Abstract
Correct development and maturation of the enteric nervous system (ENS) is critical for survival1. At birth, the ENS is immature and requires considerable refinement to exert its functions in adulthood2. Here we demonstrate that resident macrophages of the muscularis externa (MMϕ) refine the ENS early in life by pruning synapses and phagocytosing enteric neurons. Depletion of MMϕ before weaning disrupts this process and results in abnormal intestinal transit. After weaning, MMϕ continue to interact closely with the ENS and acquire a neurosupportive phenotype. The latter is instructed by transforming growth factor-β produced by the ENS; depletion of the ENS and disruption of transforming growth factor-β signalling result in a decrease in neuron-associated MMϕ associated with loss of enteric neurons and altered intestinal transit. These findings introduce a new reciprocal cell–cell communication responsible for maintenance of the ENS and indicate that the ENS, similarly to the brain, is shaped and maintained by a dedicated population of resident macrophages that adapts its phenotype and transcriptome to the timely needs of the ENS niche.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The scRNA-seq dataset generated and analysed during the current study is available in the ArrayExpress repository with accession no. E-MTAB-11453. We further analysed published datasets from Morarach et al.16, which are available at the GeoGene Expression Omnibus database under identifier GSE149524. In addition we performed analysis using the dataset published by Elmentaite et al.34, which is available at ArrayExpress with accession no. E-MTAB-9543, and by Drokhlyansky et al.33, which is deposited in the Single Cell Portal (SCP1038). Source data are provided with this paper. Should further clarification be required, requests can be directed to the corresponding author.
Change history
24 July 2023
In the version of this article initially published, there was an error in the metadata associated with Pieter Vanden Berghe’s name, which is now amended in the online version of the article.
References
Fung, C. & Vanden Berghe, P. Functional circuits and signal processing in the enteric nervous system. Cell. Mol. Life Sci. 77, 4505–4522 (2020).
Parathan, P., Wang, Y., Leembruggen, A. J., Bornstein, J. C. & Foong, J. P. The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Dev. Biol. 458, 75–87 (2020).
Michel, K. et al. How big is the little brain in the gut? Neuronal numbers in the enteric nervous system of mice, Guinea pig, and human. Neurogastroenterol. Motil. 34, e14440 (2022).
Terra, S. A., De Arruda Lourenção, P. L., Silva, M. G., Miot, H. A. & Rodrigues, M. A. M. A critical appraisal of the morphological criteria for diagnosing intestinal neuronal dysplasia type B. Mod. Pathol. 30, 978–985 (2017).
Rao, M. & Gershon, M. D. The bowel and beyond: the enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 13, 517–528 (2016).
Vries, P., de, Soret, R., Suply, E., Heloury, Y. & Neunlist, M. Postnatal development of myenteric neurochemical phenotype and impact on neuromuscular transmission in the rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 299, 539–547 (2010).
Bergner, A. J. et al. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J. Comp. Neurol. 522, 514–527 (2014).
Nobs, S. P. & Kopf, M. Tissue-resident macrophages: guardians of organ homeostasis. Trends Immunol. 42, 495–507 (2021).
Ginhoux, F. & Guilliams, M. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44, 439–449 (2016).
Guilliams, M., Thierry, G. R., Bonnardel, J. & Bajenoff, M. Establishment and maintenance of the macrophage niche. Immunity 52, 434–451 (2020).
Viola, M. F. & Boeckxstaens, G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut https://doi.org/10.1136/gutjnl-2020-323121 (2020).
De Schepper, S. et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175, 400–415 (2018).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013).
Muller, P. A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014).
Gabanyi, I. et al. Neuro-immune interactions drive itssue programming in intestinal macrophages. Cell 164, 378–391 (2016).
Morarach, K. et al. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat. Neurosci. 24, 34–46 (2021).
Summers, K. M., Bush, S. J. & Hume, D. A. Network analysis of transcriptomic diversity amongst resident tissue macrophages and dendritic cells in the mouse mononuclear phagocyte system. PLoS Biol. 18, e3000859 (2020).
