic rats with a head-mounted widefield macroscope. Neuron 100, 1045–1058 (2018).
Rynes, M. L. et al. Miniaturized head-mounted microscope for whole-cortex mesoscale imaging in freely behaving mice. Nat. Methods 18, 417–425 (2021).
Guo, C. et al. Miniscope-LFOV: a large field of view, single cell resolution, miniature microscope for wired and wire-free imaging of neural dynamics in freely behaving animals. Preprint at bioRxiv https://doi.org/10.1101/2021.1111.1121.469394 (2021).
Leman, D. P. et al. Large-scale cellular-resolution imaging of neural activity in freely behaving mice. Preprint at bioRxiv https://doi.org/10.1101/2021.01.15.426462 (2022).
Sekiguchi, K. J. et al. Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nat. Commun. 7, 11450 (2016).
Kohro, Y. et al. Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hypersensitivity. Nat. Neurosci. 23, 1376–1387 (2020).
Xu, Q. et al. Astrocytes contribute to pain gating in the spinal cord. Sci. Adv. 7, eabi6287 (2021).
Nam, Y. et al. Reversible induction of pain hypersensitivity following optogenetic stimulation of spinal astrocytes. Cell Rep. 17, 3049–3061 (2016).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Merten, K., Folk, R. W., Duarte, D. & Nimmerjahn, A. Astrocytes encode complex behaviorally relevant information. Preprint at bioRxiv https://doi.org/10.1101/2021.10.09.463784 (2021).
Zhou, P. et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 7, e28728 (2018).
Giovannucci, A. et al. CaImAn an open source tool for scalable calcium imaging data analysis. eLife 8, e38173 (2019).
Ceto, S., Sekiguchi, K. J., Takashima, Y., Nimmerjahn, A. & Tuszynski, M. H. Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after spinal cord injury. Cell Stem Cell 27, 430–440 (2020).
Kondo, T. et al. Calcium transient dynamics of neural ensembles in the primary motor cortex of naturally behaving monkeys. Cell Rep. 24, 2191–2195 (2018).
Rieder, P. et al. Astrocytes and microglia exhibit cell-specific Ca2+ signaling dynamics in the murine spinal cord. Front. Mol. Neurosci. 15, 840948 (2022).
Goldey, G. J. et al. Removable cranial windows for long-term imaging in awake mice. Nat. Protoc. 9, 2515–2538 (2014).
Dubbs, A., Guevara, J. & Yuste, R. moco: fast motion correction for calcium imaging. Front. Neuroinform. 10, 6 (2016).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018).
Acknowledgements
We thank J. E. Ford for advice on optical design, B. Piatt and J. Warda for help with tolerancing and lens fabrication, G. Zera for feedback on housing design, M. Ikeda for guidance on optical characterizations, B. Temple and E. Sanders for help with linear treadmill testing, N. Andrews of the Salk Behavior Testing Core and the Salk machine shop for technical support, J. Chambers for mouse colony management and members of the Nimmerjahn lab for feedback and suggestions. Artwork in Fig. 2a and Extended Data Figs. 4a,c and 6a was created with Biorender.com. This work was primarily supported by the NIH grant R01NS108034 (A. Nimmerjahn) and was partially supported by the NIH grants U01NS103522, U19NS112959 and U19NS123719, a Salk Innovation Grant, The Sol Goldman Charitable Trust and equipment funds from C. and L. Greenfield (A. Nimmerjahn). P. Shekhtmeyster was supported by a Rose Hills Foundation graduate fellowship, and N. A. Nelson was supported by funds from an NIH T32/CMG Training Grant, a Burt and Ethel Aginsky Research Scholar Award, a Kavli-Helinski Endowment Graduate Fellowship and an NIH individual predoctoral fellowship (F31NS120619). The content is solely the authors’ responsibility and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
P.S., D.D. and A. Nimmerjahn conceived and designed the study with input from E.M.C. and N.A.N. P.S. developed and characterized the wearable macroscopes and wrote ImageJ-based data analysis code. D.D. performed the in vivo imaging experiments. E.M.C. conducted the motor behavior experiments. P.S., D.D. and E.M.C. analyzed the in vitro and in vivo data. A. Ngo, G.G. and J.A.O. developed MATLAB- and ImageJ-based data analysis code. A. Nimmerjahn supervised the study, helped with experiments and wrote the initial manuscript draft. All authors contributed to the text and figures, discussed the results or provided input and edits on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Wearable macroscopes with custom-compound microlenses have a predicted ~3–4 μm lateral resolution across a millimeter-scale FOV based on Zemax simulations.
