Abstract
Ubiquitylation is an essential post-translational modification that regulates intracellular signalling networks by triggering proteasomal substrate degradation, changing the activity of substrates or mediating changes in proteins that interact with substrates. Hundreds of enzymes participate in reversible ubiquitylation of proteins, some acting globally and others targeting specific proteins. Ubiquitylation is essential for innate immune responses, as it facilitates rapid regulation of inflammatory pathways, thereby ensuring sufficient but not excessive responses. A growing number of inborn errors of immunity are attributed to dysregulated ubiquitylation. These genetic disorders exhibit broad clinical manifestations, ranging from susceptibility to infection to autoinflammatory and/or autoimmune features, lymphoproliferation and propensity to malignancy. Many autoinflammatory disorders result from disruption of components of the ubiquitylation machinery and lead to overactivation of innate immune cells. An understanding of the disorders of ubiquitylation in autoinflammatory diseases could enable the development of novel management strategies.
Key points
-
Autoinflammatory diseases are disorders of overactivation of the innate immune system, and some of these conditions are caused by pathogenic mutations affecting components of the ubiquitylation pathways.
-
Ubiquitylation is a reversible post-translational modification that is essential for regulation of cellular responses to immune signals, in particular NF-κB and interferon pathways, as well as the unfolded protein response.
-
Dysregulation of ubiquitylation leads to autoinflammation through both disruption of specific signalling networks and induction of generalized stress pathways.
-
Mutations affecting ubiquitylation pathway components that regulate large sets of substrates (UBA1 and the proteasome) and components regulating specific substrates (E3 ligases and deubiquitylases) can result in overlapping phenotypes.
-
Consideration of ubiquitylation disorders as a group could provide additional insights into these diseases and suggest novel treatment avenues.
Similar content being viewed by others
Introduction
Since the identification in the early 1980s of the ubiquitin pathway as the major component of intracellular proteolysis, it has emerged as an essential regulator of most cellular systems1,2,3,4,5. Ubiquitylation is a post-translational modification (PTM) that temporally, spatially and specifically regulates substrate stability and signalling, and is therefore fundamental to health and disease6. Ubiquitylation is central to many dynamically regulated processes such as cell-cycle progression, DNA-damage response and inflammatory and immune signalling pathways. Not surprisingly, dysregulation of the ubiquitylation cascade is associated with many disease states, from infantile neurodevelopmental disorders to inborn errors of immunity7,8.
In the past decade, evidence has linked monogenic autoinflammatory diseases, which result from the hyperactivation of the innate immune system, to alterations in the ubiquitin–proteasome machinery. Although these disorders are rare, they provide important insights into the complex mechanisms regulating innate immune responses9. A broader understanding of the multifactorial role of ubiquitylation in chronic inflammatory disease could enable the development of new therapeutic modalities for both rare and common rheumatological diseases. In this Review, we outline the known disorders of ubiquitylation that lead to excessive innate immune activation, describe the molecular pathogenesis of these conditions, and propose that disruption of the ubiquitin pathway is a key contributor to inflammation through both dysregulation of specific signalling networks and induction of generalized stress pathways.
Ubiquitin, inflammation and disease
Ubiquitin machinery
Ubiquitin is a 76-amino acid protein that is covalently attached to substrates through the concerted activities of three different sets of enzymes (Box 1). First, structurally related ubiquitin E1 enzymes (ubiquitin-like modifier-activating enzymes UBA1 and UBA6) utilize ATP to activate ubiquitin10. Second, the activated ubiquitin moiety is transferred to the cysteine residue in the active site of one of around 40 human ubiquitin E2 carrier enzymes. In the final step, ubiquitin-charged E2 enzymes co-operate with ubiquitin E3 ligases (a protein family with around 600 members in humans), which catalyse ubiquitin transfer to specific substrate proteins by forming an isopeptide bond between the C-terminal carboxyl group of ubiquitin and the ε-amino group of a lysine residue of the target protein10. Ubiquitin can also be conjugated via a peptide bond to the N-terminal amino group of a substrate, or via esterification to a threonine or serine residue of a protein substrate, and ubiquitin can even be joined to non-proteinaceous substrates11,12,13. Typically, one E3 ligase can modify several substrates, and conversely one protein can be ubiquitylated by several E2/E3 combinations, resulting in a diversity of ubiquitylation sites and patterns14.
The ubiquitylation cascade is organized in a hierarchical manner (Box 1). Ubiquitin activation by E1 enzymes is essential for all cellular ubiquitylation, whereas activity of E2 enzymes that bind specific sets of E3 ligases is required for ubiquitylation of many substrates and contributes to generation of particular ubiquitylation patterns. The nature of the ubiquitin modification determines the fate of a particular substrate: ubiquitin can be attached as a monomer to one or several sites within a substrate (monoubiquitylation or multi-monoubiquitylation), or it can be attached in the form of ubiquitin chains (polyubiquitylation). Polyubiquitin chains can be interconnected by ubiquitin conjugation to one or more of the seven lysine residues or the N-terminal methionine (K6, K11, K27, K29, K33, K48, K63, M1) of any ubiquitin monomer, resulting in a staggering complexity of homotypic and heterotypic architectures, each of which has the biochemical capacity to elicit distinct downstream functional consequences15 (Box 1). Notable ubiquitin modifications are the homotypic K48-linked ubiquitin chains that mediate degradation by the proteasome16, and the homotypic and heterotypic M1–K63-linked ubiquitin chains that assemble multi-protein signalling platforms during immune responses17,18. Different types of ubiquitylation are recognized by a variety of proteins with ubiquitin-binding domains (ubiquitin receptors) that translate specific ubiquitin linkages into cellular responses19,20. Ubiquitylation is reversed or edited by deubiquitylases (a family of around 100 proteins in humans), which catalyse either the removal of ubiquitin from substrates or the trimming of polymeric modifications21. In addition, there are many ubiquitin-like modifications that use analogous biochemical pathways for activation and conjugation, including SUMO (small ubiquitin-like modifier), FAT10 (human leukocyte antigen-F adjacent transcript, also known as ubiquitin D), ISG15 (interferon-stimulated gene 15), UFM1 (ubiquitin fold modifier 1), URM1 (ubiquitin-related modifier 1) and NEDD8 (neuronal precursor cell-expressed developmentally down-regulated protein 8)22.
The consequences of the ubiquitylation pathways even within a single cell are hard to fathom, with thousands of proteins undergoing modification at a time, each at a particular combination of target residues, with varying chain types and lengths, to achieve precisely coordinated functions. In addition, cell-specific and temporally dynamic patterns of ubiquitylation indicate the importance of ubiquitylation as a regulatory mechanism for interpretation of signalling changes and changes in the cellular environment. These precise functions are demonstrated by the subtle perturbations that can happen in human disease following the loss of a specific enzyme23,24. In many ways, this requirement for proper ubiquitylation to maintain continuity of physiological functions is an elegant example of “destruction for the sake of construction”25.
Autoinflammation and ubiquitylation
Dysregulation of ubiquitylation is linked to many distinct disorders of recurrent systemic inflammation that are collectively known as systemic autoinflammatory diseases (SAIDs)26. Studies of patients with SAIDs and of animal models that phenocopy these conditions have revealed basic mechanisms related to innate immunity that are widely applicable across many different disease states9. SAIDs are caused by genetic mutations that lead to enhanced or constitutive inflammation, either gain-of-function mutations in activators or sensors of immune responses (such as NLRP3, mutation of which results in cryopyrin-associated periodic syndrome)27,28, or loss-of-function mutations in inflammatory repressors (such as IL1RN, mutation of which results in deficiency of IL-1 receptor antagonist)29,30. Identification of the molecular causes of such diseases is important to enable correct clinical diagnosis and selection of targeted therapies.
SAIDs can be categorized according to a number of parameters, such as clinical manifestation31, genetics32 and molecular pathogenesis33; however, in this Review, we focus on the biochemical pathways of ubiquitylation in autoinflammatory diseases as a defining feature. Depending on which step in the ubiquitylation hierarchy is compromised, dysregulation of ubiquitylation can directly affect a particular signalling pathway (for example, through mutations affecting E3 ligases and deubiquitylases) that will ultimately result in activation of cellular stress responses, or it can cause general cellular stress as an immediate consequence (for example, through mutations affecting E1 enzymes or the proteasome). We focus here on existing data from human genetic diseases and supporting evidence from relevant model organisms to review the current understanding of the pathology and molecular basis of associated phenotypes. Strikingly, defects in general components of the ubiquitin–proteasome system (UPS) and in highly substrate-specific ubiquitin ligation and deconjugation enzymes can lead to overlapping clinical presentations, suggesting commonalities that are not apparent at the level of known molecular aetiology. Given the highly targetable nature of the ubiquitin pathway34, conceptually connecting these diseases may be important for designing rational therapeutics across diagnoses.