Wang, P. L. et al. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat. Commun. 11, 2552 (2020).
Ydens, E. et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat. Neurosci. 23, 676–689 (2020).
Chakarov, S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019).
Luissint, A.-C., Nusrat, A. & Parkos, C. A. JAM-related proteins in mucosal homeostasis and inflammation. Semin. Immunopathol. 36, 211–226 (2014).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Liu, Z. et al. Fate mapping via Ms4a3-expression history traces monocyte-derived cells. Cell 178, 1509–1525 (2019).
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Joseph, N. M. et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest. 121, 3398–3411 (2011).
Sakata, K., Kunieda, T., Furuta, T. & Sato, A. Selective destruction of intestinal nervous elements by local application of benzalkonium solution in the rat. Experientia 35, 1611–1613 (1979).
Sato, A. et al. Pathophysiology of aganglionic colon and anorectum: an experimental study on aganglionosis produced by a new method in the rat. J. Pediatr. Surg. 13, 399–435 (1978).
Gombash, S. E. et al. Intravenous AAV9 efficiently transduces myenteric neurons in neonate and juvenile mice. Front. Mol. Neurosci. 7, 81 (2014).
Zöller, T. et al. Silencing of TGFβ signalling in microglia results in impaired homeostasis. Nat. Commun. 9, 4011 (2018).
Wrana, J. L., Attisano, L., Wieser, R., Ventura, F. & Massagué, J. Mechanism of activation of the TGF-β receptor. Nature 370, 341–347 (1994).
Bain, C. C. et al. TGFβR signalling controls CD103+CD11b+ dendritic cell development in the intestine. Nat. Commun. 8, 620 (2017).
Drokhlyansky, E. et al. The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622 (2020).
Elmentaite, R. et al. Cells of the human intestinal tract mapped across space and time. Nature 597, 250–255 (2021).
Cipriani, G. et al. Muscularis propria macrophages alter the proportion of nitrergic but not cholinergic gastric myenteric neurons. Cell. Mol. Gastroenterol. Hepatol. 7, 689–691 (2019).
Ahrends, T. et al. Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections. Cell 184, 5715–5727 (2021).
Wang, P. et al. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 10, 755 (2019).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Walton, N. M. et al. Microglia instruct subventricular zone neurogenesis. Glia 54, 815–825 (2006).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016).
Lenz, K. M. & Nelson, L. H. Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 9, 698 (2018).
Orłowski, D., Sołtys, Z. & Janeczko, K. Morphological development of microglia in the postnatal rat brain. A quantitative study. Int. J. Dev. Neurosci. 21, 445–450 (2003).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
Nelson, L. H. & Lenz, K. M. Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav. Brain Res. https://doi.org/10.1016/j.bbr.2016.09.006 (2017).
Athow, A. C., Filipe, M. I. & Drake, D. P. Hyperganglionosis mimicking Hirschsprung’s disease. Arch. Dis. Child. 66, 1300–1303 (1991).
Wedel, T. et al. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology 123, 1459–1467 (2002).
Bassotti, G., Villanacci, V. & Nejad, M. R. Chronic constipation: no more idiopathic, but a true neuropathological entity. Gastroenterol. Hepatol. Bed Bench 4, 109–115 (2011).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Hao, M. M. et al. Early emergence of neural activity in the developing mouse enteric nervous system. J. Neurosci. 31, 15352 (2011).
Vanden Berghe, P., Kenyon, J. L. & Smith, T. K. Mitochondrial Ca2+ uptake regulates the excitability of myenteric neurons. J. Neurosci. 22, 6962–6971 (2002).
Schafer, D. P., Lehrman, E. K., Heller, C. T. & Stevens, B. An engulfment assay: a protocol to assess interactions between CNS phagocytes and neurons. J. Vis. Exp. https://doi.org/10.3791/51482 (2014).