a–e, Zemax simulations showing the optical system’s PSF (center) and spot diagrams (right) at different FOV positions (left), including 0 mm (a), 0.8 mm (b), 1.4 mm (c), 1.6 mm (d), and 2.0 mm (e) from the center. At a given FOV location, the RMS spot diagrams for 490 nm (blue), 525 nm (green), and 550 nm (red) are overlaid. RMSavg provides the average radius for these three wavelengths, falling within the wearable macroscope’s achromatic range (450–570 nm). f, RMS radius across the FOV for 490 nm (blue), 525 nm (green), and 550 nm (red). The dotted lines indicate the FOV positions shown in panels a–e. The small hump at field position 0.8 mm corresponds to the location of the field stop used to reduce aberrations in the outer portion of the FOV. g, Optical vignetting across the FOV. The dotted lines indicate the FOV positions shown in panels a–e. The decrease in light collection beyond field position 0.8 mm corresponds to the location of the field stop.
Extended Data Fig. 2 Wearable macroscopes with custom-compound microlenses offer ~2.4–4.1 μm lateral and ~17.7–19.4 μm axial resolutions based on point source measurements.
a–e, Experimental approach for characterizing the wearable macroscope’s lateral and axial PSFs at different FOV positions (left), including 0 mm (a), 0.8 mm (b), 1.4 mm (c), 1.6 mm (d), and 2.0 mm (e) from the center, using a test target with a 1 μm-diameter pinhole (right) (Methods). Image z stacks were acquired at each FOV position. The example images (right) are from the z-positions where the PSF had minimal x–y extent. Scale bars, 500 μm. f–j, Left, lateral PSFs at the different FOV positions. The images are zoom-ins of the indicated areas in a–e (green dashed squares). Right, x cross-section showing the Gaussian fit-based FWHM. Scale bars, 10 μm. k–o, Left, axial PSFs at the different FOV positions. Each image is a maximum-intensity projection through the corresponding z stack. Right, z cross-section showing the Gaussian fit-based FWHM. Scale bars, 10 μm. The red dotted and black solid lines in f-o show the measured intensity along the red dashed lines in the PSF images and the Gaussian fit profile, respectively. FWHM values on the left and right sides of the FOV were comparable. All images are representatives from one sample. Images with similar properties were obtained across multiple independent samples. For LOR and contrast measurements across the FOV, see Fig. 1c and Extended Data Fig. 3, respectively.
Extended Data Fig. 3 Wearable macroscopes with custom-compound microlenses provide ~3–4 μm contrast across a millimeter-scale FOV based on MTF measurements.
a, MTF of the integrated macroscope measured in the center of the FOV using the slanted edge test. b, MTF at different indicated FOV positions relative to the center. c, MTF contrast at 10% (MTF10) across the FOV. Displayed values are averages across similar FOV locations and horizontal and vertical slanted edge measurements. Spatial frequencies were converted to line widths. The data in c are from n = 2 and n = 4 measurements at 0 μm, and ±804 μm and ±1482 μm, respectively. The bar plot is presented as mean ± s.e.m. The larger error bars toward the FOV edge likely indicate sample tilt.
Extended Data Fig. 4 Wearable macroscopes with custom-compound microlenses offer an extended depth of field.
a,c, Schematics of the experimental approach. A wild-type mouse was prepared with a lumbar spinal window, injected retroorbitally with FITC-dextran (2% w/v), and imaged with both the wearable macroscope (a–b) and a two-photon microscope (c–d) under anesthesia. b, Average intensity image from a time-lapse recording acquired with the wearable macroscope at a set focal depth. Images were acquired at 45 fps (Supplementary Video 1). A 45-frame/1-s average is shown. Scale bar, 500 μm. d, Right, example images from a two-photon z stack acquired with 1 μm axial step size at the FOV location indicated in a (yellow box). z = 0 μm denotes the tissue surface. Each image represents a 2-frame/1-s average. Scale bar, 500 μm. Left, maximum-intensity projection image over a 70 μm-thick tissue volume. Scale bar, 250 μm. e, Comparison of the macroscope (top) and two-photon maximum intensity projection images of various z extent (bottom; 40 μm thickness) reveals that the wearable device has a ~40 μm depth of field. The green box indicates an example microvessel seen in both the one- and two-photon data, whereas the blue box indicates a capillary seen only in two-photon images at tissue depths exceeding 40 μm. Scale bars, 250 μm. All images are representatives from one sample. Images with similar properties were obtained across multiple independent samples or regions.