In the following sections, we describe multiple specific inflammatory pathways that are known to be dysregulated in inflammatory disease, organized by the type of ubiquitylation enzyme that is affected in human disease, with information on clinical manifestations, genetics and molecular signalling (Table 1). Importantly, given the pleiotropic role of many of these regulatory enzymes, we briefly highlight supporting evidence relating to other genes where possible. In many cases, ubiquitylation coordinates multiple signalling pathways during immune responses, and mutations that affect ubiquitylation enzymes often alter various cellular processes that contribute to disease manifestations.
UBA1 E1 enzyme, mutations and disease
VEXAS syndrome
Vacuoles, E1 activating enzyme, X-linked autoinflammatory somatic (VEXAS) syndrome is the first example of an autoinflammatory disease arising exclusively from somatic mutations, and one of an emerging class of acquired errors of immunity. Although this disorder was only first reported in 2020, hundreds of patients have already been identified35. VEXAS syndrome is caused by pathogenic variants in UBA1, a gene located on the X chromosome that encodes ubiquitin-like modifier activating enzyme 1 (UBA1); these mutations are restricted to myeloid-lineage cells35 (Fig. 1). UBA1 is the E1 enzyme that is required for initiation of most cellular ubiquitylation, and it has two well-characterized isoforms, UBA1a and UBA1b36,10. UBA1a is a long isoform that is localized to the nucleus, whereas UBA1b is a shorter isoform, without a nuclear localization signal. Individuals with VEXAS have acquired missense mutations that abrogate expression of UBA1b and initiate translation of a new catalytically impaired isoform in mutant cells. These loss-of-function mutations are primarily found at, or around, the start codon for UBA1b (p.Met41), leading to a reduction in protein isoform production35. Individual variants at p.Met41 confer particular phenotypes and levels of disease severity through differential UBA1b translation37 (D. Beck, personal communication). Several other deleterious mutations that affect the sequence of the N-terminal domain can also reduce UBA1b protein expression or activity38,39,40. Nearly all instances of VEXAS syndrome are identified in men, but it can also occur in women with inherited or acquired monosomy of the X chromosome41,42,43,44,45. UBA1 function is essential in model organisms, and pathogenic mutations are only physiologically tolerated in somatic form in haematopoietic stem cells and myeloid lineages, whereas they are lethal in other tissues35,46.
Reduction of cytoplasmic ubiquitylation by mutations affecting ubiquitin-like modifier-activating enzyme UBA1 (resulting in vacuoles, E1 activating enzyme, X-linked autoinflammatory somatic (VEXAS) syndrome) or of proteasome activity by mutations affecting particular proteasomal subunits (resulting in proteasome-associated autoinflammatory syndromes (PRAAS)) decreases the efficiency of endoplasmic reticulum (ER)-associated degradation (ERAD). This alteration results in imbalances in cellular proteostasis and accumulation of unfolded and unnecessary proteins, which activate the three different sensors of the unfolded protein response (UPR), PERK, IRE1α and ATF6. Sensor activation initiates signalling cascades that culminate in the production of specific transcription factors (TFs) that mediate upregulation of expression of ERAD components and chaperones as well as type I interferons, which drive the autoinflammation in VEXAS syndrome and PRAAS. Red stars denote proteins affected by mutation. Blue circles denote K48 linked polyubiquitin chains destined for proteasomal degradation.
Patients with VEXAS syndrome have multisystem inflammation involving primarily the skin, cartilage and lungs (although the clinical spectrum also includes many other disease manifestations), with onset in middle age or later47,48,49. VEXAS syndrome can present in a similar manner to a variety of haematological and inflammatory conditions50, and patients with VEXAS syndrome are likely to meet diagnostic criteria for relapsing polychondritis, systemic lupus erythematosus, rheumatoid arthritis, polyarteritis nodosa and Sweet’s syndrome, along with haematological diseases including multiple myeloma and myelodysplastic syndrome49. However, VEXAS syndrome can be distinguished by the presence of the distinct genetic mutation, and by unique clinical characteristics including histopathological vacuoles and late-onset treatment-refractory disease.
The mechanism by which mutation in UBA1 causes disease has remained elusive, although preliminary evidence suggests activation of the unfolded protein response (UPR) and of multiple inflammatory pathways, including the IFNα and IFNβ pathways. Activation of the UPR can lead to a type I interferon response, which is a feature of proteasomopathies (Fig. 1). The proteasome acts at the completion of the degradative ubiquitylation pathway, and, as with the initiation of the cascade by UBA1, it represents a bottleneck in the regulation of the vast majority of ubiquitylated proteins, so that loss of either step disrupts protein homeostasis (proteostasis)51. Therefore, it is no surprise that similar clinical manifestations occur in patients with different proteasomopathies (such as proteasome maturation protein (POMP)-related autoinflammation and immune dysregulation, a disease with prominent neutrophilic dermatosis), interferonopathies (such as STING-associated vasculopathy with onset in infancy or COPA syndrome, inherited disorders with prominent interstitial lung disease)52,53 and VEXAS syndrome. Finally, there is emerging evidence that blocking Janus kinases (JAKs), which are downstream effectors in interferon signalling, might be an important approach to treatment for VEXAS syndrome39. However, as is seen with other disorders of constitutive inflammation, and depending on the disease stage in individual patients, allogenic haematopoietic stem cell transplantation (HSCT) could end up being the only therapy with sustained efficacy43,45,54.
UBA1 and spinal muscular atrophy
In contrast to VEXAS syndrome, germline missense and synonymous mutations affecting the C-terminal portion of UBA1 occur in patients with a neuromuscular disease that resembles spinal muscular atrophy (SMA)55. The reported mutations cluster within the active adenylation domain that is required for catalytic activity in UBA1a and UBA1b, although not all of the mutations have a demonstrable effect on enzyme activity56. Loss of Uba1 in zebrafish causes impairment of movement, and exogenous expression of UBA1 can improve symptoms related to classic SMA in mouse models with pathogenic hypomorphic variants in the spliceosome biogenesis factor survival motor neuron protein (Smn1)57,58. The precise molecular connection between UBA1-dependent ubiquitylation and SMN1-driven spliceosome assembly remains elusive. However, given the manifold mechanisms by which ubiquitylation regulates spliceosome function59, it is likely that an essential ubiquitin-dependent pathway coordinates splicing during neurodevelopment.
Predisposition to phenotypically varied disorders as a result of mutations affecting differentially expressed protein isoforms (such as UBA1) also occurs in other diseases such as CDC42-associated or POMP-associated phenotypes60,61,62,63. For VEXAS syndrome and SMA, the relationship between UBA1 functions in neurodevelopment and innate immunity is still unclear and it remains to be seen whether UBA1-related SMA is effected by severe inflammation. Further characterization of these disparate UBA1-related conditions may provide a better understanding of the cell-specific and tissue-specific functions of this protein.
Proteasome, mutations and disease
Proteasome-associated SAIDs
The proteasome is a multisubunit complex that is required for degradation of ubiquitylated substrates, enabling the removal of misfolded and unnecessary proteins64. The proteasome consists of a 20 S core particle with proteolytic activity (caspase-like, trypsin-like and chymotrypsin-like)65 and 19 S regulatory particles that can associate with either or both ends of the core particle. The regulatory particle consists of ‘base’ and ‘lid’ sub-complexes and serves to recruit ubiquitylated substrates. The proteasome is constitutively expressed, but the core particle can also contain multiple tissue-specific subunits, generating immune-specific, thymus-specific and testis-specific proteasomes66.
Biallelic loss-of-function mutations affecting components of the proteasome lead to a class of SAIDs called proteasome-associated autoinflammatory syndromes (PRAAS), which are also known as Nakajo–Nishimura syndrome, CANDLE (chronic atypical neutrophilic dermatosis and lipodystrophy and elevated temperature) or joint contractures, muscular atrophy, microcytic anaemia and panniculitis-induced lipodystrophy67. Patients with these conditions present with symptoms that include early-onset fevers, skin manifestations, intracranial calcifications, neurological impairment, arthritis and lipomuscular dystrophy68.
Most of the identified causative genetic changes affect subunits that constitute the immunoproteasome (such as PSMB8, PSMB9 and PSMB10), although some affect core proteasome subunits (such as PSMA3 and PSMB4) or regulatory proteins (such as POMP, PSMG2 and PSMD12)60,69,70. This observation suggests the involvement of tissue-specific expression of proteasome core and/or regulatory subunits, and/or a high demand for proteasome activity in specific cells71, which could explain why, even though proteasome activity is essential for most cellular pathways, loss of proteasome function can lead to inflammation without other broad pathological conditions.