Becker, L. et al. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut https://doi.org/10.1136/gutjnl-2016-312940 (2017).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Cifone, M. G. et al. Dexamethasone-induced thymocyte apoptosis: apoptotic signal involves the sequential activation of hyposphoinositide-specific phospholipase C, acidic sphingomyelinase, and caspases. Blood 93, 2282–2296 (1999).
Cooper, H. S., Murthy, S. N., Shah, R. S. & Sedergran, D. J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69, 238–249 (1993).
Boirivant, M., Fuss, I. J., Chu, A. & Strober, W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med. 188, 1929–1939 (1998).
Kennedy, R. J. et al. Interleukin 10-deficient colitis: new similarities to human inflammatory bowel disease. Br. J. Surg. 87, 1346–1351 (2000).
Acknowledgements
We thank P. A. Penttila and R. Chinnaraj from the KU Leuven FACS core for their excellent support and cell sorting. We also thank the Cell & Tissue Imaging Core (P. Vanden Berghe and T. Martens, KU Leuven) for confocal imaging (supported by AKUL 15/37 and FWO I001918N). We also thank A. Escamilla-Ayala of the VIB Leuven BioImaging Core for her support and assistance in this work. We thank K. Kierdorf, M. Prinz, A. Schlitzer, F. Ginhoux, Z. Liu and M. Azhar for providing mice. M.A. is supported by NIH grant nos. R01HL126705, R01 HL157017-01A1 and R01 HL145064-01. M.F.V. is supported by FWO PhD fellowship no. 11C2219N. G.B. is funded by ERC Advanced grant no. 833816-NEUMACS.
Author information
Authors and Affiliations
Contributions
M.F.V. designed and conducted experiments and wrote the manuscript. M.P. performed histological analyses. E.M. and A. Sifrim performed analysis of scRNA-seq data. I.A. and N.F. provided excellent technical support throughout all experiments. M.D., N.S., J.V.H., H.T. and M.C.E. performed experiments. T.M. performed ex vivo time-lapse imaging. K.V. performed HCR experiments. S.V., S.D.S., P.V.B., T.V. and A. Sifrim provided intellectual input. K.K., M.P., A. Schlitzer, Z.L. and F.G. provided mice. P.P. performed genotyping and tamoxifen injections. M.A. generated Tgfb2fl/fl and Tgfb3fl/fl mice and genetically combined them to create Tgfb2-3fl/fl mice. G.M. performed histological evaluation of haematoxylin and eosin stainings. G.B. led the project and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ulrika Marklund, Elvira Mass and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Postnatal maturation of the ENS.
Neuronal density (A), ganglia (> 5 neurons)/mm2 (B) and single extra-ganglionic neurons/mm2 (C) in the myenteric plexus, corrected for tissue growth at P10 (n = 5-9), P14 (n = 6), P21 (n = 5) and P56 (n = 4-6). (D) NOS+ neurons, expressed as a percentage of HuC/D+ cells (n = 4-5). (E) ChAT+ area, normalised to HuC/D+ neurons (n = 4-5). (F) Synapsin-I volume/µm3, normalised to tissue growth (n = 4). Total length of MMϕ filopodia (G), number of dendrites (H) and cell volume (I) (Imaris) (n = 3). (J) Percentage of MMϕ with at least one phagocytic cup (n = 3). (K) Confocal images displaying MMϕ morphology. Scale bar: 10 µm. (L) Confocal image of the myenteric plexus at P14 of Synapsin-I or isotype control. Scale bar: 20 µm. (M) Percentage of synaptic engulfment per cell (Synapsin-I+ volume/MMϕ volume normalised to synaptic density) (n = 4). (N) Digital reconstruction depicting synapses within CD68+ lysosomes of MMϕ. Arrowheads indicate engulfed synapses. (O) Orthogonal view depicting MMϕ engulfing synaptic machinery at P14. (P) Confocal images displaying MMϕ engulfing neuronal cell bodies. Scale bar: 20 µm. (Q) Digital reconstructions of MMϕ engulfing enteric neurons. (R) MMϕ engulfing tdTomato+ neural debris (orthogonal view). (S) Mean fluorescence intensity of MMϕ engulfing neural cells at P14 (n = 7) and P56 (n = 6). (T) Graph and contour plot of tdTomato+ debris uptake by MMϕ in vivo (GFP+) and ex vivo (GFP-) (n = 4). (U) MMϕ engulfing Wnt1-tdTomato+ debris (orthogonal view). (V) MMϕ engulfing Baf53b-tdTomato+ neuronal debris (orthogonal view). Arrowhead indicates engulfed tdTomato+ debris. Scale bar: 5 µm unless otherwise indicated. Unpaired two-tailed t-tests (G-J, S) or Ordinary One-Way ANOVAs with Tukey’s multiple comparisons (A-E, M). Data are shown as Mean ± SD. Images shown are representative of at least 3 biological replicates, apart from L, R, U-V (2 biological replicates). Single points indicate biological replicates, apart from F-M (technical replicates). n = x biologically independent samples.