Extended Data Fig. 5 Wearable macroscopes with custom-compound microlenses offer a ~2.7 mm working distance permitting imaging through implanted microprisms.
a,b, Schematics showing the experimental approach for characterizing and comparing two imaging conditions in scattering tissue phantoms (Methods): Top, imaging through a coverslip; bottom, imaging through a coverslip with an attached 2.0 mm × 2.0 mm × 2.0 mm microprism (W × D × H) with a reflective, enhanced aluminum coating on the hypotenuse. c,d, Example images of tissue phantom embedded 6 μm-diameter fluorescent beads. Each image is a maximum intensity projection through a z stack acquired as shown in a–b by translating the wearable macroscope axially. Scale bar, 500 μm. e,f, Maximum intensity side projections of the acquired z stacks. Scale bar, 200 μm. g,h, Bead contrast as a function of imaging depth. Michelson contrast is defined as (peak − background)/(peak + background) and, therefore, unitless. i,j, Lateral FWHM of the 6 μm-diameter fluorescent beads as a function of imaging depth. All images are representatives from one sample. Images with similar properties were obtained across multiple independent samples.
Extended Data Fig. 6 Spine-mounted macroscopes of <10 g and low center of gravity have little effect on mouse open-field behavior.
a,e,g, Schematics of the open-field test and analysis approach used to compare the animal’s general locomotor activity with or without the ~9.8 g macroscope mounted to its lumbar spinal cord following a 3–5 d habituation period. b–d,f,h, Population data from all animals (N = 5) and analysis periods (six consecutive 5-min recordings per animal) (left) or only the first and last five minutes (right) of the 35–40 min recordings for each condition (mounted, unmounted) (Methods). Evaluated parameters included the animal’s total distance traveled (b), average running speed (c), rearing activity (d), inner versus outer zone occupancy (f), and quadrant/zone 1–4 occupancy (h). Paired two-sided t-tests determined P values (b, left: 0.1226; b, right: 0.0944, 0.0814; c, left: 0.1465; c, right: 0.5371, 0.3636; d, left: 0.1214; d, right: 0.6317, 0.3271; f, left: 0.0158, 0.1456, 0.2056, 0.0146; f, right: 0.8970, 0.7451, 0.7738, 0.7166; h, left: 0.6676, 0.7382, 0.7114, 0.7215; h, right: 0.5705, 0.8128, 0.6599, 0.8191, 0.1716, 0.6295, 0.2200, 0.2402). All data are presented as mean ± s.e.m.
Extended Data Fig. 7 Wearable macroscopes with custom-compound microlenses resolve cell bodies and processes in live CX3CR1-GFP mice.
a, Fluorescence image showing microglia in an anesthetized CX3CR1-GFP mouse acquired with the wearable macroscope. Scale bar, 250 μm. b, Zoom-ins of the two subregions indicated in a. Scale bars, 100 μm. c, Zoom-ins of the four subregions indicated in b. Individual microglial cell bodies and processes are indicated (closed and open arrowheads, respectively). Scale bars, 10 μm. All images are representatives from one sample. Images with similar properties were obtained across multiple independent samples. For corresponding data from freely behaving mice, see Extended Data Fig. 8g–i and Supplementary Video 2.