Initial clues to the pathophysiological mechanism in PRAAS came from the demonstration that defects in the immunoproteasome, both in patient-derived cells and in cells from healthy individuals with proteasome knockdown or inhibition, lead to production of type I interferons69 (Fig. 1). Further studies of POMP-related autoinflammation and immune dysregulation, with heterozygous dominant-negative mutations in POMP, demonstrated that the UPR is activated in cells from affected patients, thereby providing further evidence for a link between proteasome-dependent quality control and interferon production, which is consistent with the observation of clinical response to JAK inhibition in patients with PRAAS60. Furthermore, evidence indicates that protein kinase R (PKR) functions as an innate immune sensor for proteotoxic stress triggered by proteasome dysfunction72. Interestingly, knockout of the catalytic β5i/LMP7 subunit of the immunoproteasome in mice does not result in spontaneous inflammation, limiting efforts to further model these diseases73.
Neurodevelopmental disease
Mutations in PSMD12, which encodes one of the 19 S regulatory proteasomal subunits, are linked to neurodevelopmental disease, with heterozygous loss-of-function alleles identified in patients with intellectual disability, congenital malformations and autistic features74. Interpretation of the effects of these mutations is complicated by the variability of the severity of disease presentation among affected family members75,76. Reduced penetrance of pathogenic variants is a common feature of many primary interferonopathies77. In the same way that distinct UBA1 mutations can lead to either VEXAS syndrome or SMA, there may be a link between the neurodevelopmental and inflammatory phenotypes associated with PSMD12 (ref.70). Proteasomal regulatory proteins, such as PSMD12, might also have previously unrecognized cell-specific or tissue-specific functions, which are now being discovered by human genetics studies. Whether inflammation associated with the haploinsufficiency of PSMD12 contributes to the neurodevelopmental phenotype has yet to be identified but might be an important consideration for optimization of treatment strategies.
E3 ligases and deubiquitylases
Ensuring a proper innate immune response requires rapid and highly regulated remodelling of signalling networks, for which immune cells often utilize PTMs. In particular, ubiquitylation has emerged as a critical means of fine-tuning the strength and duration of immune signals. In this respect, it is not surprising that mutations that affect the enzymatic machinery catalysing ubiquitylation (E3 ligases) and deubiquitylation (deubiquitylases) can lead to alteration of many cytokine pathways.
NF-κB signalling pathway
NF-κB is a transcription factor that lies at the centre of multiple innate and adaptive immune pathways78. NF-κB has both canonical and non-canonical pathways that regulate activation of immune responses79. In general, the canonical NF-κB pathway utilizes an abundance of cell-specific and stimulus-specific regulators to activate immune responses, including pathogen-associated molecular patterns (PAMPs) and cytokines such as TNF and IL-1β, whereas the non-canonical pathway responds mainly to ligands of the TNF superfamily. Although the non-canonical pathway has important roles in inflammation, it is canonical signalling that is essential for rapid regulation of host responses. Inhibitors of the pathways (IκB proteins) directly bind to NF-κB family members and prevent activation of signalling80. Activity of NF-κB pathways is extensively regulated by a variety of PTMs, including phosphorylation and ubiquitylation. The central importance of NF-κB to human disease is supported by an abundance of evidence from in vitro experiments, cellular systems, model organisms and observations in human disease81. Pathogenic variants of proteins that are involved in NF-κB pathways can behave either as gain-of-function or as loss-of-function alleles and, depending on the affected domain and the function of the altered protein, can cause either immunodeficiency or systemic inflammation. Nevertheless, all the diseases resulting from disruption of NF-κB pathways present with a continuum of clinical features, including immunodeficiency, autoinflammation, autoimmunity and atopy78. A strong additional piece of evidence comes from identification of an autoinflammatory disease caused by haploinsufficiency of RELA (encoding a subunit of NF-κB)82, which has overlapping clinical features with the disease caused by dysregulated ubiquitylation, termed haploinsufficiency of A20 (HA20; A20 is encoded by TNFAIP3). This condition is discussed in greater detail in the following section.
TNFAIP3-associated diseases
A20 is a bi-functional ubiquitin-modulating enzyme with both ubiquitin E3 ligase and deubiquitylase activities, which can also act as a linear ubiquitin effector83,84 (Fig. 2a). Because A20 functions as an inhibitor of inflammation, mutations that result in loss of function of this protein are linked to autoimmune and autoinflammatory phenotypes. A20 has deubiquitylase activity towards K63-linked ubiquitin chains on various proteins, including RIPK1 (receptor-interacting serine/threonine protein kinase 1) and NEMO (NF-κB essential modulator), and this activity results in disassembly of inflammatory complexes and suppression of immune responses26. Similarly, A20 has ubiquitin ligase activity, and modifies RIPK1 and other substrates with proteasome-targeting K48 chains, again halting NF-κB signalling. These contrasting, yet synergistic, functions of A20 are carried out by specific protein domains. The ovarian tumour (OTU) domain harbours deubiquitylase activity, whereas C-terminal zinc finger (ZnF) domains have ubiquitin-binding functions, with the ZnF4 domain binding K63 and K48 chains, and the ZnF7 domain binding M1 ubiquitin chains85. Although both the deubiquitylase and ubiquitin-ligase domains of A20 are implicated in regulatory functions, results from in vitro experiments and transgenic knock-in mice deficient in one or other of these activities fail to recapitulate the spontaneous inflammatory phenotype of Tnfaip3 knockout mice or of patients with TNFAIP3 loss-of-function mutations86. Results from studies of mouse models indicate that the anti-inflammatory role of A20 is also the result of its non-catalytic function in protecting cells from cell death86. Specifically, the activity of ZnF7 is essential for the inhibitory function of A20 by preventing TNF-induced necroptosis via inflammasome activation84,87. Although these models do not completely recapitulate the knockout mouse, they clearly demonstrate the importance of linear ubiquitin binding in A20 function in vivo. Moreover, only the combined inactivation of ZnF4 and ZnF7 phenocopied the perinatal lethality and severe multiorgan inflammation of A20-deficient mice87. In addition, A20 phosphorylation is critical for its anti-inflammatory function88. Hypomorphic missense mutations that diminish A20 phosphorylation can be evolutionarily advantageous to humans because they induce a subtle hyperinflammatory phenotype in leukocytes. The ancient Denisovan A20 haplotype carrying p.Thr108Ala and p.Ile207Leu variants, which are common in populations in Southeast Asia and Oceania, are associated with greater NF-κB response and heightened immune responsiveness89.
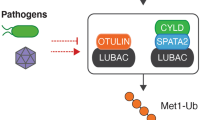
a | Simplified model of how the linear-ubiquitylation pathway regulates NF-κB and cell-death signalling during responses to immune stimuli. Upon immune stimulation, the linear-ubiquitin assembly complex (LUBAC), which consists of HOIL-1 (haem-oxidized IRP2 ubiquitin ligase 1), HOIP (HOIL-1-interacting protein) and SHARPIN (SHANK-associated RH domain interactor), assembles linear ubiquitin chains on specific substrates. This is counteracted by the linear-ubiquitin-specific deubiquitylase OTULIN, which also reverses autoubiquitylation and thus stabilizes LUBAC in a cell-type-specific manner. Linear ubiquitin chains are recognized and interpreted by dedicated binding proteins that initiate NF-κB or cell-death signalling pathways. One such linear-ubiquitin reader is the hybrid deubiquitylase–E3 ligase enzyme A20. In this model, LUBAC is a ubiquitin ‘writer’, OTULIN is a ubiquitin ‘eraser’ and A20 is a ubiquitin ‘reader’. b | Loss-of-function mutations affecting subunits of the linear-ubiquitin writer LUBAC, the linear-ubiquitin eraser OTULIN and the linear-ubiquitin effector protein A20 result in autoinflammatory diseases. HA20, haploinsufficiency of A20; ORAS, OTULIN-related autoinflammatory syndrome.