Extended Data Fig. 2 MMϕ mature transcriptionally after weaning.
(A) Dot plot in αCSF1R-treated mice and controls to show efficient depletion of MMϕ. Cells shown are pre-gated on fsc/ssc, single cells, live and CD45+. (B) Quantification of immune cells in αCSF1R-treated mice (n = 4) and controls (n = 5). Macrophages are defined as CD45+CD11b+CD64+, B cells as CD45+CD3e-CD64−CD11b−CD19+ and T cells as CD45+CD3e+. All populations were pre-gated on fsc/ssc and live cells. Unpaired two-tailed t-tests. Data are shown as Mean ± SD. Each data point indicates a separate biological replicate. *** p < 0.001; ns = not significant. (C) Gating strategy used to isolate Cx3cr1+ MMϕ from the muscularis externa for scRNAseq. (D) UMAP of data prior to exclusion of contaminating cluster (pink). (E) UMAP with heatmap colour-coding to show expression for canonical macrophage markers and CCR2 within the scRNAseq dataset. (F) Violin plots showing the upregulation of extracellular matrix genes in the contaminating cluster (pink). (G) Representative confocal image of the muscularis externa at P10, showing Cx3cr1+ MMϕ embedded within the extracellular matrix (Dcn, Decorin). Image is representative of 3 biological replicates. Scale bar is 20 µm. (H) Volcano plot depicting globally differentially expressed genes at P10 and P56. Coloured dots represent significantly (p < 0.05) upregulated genes over Log2FC > 0.5. (I) Dot plot to depict expression of top 10 upregulated genes within each cluster. Colour of dots represents expression level, while size of dots represents percentage of cells expressing the gene identified. Only genes significantly (p ≤ 0.05) upregulated (logFC ≥ 0.2) are shown. (J) Violin plots showing the expression of Bmp2 in the identified MMϕ clusters. n = x biologically independent samples.
Extended Data Fig. 3 MMϕ subset phenotype and function prior to and after weaning.