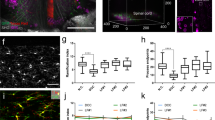
Extended Data Fig. 8 Stable trans-segmental imaging in focally restrained and freely moving CX3CR1-GFP mice.
a,d,g, Average intensity projection images from time-lapse recordings in focally restrained (a,d) or freely behaving CX3CR1-GFP mice (g) (Supplementary Videos 2, 4) overlaid with ~10 μm × 10 μm ROIs. Only ‘active’ ROIs above the indicated ΔF/F thresholds are shown. The chosen ΔF/F thresholds are based on image noise levels, depend on fluorescent indicator expression and were consistently applied across all animals of the same strain. b,e,h, Top, animal behaviors (turning, locomotion) and sensory stimulus application (tail pinch) during the example recordings shown in a,d,g and Supplementary Videos 2, 4. Center, all computationally identified ‘active’ ROIs. Bottom, 500 representative inactive ROIs. Locomotor activity evoked sparse, distributed, and synchronized transients with onset, offset, and plateau kinetics in CX3CR1-GFP mice distinct from cellular activity seen in CX3CR1-GCaMP5g-tdTomato animals (Extended Data Fig. 9). These false positives tended to occur more frequently or with larger amplitude in focally restrained (a and d) compared to freely behaving mice (g), likely due to the larger forces acting on the spinal implant during running when restrained. c,f,i, Population analysis of false positive ratios (‘active’ ROIs/total ROIs) for focally restrained or freely behaving CX3CR1-GFP mice. The data in c, f, and i are from 4, 13, and 19 trials with comparable behavior or stimuli. All data are presented as mean ± s.e.m. (c, 0.82 ± 0.38; f, 0.57 ± 0.17; i, 0). Data with functional calcium indicator expression are shown in Extended Data Fig. 9 and Supplementary Video 5.
Extended Data Fig. 9 High-speed trans-segmental imaging of microglia calcium activity in freely moving CX3CR1-GCaMP5g-tdTomato mice.
a,c,e,g, Maximum intensity projection images from four example time-lapse recordings in a freely behaving CX3CR1-GCaMP5g-tdTomato mouse ~5 weeks after tamoxifen injection (Supplementary Fig. 1; Supplementary Video 5) overlaid with ~10 μm × 10 μm ROIs. The recordings, which show microglia calcium activity during animal rest, tail pinch, locomotion, and turning were acquired over a ~105 min period after macroscope mounting. Only active ROIs above the indicated ΔF/F thresholds are shown. The chosen ΔF/F thresholds are based on image noise levels, depend on fluorescent indicator expression, and were consistently applied across all animals of the same strain. b,d,f,h, Top, turning motion, pressure stimulus amplitude, and locomotor activity during the example recordings shown in a, c, e, and g and Supplementary Video 5. Center, all computationally identified active ROIs. Bottom, 500 representative inactive ROIs. The spatiotemporal properties of these microglial single-cell and population calcium activities are distinct from motion-induced artifacts in CX3CR1-GFP mice (Extended Data Fig. 8; Supplementary Video 2).
Extended Data Fig. 10 High-speed trans-segmental imaging of sensory-evoked calcium activity in freely behaving GFAP-GCaMP6f mice.
a,b, Average intensity projection images from a time-lapse recording in a freely moving GFAP-GaMP6f mouse (Supplementary Video 8) taken at ~50 μm focal depth below the pia overlaid with ~10 μm × 10 μm ROIs. Only active ROIs above the indicated ΔF/F thresholds in response to an innocuous tail pinch/touch (p < 200 g) (a) or a noxious tail pinch (>500 g) (b) are shown. Like in focally restrained mice (Fig. 2), innocuous tail pinch/touch evoked only sparse activity across spinal segments, while noxious pinch resulted in widespread, bilateral astrocyte excitation. c, Innocuous tail pinch/touch and noxious tail pinch-evoked calcium activity (bottom) for the example recording shown in a–b. Each row depicts the percent of active ROIs across a given mediolateral (Y) position. The corresponding pressure stimulus, locomotor activity, and average calcium transient across the FOV are shown above the activity heat map. Innocuous tail touch/pinch and noxious pinch onsets are indicated by gray vertical lines. d, Population data showing the percent of active ROIs for innocuous and noxious stimulus trials. e, Population data showing the average calcium transient onset latency for noxious pinch trials across all, anterior, or posterior regions (Methods). f,g, Population data showing the individual ROI and average calcium response amplitude (f) and duration (g) for noxious pinch trials. Only 0.4% of ROIs had a transient longer than 6 s. h,i, Population data showing the average calcium transient amplitude (h) and duration (i) on the left and right sides of the spinal cord for noxious pinch trials. The data in d–i are from 11 innocuous and 8 noxious pinch trials in 2 mice. The data in f and g (left) are from 124,928 ROIs with ∆F/F > 20%, 8 recordings, and 2 mice. Paired two-sided t-tests determined P values (e: 0.2431, 0.1231, 0.2131; h: 0.3521; i: 0.5216), and all bar plots are presented as mean ± s.e.m. The box and whisker plots mark the median and the 25th and 75th percentiles, and the whiskers cover the minimum and maximum of the data.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Tables 1–3.