TNFAIP3 coding and non-coding genetic variants are implicated in many different human diseases90, at the level of both common, low-penetrance (single-nucleotide polymorphisms (SNPs)), and rare, high-penetrance variants. TNFAIP3 SNPs are associated with conditions including systemic lupus erythematosus91, rheumatoid arthritis92 and psoriatic arthritis93. Some of these SNPs result in downregulation of A20 protein expression. Rare nonsense and frameshift variants are associated with a severe dominantly inherited autoinflammatory disease, HA20 (ref.94); somatic TNFAIP3 variants are also found in up to 30% of B cell lymphomas95. Patients with HA20 present with childhood-onset systemic disease manifesting with oral and genital ulcers, musculoskeletal, gastrointestinal and integumentary (skin) inflammation, and features of autoimmunity96. Immune profiling of these patients has identified high concentrations of numerous cytokines, many of which result directly from upregulation of NF-κB signalling94 (Fig. 2b). Patients with HA20 tend to respond to cytokine inhibitors, although severe, refractory cases may require more extensive combined therapies97 or HSCT. What is abundantly clear is that A20 acts as a central regulator of many pathways with various functions in particular cell types, and its perturbations underlie a spectrum of rheumatological and haematological diseases. In summary, although ‘mild’ hypomorphic A20 variants can be beneficial to human health by enhancing immune responses, more-detrimental variants can overactivate inflammatory signalling, resulting in a spectrum of autoinflammatory and autoimmune phenotypes87.
Diseases linked to dysregulation of linear ubiquitylation
Ubiquitylation frequently involves the addition of protein chains, with the most proximal ubiquitin attached to the substrate, typically to a lysine reside. An important chain subtype is the linear ubiquitin chain, in which the N-terminal α-amino group of Met1 of the growing chain is used for linkage to the C terminus of the previous ubiquitin moiety. Linear (M1) ubiquitin chains are critical for innate immune signalling and are conjugated by the E3 ligase complex LUBAC (linear ubiquitin chain activating complex) and removed by the deubiquitylase OTULIN (ubiquitin thioesterase otulin), which exclusively hydrolyses M1 ubiquitin chains, and CYLD (ubiquitin carboxyl-terminal hydrolase), which is less specific and can hydrolyse K63-linked chains and other ubiquitin linkages98 (Fig. 2a). SPATA2 (spermatogenesis-associated protein 2) acts as activator for the K63 and Met1 deubiquitylase function of CYLD in the TNFR1 signalling pathway99. Intriguingly, deficiencies of either LUBAC or OTULIN are implicated in monogenic autoinflammatory diseases98. LUBAC is composed of three proteins, HOIL-1 (haem-oxidized IRP2 ubiquitin ligase 1), HOIP (HOIL-1-interacting protein) and SHARPIN (SHANK-associated RH domain interactor), and mutations affecting HOIL-1 and HOIP lead to an autosomal-recessive immunodeficiency with concomitant autoinflammation100,101. Autosomal-recessive mutation resulting in the loss of OTULIN expression causes a rare, potentially fatal disease manifesting with constitutive severe systemic inflammation102,103,104,105. Notably, although LUBAC and OTULIN have opposing functions, their deficiencies lead to overlapping clinical manifestations, possibly (at least partly) as a result of the role of OTULIN in regulation of LUBAC activity. In the mouse model, OTULIN was shown to prevent autoubiquitylation of LUBAC, thereby promoting its E3 ligase function106 (Fig. 2a). Many components of TNF receptor (TNFR) signalling cascades are modified with, or can directly bind to, linear ubiquitin chains, so linear ubiquitylation is an important regulator of many aspects of innate and adaptive immunity. M1-linked ubiquitin chains are not only essential for TNFR-dependent NF-κB signalling but are also required for NOD2 (nucleotide-binding oligomerization domain-containing protein 2)-dependent NF-κB signalling and proteasome function107,108,109. Patients with otulipenia present with severe inflammation of the skin, adipose tissue and joints, whereas immunodeficiency is a more prominent feature of LUBAC deficiencies98. These differences reflect cell-specific and tissue-specific functions of individual components of the linear ubiquitin system. In general, patients with OTULIN deficiency respond to therapy with cytokine inhibitors, provided that treatment is initiated early in life. Alternatively, a substantial resolution of systemic inflammation and/or immunodeficiency is possible using HSCT110.
As described above, because the binding of A20 to linear ubiquitin chains is important for its anti-inflammatory function, we hypothesize that HA20 manifestations might in part result from dysregulation of linear ubiquitylation (Fig. 2).
Disease linked to dysregulation of the E3 ligase XIAP
E3 ubiquitin-protein ligase XIAP (also known as X-linked inhibitor of apoptosis) regulates cell-death pathways and NF-κB-dependent immune signalling events111. The multiple functions of XIAP are mediated by its modular domain architecture, which harbours three zinc-binding baculovirus IAP repeat (BIR) domains, a ubiquitin-associated (UBA) domain and a C-terminal RING ubiquitin E3 ligase domain. The BIR2 and BIR3 domains bind to and inhibit caspases, enabling XIAP to exert anti-apoptotic effects that are important during expansion of adaptive immune cells in the setting of viral infection112. The UBA domain of XIAP binds directly to K63-linked ubiquitin chains on NEMO, to activate NF-κB signalling113. Through its ubiquitin E3 ligase activity, XIAP prevents TNF-mediated and RIPK3-dependent cell death (via ubiquitylation of RIPK1)114 and promotes NOD2 pro-inflammatory signalling by mediating K63-linked polyubiquitylation of RIPK2 within the NOD2 signalling complex, to recruit LUBAC and to enhance NF-κB-dependent and MAPK-dependent cytokine production107,115. Thus, XIAP is important for the clearance of pathogens and for broad regulation of inflammatory and immune responses.
The XIAP gene is located on the X chromosome, and loss-of-function mutations (including point mutations affecting the RING domain) in males lead to a broad spectrum of immune dysregulation characterized by cytopenia, hypogammaglobulinaemia and/or recurrent infection, splenomegaly, colitis and a propensity to haemophagocytic lymphohistiocytosis, often triggered by Epstein–Barr virus infection112. The inflammatory phenotypes are in part attributed to inflammasome activation and secretion of various pro-inflammatory cytokines and chemokines. Disease onset and presentation is highly variable, and some men with loss-of-function mutations in XIAP remain asymptomatic, suggesting that environmental factors, such as exposure to infection, contribute to the disease expressivity. Elucidation of these factors will be important, as HCST is the only curative treatment described to date, and it has poor outcomes116.
Interferon signalling pathway
Type I interferons are cytokines that mediate host responses to pathogens, in particular viruses. These cytokines are produced in response to stimulation of host pathogen sensors by binding of specific PAMPs, primarily through nucleic acid sensing117,118. PAMP recognition initiates a cascade of signalling, through JAK–signal transducer and activator of transcription (STAT) pathways that lead to activation of ISGs, which is a hallmark of type I interferon-mediated inflammation119. This transcriptional programme leads to antiviral activities including apoptosis of infected cells, and maturation and proliferation of lymphocytes as a coordinated means of clearing pathogens. Furthermore, secretion of type I interferons leads to autocrine and paracrine signalling, which enhances anti-viral effects. In this pathway, inherited genetic defects or acquired defects in the form of anti-interferon autoantibodies result in a compromised responsiveness to infections, including SARS-CoV-2 (refs120,121,122). Notably, some viruses have evolved strategies whereby, upon infection, they attenuate interferon responses by use of specific enzymes that can cleave the ubiquitin-like modifier ISG15 from proteins such as IRF3 (ref.123).
Type I interferonopathies
Type I interferonopathies are a class of monogenic autoinflammatory diseases characterized by mutations that lead to constitutive activation of the type I interferon pathway. These disorders have substantial overlap of phenotypes, including arthritis, necrotizing vasculitis, intracranial calcifications, interstitial lung disease and lipodystrophy, with similar molecular signatures at the transcriptional and cytokine level124. Evidence of the importance of this signalling pathway is abundant, and derives from both inherited diseases and acquired conditions that lead to hyperinflamed or immunodeficient states125. Similar to the activation in NF-κB signalling, mutations in ubiquitin E3 ligases and deubiquitylases can cause constitutive activation of the type I interferon pathway126.
The OTU domain-containing protein 1 (OTUD1) deubiquitylase was implicated as a potential disease-causing gene in multiple systemic inflammatory diseases in a small-scale case–control study127. OTUD1 enzymatically controls inflammation by inhibiting IRF3-dependent transcriptional activity and activation of RIG-I (antiviral innate immune response receptor RIG-I). In experiments in vitro and in vivo in mice, OTUD1 repressed type I interferon-mediated disease128. Other ubiquitylation enzymes identified in mice as being critical to regulation of interferon signalling are not yet known to be associated with human disease129. Upregulation of type I interferon pathways also occurs in OTULIN-deficient humans and mice106, and in patients with HA20 (ref.97).