(A) Representative gating strategy used to identified MMϕ subsets via flow cytometry. (B) Heatmap depicting expression of genes identified by Chakarov et al., within each cluster, cells pooled. (C) Representative contour plots of identified MMϕ subsets at P10 and P56. (D) Relative frequency of Lyve1+ MMϕ quantified via immunohistochemistry, in the different micro-anatomical niches of the muscularis externa (n = 4). (E) Phagocytic index of each MMϕ subset phagocytosing pHRodo-labelled apoptotic thymocytes, and representative histograms from the same sample incubated at 4 °C and at 37 °C with phRodo-labelled apoptotic cells (n = 5). (F) Representative confocal images showing CD68+ lysosomes in NA-MMϕ and Lyve1+ MMϕ. Images shown are representative of two independent experiments. Scale bar is 20 µm. (G) Relative frequency of NA-MMϕ quantified via immunohistochemistry, in the different micro-anatomical niches of the muscularis externa (n = 4). (H) Dot plot depicting expression of top 5 transcription factors identified via SCENIC within each cluster. Colour of dots represents expression level, while size of dots represents percentage of cells expressing the transcription factor identified. Only genes significantly (p ≤ 0.05) upregulated (logFC ≥ 0.2) are shown. (I) Quantification of the percentage of Ms4a3+ Cx3cr1+ MMϕ in the muscularis externa throughout early postnatal life and adulthood (n = 5-6). (J) Quantification of the percentage of Ms4a3+ Cx3cr1+ MMϕ in the identified MMФ subsets in the muscularis externa throughout early postnatal life and adulthood (n = 5-6). (K) Quantification via flow cytometry of population frequency at 8 weeks (P56) and 20 weeks (n = 5). Data are shown as Mean ± SD, analysed by multiple unpaired t-tests (D-G) or two-way ANOVA followed by Tukey’s (J) or Šídák’s (K) multiple comparison. Each data point indicates a separate biological replicate. n = x biologically independent samples.
Extended Data Fig. 4 NA-MMϕ phenotype is instructed by ENS-derived TGFβ.
(A) Gene expression of TGFβ within the muscularis externa at P10 (n = 4) and P56 (n = 7). (B) Percentage of enteric neurons (Wnt1+Cd49b-) and enteric glia (Wnt1+ Cd49b+) expressing TGFβ (n = 4). (C) Representative contour plot showing TGFβ expression in immune cells (CD45+) and Wnt1-CD45- cells. Only live cells are shown. The graph depicts the percentage of cells of each population expressing TGFβ (n = 4). (D) Expression of Tgfb1, 2 and 3 in enteric neurons, assessed in the published dataset of Morarch et al., 2021. (E) Gene expression of Tgfb1, Tgfb2 and Tgfb3 in immune cells (CD45+) at P10, P14, P28 and P56, FACS-sorted from the muscularis externa of Wnt1cre/wt Rosa26tdT/WT mice (n = 3-4). (F) Hybridization Chain Reaction (HCR) combined with immunohistochemistry showing transcripts of Tgfb2 and Tgfb3, or no probe, in the ENS. Images are representative of 3 biological replicates. Scale bar is 5 µm. (G) Heatmap indicating expression of key marker genes identified in MMϕ subsets in BMDMs stimulated for 24 h with TGFβ1, 2 or 3. Coloured line indicates the MMϕ subset (n = 6-10). Data is representative of two pooled experiments. (H) Frequency of NA-MMϕ throughout early postnatal development to adulthood (n = 5-6). Dotted line indicates time of weaning. (I) Frequency of identified MMϕ subsets in 8 week-old germ-free mice compared to conventionally colonised mice (n = 5). Data are shown as Mean ± SD, analysed using unpaired two-tailed T-tests or ANOVA followed by Šídák (A, I) or Holm-Šídák (E, H) multiple comparisons. Each data point indicates a separate biological replicate. n = x biologically independent samples.
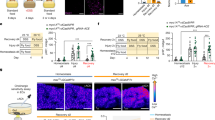
Extended Data Fig. 5 Characterisation of the TGFβ-TGFBR axis in the GI tract.