High-speed trans-segmental blood flow imaging in the spinal cord of an anesthetized FITC-dextran-injected wild-type mouse. Top, time-lapse recording acquired with the wearable macroscope in a wild-type mouse injected retroorbitally with FITC-dextran (2% (wt/vol)) and prepared with a lumbar spinal window. Images were acquired at 45 fps; scale bar, 500 μm. Bottom, zoom-in videos of the three indicated regions, demonstrating that the wearable macroscope enables high-speed, high-resolution measurements across the millimeter-scale FOV; scale bar, 50 μm. For high-speed, high-resolution measurement of cellular calcium activity in freely moving mice, see Extended Data Figs. 9 and 10 and Supplementary Videos 5 and 8.
Stable trans-segmental imaging of microglial cell bodies and processes in freely behaving CX3CR1–GFP mice. Top left, example videos from a 4-min recording showing an 11-week-old male CX3CR1–GFP mouse with a mounted wearable macroscope exploring an open field arena. The mouse could carry the device on its back without extensive training (30 min of habituation). A littermate was placed in the same area for behavioral comparison. Both mice were naive to the open field apparatus, having never seen the arena before. Bottom, simultaneously acquired time-lapse recording (23 fps) showing GFP-labeled spinal microglia across a 2.75 mm × 1.25 mm FOV. The data were obtained at a focal depth of ~50 μm below the pia. Elapsed time is indicated. Top right, zoom-in of the subregion indicated on the bottom. Note that individual microglial cells remained in focus throughout the recording, including during rest periods, running and turning (Extended Data Fig. 8g–i); scale bars, 250 μm (bottom) and 10 μm (top right). For the behavior analysis of trained mice (3–5 d of habituation) in the open field arena, see Extended Data Fig. 6.
Spine-mounted macroscopes of <10 g and low center of gravity have little effect on mouse linear treadmill behavior. Example videos showing the same mouse on a linear treadmill with (top) and without (bottom) the wearable macroscope. The mouse was habituated to carrying the microscope for 5 d before testing. The videos were recorded at 200 fps but are replayed at reduced speed to allow comparison of mouse gait.
Stable trans-segmental imaging of microglial cell bodies and processes in focally restrained behaving CX3CR1–GFP mice. Top left, example behavior videos from a pinch and run trial of a 12-week-old female CX3CR1–GFP mouse focally restrained on a spherical treadmill. Bottom, simultaneously acquired time-lapse recording (23 fps) showing GFP-labeled spinal microglia across a 2.25 mm × 0.98 mm FOV. The data were obtained at a focal depth of ~50 μm below the pia. Elapsed time is indicated. Top right, zoom-in of the subregion indicated on the bottom. Individual microglial cells remained in focus throughout the recordings, including during rest periods and running (Extended Data Fig. 8a–f); scale bars, 250 μm (bottom) and 50 μm (top right).
High-speed trans-segmental imaging of microglia calcium activity in freely behaving CX3CR1-GCaMP5g-tdTomato mice. Top left, example behavior videos showing four different trial types (rest, pinch, locomotion and turning; Extended Data Fig. 9) in a 16-week-old male CX3CR1-GCaMP5g-tdTomato mouse with a mounted wearable macroscope exploring an open field arena. Note that the mouse could carry the device on its back without extensive training (30 min of habituation). It had not seen the arena before. The shown data were acquired over a ~105-min period after macroscope mounting. Bottom, simultaneously acquired time-lapse recording (45 fps) showing GCaMP5g-labeled spinal microglia across a FOV of 2.63 mm × 1.37 mm. The data were obtained at a focal depth of ~50 μm below the pia. Elapsed time is indicated. Note that the background artifacts/fluctuations are a result of image processing (for display purposes), likely caused by tissue movement under the optical window/coverslip, and do not appear in the analyzed raw data (Extended Data Fig. 9). Top right, zoom-in of two subregions indicated on the bottom. Individual microglial cells showed spontaneous somatic calcium transients throughout the recordings, including rest, noxious tail pinch, locomotion and turning periods; scale bars, 250 μm (bottom) and 50 μm (top right). For calcium activity measurements in freely behaving GFAP-GCaMP6f mice with labeled astrocytes, see Extended Data Fig. 10 and Supplementary Video 8.