Diseases linked to dysregulation of the ubiquitin-like modifier ISG15
ISG15 is expressed in response to type I interferon signalling and has important roles in anti-pathogenic responses98,130. In a similar manner to ubiquitylation, ISG15 undergoes cycles of conjugation and deconjugation to substrates, and there is thought to be considerable crosstalk between ISG15 and ubiquitylation pathways131. Historically, ISG15 was thought to function as a pro-inflammatory antiviral effector. Results from studies in patients who lack ISG15 showed that, depending on the circumstances and target substrates, ISG15 can either suppress or activate the interferon pathway. ISG15 loss by recessively inherited mutations leads to a disease manifesting with basal ganglia calcifications and sporadic seizures that is similar to Aicardi–Goutières Syndrome (AGS), as a result of upregulation of the expression of ISGs132. Interestingly, patients with ISG15 deficiency also have susceptibility to mycobacterial disease, which is likely to be explained by loss of the extracellular function of ISG15 to induce IFNγ production in lymphocytes133,134,135. Thus, patients have manifestations of both autoinflammation and immunodeficiency. Similarly, autosomal-recessive loss-of-function mutations affecting the ISG15-specific deconjugating enzyme ubiquitin specific peptidase 18 (USP18) also result in primary interferonopathy. Loss of USP18 is primarily thought to cause disease in a manner that does not rely on its catalytic activity but rather on its ability to bind and inhibit signalling through the IFNα receptor (IFNAR)136. The binding between free intracellular ISG15 and USP18 increases the stability of USP18 by preventing its degradation. Although loss of ISG15 is compatible with life, USP18 deficiency is a potentially lethal disease with a phenotype similar to that of severe AGS as a result of brain calcification. Both USP18-deficient and ISG15-deficient patients have a strong interferon gene-expression signature in their peripheral blood leukocytes and cerebrospinal fluid, which can be effectively ameliorated with JAK inhibitors137. Interestingly, deficiency of ISG15 results in specific immunodeficiency in humans but not in mice, and as there is minimal conservation between species of the gene and pathway, ISG15-deficient patients are potentially a critical resource for further studies of this pathway138.
Stress and cell death in immune cells
In contrast to the many specific pathways described above, there are a few examples of human diseases that are caused by dysregulated ubiquitylation leading to disruption of general cellular functions. These general pathways are probably not causing inflammation because of aberrant levels of a single downstream effector but instead through activation of stress or cell-death pathways with many potential triggers (Fig. 3). These factors might also have a role in some of the specific pathways, as a downstream consequence of inflammation.
Mutations that affect enzymes involved in ubiquitylation pathways (indicated here by red stars) can lead to activation of cell death, unfolded protein response and inflammatory pathways that result in inflammatory disease. Therapeutic intervention might be possible at the levels of enzyme-specific activation and inhibition, pathway-specific inhibition and cytokine inhibition. LUBAC, linear-ubiquitin assembly complex; OTULIN, ubiquitin thioesterase otulin; UBA1, ubiquitin-like modifier-activating enzyme 1.
The UPR and autoinflammation
The UPR is a cellular stress pathway that regulates immune responses139,140. The UPR is activated when protein-folding requirements exceed the processing capacity of the endoplasmic reticulum (ER), and misfolded and unfolded proteins accumulate. The UPR is composed of three major pathways that use inositol-requiring enzyme 1α (IRE1α), PRKR-like ER kinase (PERK) and activating transcription factor 6α (ATF6α) as sensors, all of which are activated by misfolded proteins139,140. Stimulation of these pathways can directly result in activation of multiple innate immune responses, including NF-κB pathways, with reciprocal activation of the UPR as a consequence of pathogen-receptor activation. Studies of autoimmune and autoinflammatory diseases such as inflammatory bowel disease have implicated the UPR and ER integrity in disease mechanisms141,142. Dysregulation of both ubiquitin activation (in VEXAS) and processing of degradative ubiquitylation signals (in PRAAS) lead to activation of the UPR. As further evidence of the importance of the UPR in inflammation, mutations in APS13 (ref.143) and COPA144, which encode proteins that maintain Golgi–ER trafficking of TLR3 and STING proteins, respectively, can lead to monogenic autoinflammatory diseases with distinct features, likely through altered proteostasis. How these proteins directly contribute to specific forms of inflammation (such as pustular psoriasis in APS13 deficiency) remains unclear, but it might involve inappropriate processing of a key factor, such as STING145, or regulation of a number of other cell-specific proteins.
Cell death and autoinflammation
Cell death is an essential process that is required for proper tissue homeostasis and development. Many forms of programmed cell death exist, including apoptosis, necroptosis, pyroptosis and NETosis, and extensive reviews of these processes are available elsewhere146,147. Instead of death bringing finality, it has manifold roles, depending on the context of cell type and stimuli, in preventing, causing and perpetuating inflammation146. Programmed cell death can result directly in the release of cytokines, as is the case for pyroptosis, or it can result in the presentation of damage-associated molecular patterns (DAMPs), which then promote inflammation. In particular, apoptosis and necroptosis integrate external signals, such as binding of death-receptor ligands (including TNF, FASL and TRAIL), either directly or indirectly resulting in a pro-inflammatory milieu. These mechanisms can involve cell-intrinsic and cell-extrinsic processes.
A number of inborn errors of immunity are caused by mutations in genes encoding key regulators of cell death, including CASP8 (ref.148) and RIPK1 (refs149,150,151), or receptor–ligand combinations, such as FAS and FASLG (also known as TNFSF6)152. These disorders can present with a combination of immunodeficiency and/or autoinflammation, similar to LUBAC-related disorders. FAS and TNFR1 directly mediate immune signalling upon specific cytokine binding and activate cell-death pathways, and dysregulation of either activity contributes to disease pathogenesis. These diseases directly involve cell death, whether through lack of apoptosis in autoimmune lymphoproliferative syndrome caused by FAS mutations152,153, or via increased cell death in RIPK1-related disorders. In addition, many of the downstream ubiquitylation enzymes and ubiquitylated substrates in programmed cell death are important for regulation of inflammation as they mediate the balance between TNFR1-induced apoptosis complex 1 (membrane-bound TNFR1 complex) and complex 2 (cytoplasmic RIPK1-associated complex)9. Notably, because of the overlap between the signals that modulate inflammation and cell death, many factors have pleiotropic effects on inflammation.
PTMs such as ubiquitylation are important downstream changes that enable dynamic modulation of cell-death processes, and alteration of PTM machinery has varied physiological consequences, particularly in immunity. For example, loss of the E3 ligase LUBAC leads to autoinflammation, although the reduction of NF-κB signalling is a major feature of disease in patients with HOIL-1 loss of function100, which seems paradoxical, given that increased NF-κB signalling would be expected in autoinflammation. Nevertheless, the downregulation in NF-kB pro-survival signalling can shift the balance towards RIPK1-mediated pro-death pathways, resulting in increased sensitivity of LUBAC-deficient cells to TNF-induced cell death. Evidence from mouse models indicates that programmed cell death, whether apoptosis or necroptosis, is pro-inflammatory in these LUBAC deficiencies146. LUBAC prevents cell death in response to TRAIL, FAS, TLR and TNF signalling, and A20, in addition to its inhibitory role in TNF-dependent inflammation, is implicated in dysregulation of cell death86. Another deubiquitylase, CYLD, has a role in an autoinflammatory disease that is caused by deficiency of TANK binding kinase 1 (TBK1)154. Although TBK1 is known to regulate the type I interferon pathway, TBK1-deficient individuals maintain hypomorphic but sufficient interferon induction via the RIG-I–MAVS pathway, and present with severe systemic inflammation resulting from dysregulation of TNF-induced RIPK1-mediated cell death154. Fibroblasts derived from patients with TBK1 deficiency display heightened sensitivity to necroptosis that results from the lack of inhibitory phosphorylation on CYLD, which increases its deubiquitylation activity towards RIPK1. CYLD is phosphorylated by TBK1 to restrain the pro-death propensity of RIPK1154. A similar mechanism may operate in patients with HA20. The contribution of dysregulated cell death to broader classes of inflammatory disease remains poorly understood, but it is likely to be an under-recognized effector of pathogenesis that could also act in VEXAS syndrome and PRAAS as a secondary effect. In the future, it will be important to study the contribution of these pathways in a cell-specific context and in human cells.
Conclusions
Innate immune sensing and activation requires the concerted ability to detect pathogens or stimuli and rapidly trigger production of cytokines or mediators of inflammation. In turn, efficient inactivation is critical to prevent prolonged inflammation once the threat is neutralized. In many cases, activation is accomplished by sensors or receptors that bind specific molecules and trigger highly targeted transcriptional, translational or post-translational programmes to promote clearance of pathogens155. In SAIDs, and rheumatological disorders as a whole, these pathways can be inappropriately co-opted and lead to recurrent or continued inflammation.