(A) Expression of Uchl1 and Gfap in the muscularis externa 5 days after BAC treatment compared to SHAM (n = 5). (B) Live Cx3cr1+ MMϕ and (C) relative percentages of Lyve1+ MMϕ and differentiating MMϕ in the muscularis externa in SHAM (n = 4) and BAC-treated (n = 5) mice. (D) Transfection efficiency (GFP+ HUC/D+/HUC/D+ cells) in ganglia of TGFb2-3wt (n = 5) and TGFb2-3fl (n = 6) mice after infection with AAV9-Cre-GFP. (E) Number of live Cx3cr1+ MMϕ in Cx3cr1creERT2/WT TGFbr2fl/fl (Cre+) (n = 7) and Cx3cr1WT/WT TGFbr2fl/fl (Cre−) (n = 5) mice. (F) Frequency of MMϕ populations and (G) Cell counts of MMϕ populations in Cre+ (n = 7) and Cre− (n = 5) mice 4 weeks after tamoxifen administration. (H) Body weight of Cre+ (n = 7) and Cre− (n = 5) mice over 4 weeks following tamoxifen administration expressed as a percentage of baseline body weight. (I) Disease activity index (DAI) of Cre+ (n = 7) and Cre− (n = 5) mice over 4 weeks following tamoxifen administration. (J) Histopathological score of colonic tissue of Cre+ (n = 7) and Cre− (n = 5) mice 4 weeks after tamoxifen administration. (K) Brightfield images of colonic tissue stained with hematoxylin and eosin obtained from Cre+ and Cre− mice 4 weeks after tamoxifen administration. Images are representative of 5-7 biological replicates. Scale bar is 100 μm. (L) Expression of TGFBR1 and TGFBR2 in human myeloid cells, obtained from Elmentaite et al., 2021. (M) Expression of TGFB1, TGFB2 and TGFB3 in human enteric neurons, obtained from Drokhlyansky et al., 2020. Data are shown as Mean ± SD, analysed using unpaired two-tailed T-tests (A-E, J) or Two-way ANOVA followed by Šídák’s multiple comparisons (F-G) or RM Two-way ANOVA with Geisser-Greenhouse correction and Bonferroni’s multiple comparisons (H–I). Each data point indicates 1 biological replicate. ns = not significant. n = X biologically independent samples.
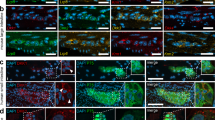
Extended Data Fig. 6 Low magnification images of the ENS.
(A) Representative low magnification confocal images of the developing ENS, from P10 to P56. (B) Representative low magnification confocal images of the ENS in in Cx3cr1WT/WT TGFbr2fl/fl (Cre−) and in Cx3cr1creERT2/WT TGFbr2fl/fl (Cre+) littermate controls. Images shown are representative of at least 2 independent experiments. Scale bars are 100 μm.
Supplementary information
Supplementary Tables
This file contrains Supplementary Tables 1–3.
Supplementary Video 1
Stitched time-lapse of ex vivo live imaging of muscularis externa of 14-day-old Wnt1Cre/WT Rosa26tdT/WT Cx3cr1GFP/WT mice. Scale bar, 20 µm.
Supplementary Video 2
Stitched time-lapse of ex vivo live imaging of muscularis externa of 56-day-old Wnt1Cre/WT Rosa26tdT/WT Cx3cr1GFP/WT mice. Scale bar, 20 µm.
Supplementary Video 3
Stitched time-lapse of ex vivo live imaging of Cx3cr1+ MMФ engulfing tdTomato+ neural debris, recorded in 14-day-old Wnt1Cre/WT Rosa26tdT/WT Cx3cr1GFP/WT mice. White arrowhead indicates engulfment of tdTomato+ neural debris. Scale bar, 10 µm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Viola, M.F., Chavero-Pieres, M., Modave, E. et al. Dedicated macrophages organize and maintain the enteric nervous system. Nature 618, 818–826 (2023). https://doi.org/10.1038/s41586-023-06200-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06200-7
This article is cited by
-
Fresh perspectives on how to build, maintain and repair the ENS
Nature Reviews Gastroenterology & Hepatology (2024)
-
Neuroimmune Connectomes in the Gut and Their Implications in Parkinson’s Disease
Molecular Neurobiology (2024)
-
Gut macrophages shape and sustain the enteric nervous system
Nature Reviews Neuroscience (2023)
-
Depletion of muscularis macrophages ameliorates inflammation-driven dysmotility in murine colitis model
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.