High-speed trans-segmental imaging of innocuous tail pinch-evoked calcium activity in focally restrained behaving GFAP-GCaMP6f mice. Top left, example behavior video showing a focally restrained GFAP-GCaMP6f mouse with a wearable macroscope on a spherical treadmill. Focal restraint allows precise placement of sensory stimuli (for example, tail pinch), while placing the mouse on the treadmill provides a quantitative readout of locomotor activity. Bottom, simultaneously acquired time-lapse recording (45 fps) showing innocuous tail pinch-evoked calcium excitation in spinal astrocytes (Fig. 2b,c and Supplementary Fig. 2, left column). The data were obtained at a focal depth of ~50 μm below the pia. Elapsed time is indicated in the upper right corner. Top right, zoom-in of the subregion indicated on the bottom, shown in both raw and ΔF/F formats. Innocuous pinch triggered sparse calcium excitation across the imaged lumbar spinal segments. Running alone did not evoke substantial calcium increases. Also, note that despite the dense tissue labeling, cellular-sized large-amplitude transients can be seen throughout the FOV; scale bars, 250 μm (bottom) and 50 μm (top right). For calcium activity measurements in freely behaving GFAP-GCaMP6f mice, see Extended Data Fig. 10 and Supplementary Video 8.
High-speed trans-segmental imaging of noxious tail pinch-evoked calcium activity in focally restrained behaving GFAP-GCaMP6f mice. Top left, example behavior video showing a focally restrained GFAP-GCaMP6f mouse with a wearable macroscope on a spherical treadmill. Focal restraint allows precise placement of sensory stimuli (for example, tail pinch), while placing the mouse on the treadmill provides a quantitative readout of locomotor activity. Bottom, simultaneously acquired time-lapse recording (45 fps) showing noxious tail pinch-evoked calcium excitation in spinal astrocytes (Fig. 2d,e and Supplementary Fig. 2, right column). The data were obtained at a focal depth of ~50 μm below the pia. Elapsed time is indicated in the upper right corner. Top right, zoom-in of the subregion indicated on the bottom, shown in both raw and ΔF/F formats. Noxious pinch triggered widespread, coordinated calcium excitation on both sides of the spinal cord and across lumbar spinal segments. Also note that despite the dense tissue labeling, cellular-sized transients can be seen throughout the FOV before and after the noxious pinch; scale bars, 250 μm (bottom) and 50 μm (top right). For calcium activity measurements in freely behaving GFAP-GCaMP6f mice, see Extended Data Fig. 10 and Supplementary Video 8.
High-speed trans-segmental imaging of innocuous and noxious tail pinch-evoked calcium activity in freely behaving GFAP-GCaMP6f mice. Left, example behavior videos showing a freely behaving GFAP-GCaMP6f mouse with a wearable macroscope in an open field arena. In example 2, a Kimwipe was placed in the arena to entice the animal to occupy the center and enable easier tail access to provide the pinch stimulus. Animal behavior (for example, locomotion) was scored manually (Extended Data Fig. 10). Right, simultaneously acquired time-lapse recording (45 fps) showing innocuous tail pinch/touch and noxious tail pinch-evoked calcium excitation in spinal astrocytes (Extended Data Fig. 10). The data were obtained at a focal depth of ~50 μm below the pia. Elapsed time is indicated in the upper right corner. Like in focally restrained mice (Fig. 2 and Supplementary Videos 6 and 7), innocuous tail pinch/touch evoked only sparse activity across spinal segments, while noxious pinch resulted in widespread, bilateral astrocyte excitation; scale bar, 250 μm.
Supplementary Data
Statistical source data.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shekhtmeyster, P., Duarte, D., Carey, E.M. et al. Trans-segmental imaging in the spinal cord of behaving mice. Nat Biotechnol 41, 1729–1733 (2023). https://doi.org/10.1038/s41587-023-01700-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-023-01700-3