Genetic discovery of Mendelian disorders of autoinflammation has revealed new insights into the molecular mechanisms that are important for fine tuning of the innate immune system. Many disorders are linked to enzymes that regulate ubiquitylation, which is a pleiotropic set of processes that regulate many different aspects of immunity. Despite being linked to specific pathways of inflammation, ubiquitylation-related disorders cannot be easily treated with single cytokine-targeting therapies. Notably, the most proximal enzyme (UBA1) and some of the most distal enzymes (A20 and OTULIN) of ubiquitylation, when affected by mutation, can have similar effects in disease manifestation (resulting in VEXAS syndrome, HA20 and otulipenia) (Fig. 3). This observation suggests that shared mechanisms of disease exist for disorders of ubiquitylation, beyond systemic cytokine production, although the nature of these shared mechanisms is not yet fully known. They could involve specific shared substrates or pathways, many different substrates with similar effects, global shifts in cytoplasmic ubiquitin machinery, or combinations of these factors. In particular, the contribution of dysregulation of cell-death pathways warrants further investigation. Studying diseases caused by ubiquitylation defects consistently in humans and in animal models will help to further our understanding of potential commonalities, and could lead to more general therapies than are available at present (Fig. 3). For example, we propose that developing small-molecule activators of UBA1 or the proteasome that counterbalance dysregulation in protein turnover might not only provide a treatment option for PRAAS and VEXAS syndromes, but these drugs might also be beneficial for amelioration of autoinflammatory phenotypes caused by mutations affecting substrate-specific ubiquitin-system components. Other general treatment strategies could involve the use of inhibitors of the UPR and cell-death pathways that are frequently overactivated in SAIDs.
The genes that are currently implicated in disorders of ubiquitylation are likely to be just the first of many, and associations of further elements of the ubiquitylation machinery with human immunological and non-immunological disorders might soon be identified. Given the identification of disease-causing variants in E1 enzymes, E3 ligases and deubiquitylases, there could also be phenotypes that are associated with variants in E2 enzymes, ubiquitin sensors or effector proteins, and other cell-specific deubiquitylases. In addition, given the disease manifestations related to ISG15 and UPS18, we predict that variants in the ISG15 conjugation or ubiquitin-like machineries could also contribute to human immunological diseases. Similarly, other forms of protein degradation, such as autophagy, are likely to be implicated in the pathogenesis of autoinflammation156. Although we have focused here on germline causes, additional, both germline and somatic, pathogenic mutations causing aberrant ubiquitylation will likely be uncovered in the future.
References
No Author Listed. A prize for protein degradation. Nat. Cell Biol. 6, 1011 (2004).
Wilkinson, K. D. The discovery of ubiquitin-dependent proteolysis. Proc. Natl Acad. Sci. USA 102, 15280–15282 (2005).
Ciechanover, A., Heller, H., Elias, S., Haas, A. L. & Hershko, A. ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc. Natl Acad. Sci. USA 77, 1365–1368 (1980).
Hershko, A., Ciechanover, A., Heller, H., Haas, A. L. & Rose, I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc. Natl Acad. Sci. USA 77, 1783–1786 (1980).
Goldstein, G. et al. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc. Natl Acad. Sci. USA 72, 11–15 (1975).
Komander, D. & Rape, M. The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012).
Rape, M. Ubiquitylation at the crossroads of development and disease. Nat. Rev. Mol. Cell Biol. 19, 59–70 (2018).
Basar, M. A., Beck, D. B. & Werner, A. Deubiquitylases in developmental ubiquitin signaling and congenital diseases. Cell Death Differ. 28, 538–556 (2021).
Manthiram, K., Zhou, Q., Aksentijevich, I. & Kastner, D. L. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat. Immunol. 18, 832–842 (2017).
Schulman, B. A. & Harper, J. W. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat. Rev. Mol. Cell Biol. 10, 319–331 (2009).
Otten, E. G. et al. Ubiquitylation of lipopolysaccharide by RNF213 during bacterial infection. Nature 594, 111–116 (2021).
Kelsall, I. R., Zhang, J., Knebel, A., Arthur, J. S. C. & Cohen, P. The E3 ligase HOIL-1 catalyses ester bond formation between ubiquitin and components of the Myddosome in mammalian cells. Proc. Natl Acad. Sci. USA 116, 13293–13298 (2019).
Pao, K. C. et al. Activity-based E3 ligase profiling uncovers an E3 ligase with esterification activity. Nature 556, 381–385 (2018).
Metzger, M. B., Pruneda, J. N., Klevit, R. E. & Weissman, A. M. RING-type E3 ligases: master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim. Biophys. Acta 1843, 47–60 (2014).
Islam, A. B., Dave, M., Amin, S., Jensen, R. V. & Amin, A. R. Genomic, lipidomic and metabolomic analysis of cyclooxygenase-null cells: eicosanoid storm, cross talk, and compensation by COX-1. Genomics Proteom. Bioinforma. 14, 81–93 (2016).
Pickart, C. M. Targeting of substrates to the 26S proteasome. FASEB J. 11, 1055–1066 (1997).
Iwai, K. Diverse ubiquitin signaling in NF-κB activation. Trends Cell Biol. 22, 355–364 (2012).
French, M. E., Koehler, C. F. & Hunter, T. Emerging functions of branched ubiquitin chains. Cell Discov. 7, 6 (2021).
Randles, L. & Walters, K. J. Ubiquitin and its binding domains. Front. Biosci. 17, 2140–2157 (2012).
Husnjak, K. & Dikic, I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 81, 291–322 (2012).
Clague, M. J., Urbe, S. & Komander, D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 20, 338–352 (2019).
Cappadocia, L. & Lima, C. D. Ubiquitin-like protein conjugation: structures, chemistry, and mechanism. Chem. Rev. 118, 889–918 (2018).
Boyden, L. M. et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482, 98–102 (2012).
Choi, J. et al. Loss of KLHL6 promotes diffuse large B-cell lymphoma growth and survival by stabilizing the mRNA decay factor roquin2. Nat. Cell Biol. 20, 586–596 (2018).
Glickman, M. H. & Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82, 373–428 (2002).
Aksentijevich, I. & Zhou, Q. NF-κB pathway in autoinflammatory diseases: dysregulation of protein modifications by ubiquitin defines a new category of autoinflammatory diseases. Front. Immunol. 8, 399 (2017).
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 29, 301–305 (2001).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Aksentijevich, I. et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N. Engl. J. Med. 360, 2426–2437 (2009).
Stone, D. L. et al. The systemic autoinflammatory diseases: coming of age with the human genome. J. Allergy Clin. Immunol. 146, 997–1001 (2020).
McGonagle, D. & McDermott, M. F. A proposed classification of the immunological diseases. PLoS Med. 3, e297 (2006).
Ben-Chetrit, E. et al. Consensus proposal for taxonomy and definition of the autoinflammatory diseases (AIDs): a Delphi study. Ann. Rheum. Dis. 77, 1558–1565 (2018).
Savic, S., Caseley, E. A. & McDermott, M. F. Moving towards a systems-based classification of innate immune-mediated diseases. Nat. Rev. Rheumatol. 16, 222–237 (2020).
Wertz, I. E. & Wang, X. From discovery to bedside: targeting the ubiquitin system. Cell Chem. Biol. 26, 156–177 (2019).
Beck, D. B. et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2026834 (2020).
Hyer, M. L. et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 24, 186–193 (2018).
Georgin-Lavialle, S. et al. Further characterization of clinical and laboratory features in VEXAS syndrome: large-scale analysis of a multicentre case series of 116 French patients. Br. J. Dermatol. https://doi.org/10.1111/bjd.20805 (2021).
Poulter, J. A. et al. Novel somatic mutations in UBA1 as a cause of VEXAS syndrome. Blood 137, 3676–3681 (2021).
Bourbon, E. et al. Therapeutic options in VEXAS syndrome: insights from a retrospective series. Blood 137, 3682–3684 (2021).
Staels, F. et al. Case report: VEXAS syndrome: from mild symptoms to life-threatening macrophage activation syndrome. Front. Immunol. 12, 678927 (2021).
Barba, T. et al. VEXAS syndrome in a woman. Rheumatology https://doi.org/10.1093/rheumatology/keab392 (2021).
Arlet, J. B., Terrier, B. & Kosmider, O. Mutant UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 384, 2163 (2021).
Beck, D. B., Grayson, P. C. & Kastner, D. L. Mutant UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 384, 2164–2165 (2021).
Luzzatto, L., Risitano, A. M. & Notaro, R. Mutant UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 384, 2164 (2021).
Diarra, A., Duployez, N. & Terriou, L. Mutant UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 384, 2163–2164 (2021).
Pfleger, C. M., Harvey, K. F., Yan, H. & Hariharan, I. K. Mutation of the gene encoding the ubiquitin activating enzyme uba1 causes tissue overgrowth in Drosophila. Fly 1, 95–105 (2007).
Lacombe, V., Kosmider, O., Prevost, M., Lavigne, C. & Urbanski, G. Severe joint involvement in VEXAS syndrome: a case report. Ann. Intern. Med. 174, 1025–1027 (2021).
Grey, A. et al. A case of VEXAS syndrome complicated by hemophagocytic lymphohistiocytosis. J. Clin. Immunol. https://doi.org/10.1007/s10875-021-01070-y (2021).
Magnol, M. et al. VEXAS syndrome in a patient with previous spondyloarthritis with favorable response to intravenous immunoglobulin anti-IL17 therapy. Rheumatology https://doi.org/10.1093/rheumatology/keab211 (2021).
Grayson, P. C., Patel, B. A. & Young, N. S. VEXAS syndrome. Blood 137, 3591–3594 (2021).
Goetzke, C. C., Ebstein, F. & Kallinich, T. Role of proteasomes in inflammation. J. Clin. Med. https://doi.org/10.3390/jcm10081783 (2021).
Fremond, M. L. & Crow, Y. J. STING-Mediated Lung Inflammation and Beyond. J. Clin. Immunol. 41, 501–514 (2021).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Diarra, A. et al. Successful allogeneic hematopoietic stem cell transplantation in patients with VEXAS syndrome: a two center experience. Blood Adv. https://doi.org/10.1182/bloodadvances.2021004749 (2021).
Ramser, J. et al. Rare missense and synonymous variants in UBE1 are associated with X-linked infantile spinal muscular atrophy. Am. J. Hum. Genet. 82, 188–193 (2008).
Balak, C. D. et al. Functional characterizations of rare UBA1 variants in X-linked Spinal Muscular Atrophy. F1000Res 6, 1636 (2017).
Groen, E. J. N. & Gillingwater, T. H. UBA1:at the crossroads of ubiquitin homeostasis and neurodegeneration. Trends Mol. Med. 21, 622–632 (2015).
Powis, R. A. et al. Systemic restoration of UBA1 ameliorates disease in spinal muscular atrophy. JCI Insight 1, e87908 (2016).
Chanarat, S. & Mishra, S. K. Emerging roles of ubiquitin-like proteins in pre-mRNA splicing. Trends Biochem. Sci. 43, 896–907 (2018).
Poli, M. C. et al. Heterozygous truncating variants in POMP escape nonsense-mediated decay and cause a unique immune dysregulatory syndrome. Am. J. Hum. Genet. 102, 1126–1142 (2018).
Lam, M. T. et al. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J. Exp. Med. 216, 2778–2799 (2019).
Endo, M., Druso, J. E. & Cerione, R. A. The two splice variant forms of Cdc42 exert distinct and essential functions in neurogenesis. J. Biol. Chem. 295, 4498–4512 (2020).
Dahlqvist, J. et al. A single-nucleotide deletion in the POMP 5′ UTR causes a transcriptional switch and altered epidermal proteasome distribution in KLICK genodermatosis. Am. J. Hum. Genet. 86, 596–603 (2010).
Bard, J. A. M. et al. Structure and function of the 26S proteasome. Annu. Rev. Biochem. 87, 697–724 (2018).
Rousseau, A. & Bertolotti, A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 19, 697–712 (2018).
Murata, S., Takahama, Y., Kasahara, M. & Tanaka, K. The immunoproteasome and thymoproteasome: functions, evolution and human disease. Nat. Immunol. 19, 923–931 (2018).
Brehm, A. & Kruger, E. Dysfunction in protein clearance by the proteasome: impact on autoinflammatory diseases. Semin. Immunopathol. 37, 323–333 (2015).
Ohmura, K. Nakajo-Nishimura syndrome and related proteasome-associated autoinflammatory syndromes. J. Inflamm. Res. 12, 259–265 (2019).
Brehm, A. et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Invest. 125, 4196–4211 (2015).
Yan, K. et al. Haploinsufficiency of PSMD12 causes proteasome dysfunction and subclinical autoinflammation. Arthritis Rheumatol. https://doi.org/10.1002/art.42070 (2022).
Aksentijevich, I. & Schnappauf, O. Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases. Nat. Rev. Rheumatol. 17, 405–425 (2021).
Davidson, S. et al. Protein kinase R is an innate immune sensor of proteotoxic stress via accumulation of cytoplasmic IL-24. Sci. Immunol. 7, eabi6763 (2022).
Opitz, E. et al. Impairment of immunoproteasome function by beta5i/LMP7 subunit deficiency results in severe enterovirus myocarditis. PLoS Pathog. 7, e1002233 (2011).
Kury, S. et al. De novo disruption of the proteasome regulatory subunit PSMD12 causes a syndromic neurodevelopmental disorder. Am. J. Hum. Genet. 100, 352–363 (2017).
Isidor, B. et al. Stankiewicz-Isidor syndrome: expanding the clinical and molecular phenotype. Genet. Med. 24, 179–191 (2022).
Khalil, R. et al. PSMD12 haploinsufficiency in a neurodevelopmental disorder with autistic features. Am. J. Med. Genet. B Neuropsychiatr. Genet. 177, 736–745 (2018).
Crow, Y. J. & Manel, N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 429–440 (2015).
Schnappauf, O. & Aksentijevich, I. Mendelian diseases of dysregulated canonical NF-κB signaling: From immunodeficiency to inflammation. J. Leukoc. Biol. 108, 573–589 (2020).
Sun, S. C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558 (2017).
Hayden, M. S. & Ghosh, S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–234 (2012).
Zhang, Q., Lenardo, M. J. & Baltimore, D. 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell 168, 37–57 (2017).
Badran, Y. R. et al. Human RELA haploinsufficiency results in autosomal-dominant chronic mucocutaneous ulceration. J. Exp. Med. 214, 1937–1947 (2017).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A2κκ0 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Polykratis, A. et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat. Cell Biol. 21, 731–742 (2019).
Tokunaga, F. et al. Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-κB regulation. EMBO J. 31, 3856–3870 (2012).
Priem, D., van Loo, G. & Bertrand, M. J. M. A20 and cell death-driven inflammation. Trends Immunol. 41, 421–435 (2020).
Martens, A. et al. Two distinct ubiquitin-binding motifs in A20 mediate its anti-inflammatory and cell-protective activities. Nat. Immunol. 21, 381–387 (2020).
Hutti, J. E. et al. IκB kinase beta phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-κB pathway. Mol. Cell Biol. 27, 7451–7461 (2007).
Zammit, N. W. et al. Denisovan, modern human and mouse TNFAIP3 alleles tune A20 phosphorylation and immunity. Nat. Immunol. 20, 1299–1310 (2019).
Vereecke, L., Beyaert, R. & van Loo, G. Genetic relationships between A20/TNFAIP3, chronic inflammation and autoimmune disease. Biochem. Soc. Trans. 39, 1086–1091 (2011).
Adrianto, I. et al. Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nat. Genet. 43, 253–258 (2011).
Westra, H. J. et al. Fine-mapping and functional studies highlight potential causal variants for rheumatoid arthritis and type 1 diabetes. Nat. Genet. 50, 1366–1374 (2018).
Stuart, P. E. et al. Genome-wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am. J. Hum. Genet. 97, 816–836 (2015).
Zhou, Q. et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 48, 67–73 (2016).
Kato, M. et al. Frequent inactivation of A20 in B-cell lymphomas. Nature 459, 712–716 (2009).
Aeschlimann, F. A. et al. A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease. Ann. Rheum. Dis. 77, 728–735 (2018).
Schwartz, D. M. et al. Type I interferon signature predicts response to JAK inhibition in haploinsufficiency of A20. Ann. Rheum. Dis. 79, 429–431 (2020).
Jahan, A. S., Elbaek, C. R. & Damgaard, R. B. Met1-linked ubiquitin signalling in health and disease: inflammation, immunity, cancer, and beyond. Cell Death Differ. 28, 473–492 (2021).
Schlicher, L. et al. SPATA2 promotes CYLD activity and regulates TNF-induced NF-κB signaling and cell death. EMBO Rep. 17, 1485–1497 (2016).
Boisson, B. et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 13, 1178–1186 (2012).
Boisson, B. et al. Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. J. Exp. Med. 212, 939–951 (2015).
Zinngrebe, J. et al. Compound heterozygous variants in OTULIN are associated with fulminant atypical late-onset ORAS. EMBO Mol. Med. 14, e14901 (2022).
Damgaard, R. B. et al. The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell 166, 1215–1230.e20 (2016).
Verboom, L., Hoste, E. & van Loo, G. OTULIN in NF-κB signaling, cell death, and disease. Trends Immunol. 42, 590–603 (2021).
Zhou, Q. et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc. Natl Acad. Sci. USA 113, 10127–10132 (2016).
Heger, K. et al. OTULIN limits cell death and inflammation by deubiquitinating LUBAC. Nature 559, 120–124 (2018).
Damgaard, R. B. et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol. Cell 46, 746–758 (2012).
Gerlach, B. et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471, 591–596 (2011).
Tao, P. et al. Deubiquitination of proteasome subunits by OTULIN regulates type I IFN production. Sci. Adv. 7, eabi6794 (2021).
Damgaard, R. B. et al. OTULIN deficiency in ORAS causes cell type-specific LUBAC degradation, dysregulated TNF signalling and cell death. EMBO Mol. Med. https://doi.org/10.15252/emmm.201809324 (2019).
Jost, P. J. & Vucic, D. Regulation of cell death and immunity by XIAP. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a036426 (2020).
Mudde, A. C. A., Booth, C. & Marsh, R. A. Evolution of our understanding of XIAP deficiency. Front. Pediatr. 9, 660520 (2021).
Gyrd-Hansen, M. et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-κB as well as cell survival and oncogenesis. Nat. Cell Biol. 10, 1309–1317 (2008).
Lawlor, K. E. et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 6, 6282 (2015).
Goncharov, T. et al. Disruption of XIAP-RIP2 association blocks NOD2-mediated inflammatory signaling. Mol. Cell 69, 551–565 e557 (2018).
Marsh, R. A. et al. Allogeneic hematopoietic cell transplantation for XIAP deficiency: an international survey reveals poor outcomes. Blood 121, 877–883 (2013).
Lee-Kirsch, M. A. The type I interferonopathies. Annu. Rev. Med. 68, 297–315 (2017).
Rodero, M. P. & Crow, Y. J. Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J. Exp. Med. 213, 2527–2538 (2016).
Liu, C., Kieltyka, J., Fleischmann, R., Gadina, M. & O’Shea, J. J. A decade of jakinibs: what have we learned and what may be the future. Arthritis Rheumatol. https://doi.org/10.1002/art.41906 (2021).
Zhang, Q. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science https://doi.org/10.1126/science.abd4570 (2020).
Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science https://doi.org/10.1126/science.abd4585 (2020).
Beck, D. B. & Aksentijevich, I. Susceptibility to severe COVID-19. Science 370, 404–405 (2020).
Shin, D. et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 587, 657–662 (2020).
d’Angelo, D. M., Di Filippo, P., Breda, L. & Chiarelli, F. Type I interferonopathies in children: an overview. Front. Pediatr. 9, 631329 (2021).
Crow, Y. J. & Stetson, D. B. The type I interferonopathies: 10 years on. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-021-00633-9 (2021).
Wang, H. T. & Hur, S. Substrate recognition by TRIM and TRIM-like proteins in innate immunity. Semin. Cell Dev. Biol. 111, 76–85 (2021).
Lu, D. et al. Mutations of deubiquitinase OTUD1 are associated with autoimmune disorders. J. Autoimmun. 94, 156–165 (2018).
Zhang, Z., Wang, D., Wang, P., Zhao, Y. & You, F. OTUD1 negatively regulates type I IFN induction by disrupting noncanonical ubiquitination of IRF3. J. Immunol. 204, 1904–1918 (2020).
Fuchs, S. Y. Ubiquitination-mediated regulation of interferon responses. Growth Factors 30, 141–148 (2012).
D’Cunha, J., Knight, E. Jr, Haas, A. L., Truitt, R. L. & Borden, E. C. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc. Natl Acad. Sci. USA 93, 211–215 (1996).
Fan, J. B. et al. Identification and characterization of a novel ISG15-ubiquitin mixed chain and its role in regulating protein homeostasis. Sci. Rep. 5, 12704 (2015).
Zhang, X. et al. Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature 517, 89–93 (2015).
Swaim, C. D. et al. Modulation of extracellular ISG15 signaling by pathogens and viral effector proteins. Cell Rep. 31, 107772 (2020).
Bogunovic, D. et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science 337, 1684–1688 (2012).
Meuwissen, M. E. et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med. 213, 1163–1174 (2016).
Basters, A., Knobeloch, K. P. & Fritz, G. USP18 — a multifunctional component in the interferon response. Biosci. Rep. https://doi.org/10.1042/BSR20180250 (2018).
Strong, W. B. Cholesterol screening in children: a consensus statement — finally. J. Med. Assoc. Ga. 80, 451–452 (1991).
Speer, S. D. et al. ISG15 deficiency and increased viral resistance in humans but not mice. Nat. Commun. 7, 11496 (2016).
Metcalf, M. G., Higuchi-Sanabria, R., Garcia, G., Tsui, C. K. & Dillin, A. Beyond the cell factory: homeostatic regulation of and by the UPR(ER). Sci. Adv. 6, eabb9614 (2020).
Grootjans, J., Kaser, A., Kaufman, R. J. & Blumberg, R. S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 16, 469–484 (2016).
Henckaerts, L. et al. Genetic variation in the autophagy gene ULK1 and risk of Crohn’s disease. Inflamm. Bowel Dis. 17, 1392–1397 (2011).
Larabi, A., Barnich, N. & Nguyen, H. T. T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 16, 38–51 (2020).
Setta-Kaffetzi, N. et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am. J. Hum. Genet. 94, 790–797 (2014).
Watkin, L. B. et al. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat. Genet. 47, 654–660 (2015).
Deng, Z. et al. A defect in COPI-mediated transport of STING causes immune dysregulation in COPA syndrome. J. Exp. Med. https://doi.org/10.1084/jem.20201045 (2020).
Anderton, H., Wicks, I. P. & Silke, J. Cell death in chronic inflammation: breaking the cycle to treat rheumatic disease. Nat. Rev. Rheumatol. 16, 496–513 (2020).
Samir, P., Malireddi, R. K. S. & Kanneganti, T. D. The PANoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis). Front. Cell Infect. Microbiol. 10, 238 (2020).
Chun, H. J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002).
Lalaoui, N. et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature 577, 103–108 (2020).
Tao, P. et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 577, 109–114 (2020).
Cuchet-Lourenco, D. et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science 361, 810–813 (2018).
McDermott, M. F. et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97, 133–144 (1999).
Molnar, E. et al. Key diagnostic markers for autoimmune lymphoproliferative syndrome with molecular genetic diagnosis. Blood 136, 1933–1945 (2020).
Taft, J. et al. Human TBK1 deficiency leads to autoinflammation driven by TNF-induced cell death. Cell 184, 4447–4463.e20 (2021).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Grumati, P. & Dikic, I. Ubiquitin signaling and autophagy. J. Biol. Chem. 293, 5404–5413 (2018).
Acknowledgements
We thank Dr Hirotsugu Oda for thoughtful comments on the manuscript. This work was supported by the intramural research program of the NIH including the National Human Genome Research Institute and the National Institute of Dental and Craniofacial Research. D.B.B. was supported in part by funding from the NIH (R00AR078205).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks G. van Loo; M. A. Lee-Kirsch; and M. McDermott, who co-reviewed with L. Pieper Pournara, for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beck, D.B., Werner, A., Kastner, D.L. et al. Disorders of ubiquitylation: unchained inflammation. Nat Rev Rheumatol 18, 435–447 (2022). https://doi.org/10.1038/s41584-022-00778-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00778-4
This article is cited by
-
Shared and distinct mechanisms of UBA1 inactivation across different diseases
The EMBO Journal (2024)
-
Relapsing polychondritis: clinical updates and new differential diagnoses
Nature Reviews Rheumatology (2024)
-
Endothelial progenitor cells systemic administration alleviates multi-organ senescence by down-regulating USP7/p300 pathway in chronic obstructive pulmonary disease
Journal of Translational Medicine (2023)
-
Neuronal-specific TNFAIP1 ablation attenuates postoperative cognitive dysfunction via targeting SNAP25 for K48-linked ubiquitination
Cell Communication and Signaling (2023)
-
Novel causative variants of VEXAS in UBA1 detected through whole genome transcriptome sequencing in a large cohort of hematological malignancies
Leukemia (2023)