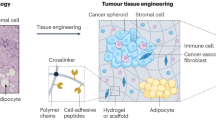
Abstract
The use of biomaterials has substantially contributed to both our understanding of tumorigenesis and our ability to identify and capture tumour cells in vitro and in vivo. Natural and synthetic biomaterials can be applied as models to recapitulate key features of the tumour microenvironment in vitro, including architectural, mechanical and biological functions. Engineered biomaterials can further mimic the spatial and temporal properties of the surrounding tumour niche to investigate the specific effects of the environment on disease progression, offering an alternative to animal models for the testing of cancer cell behaviour. Biomaterials can also be used to capture and detect cancer cells in vitro and in vivo to monitor tumour progression. In this Review, we discuss the natural and synthetic biomaterials that can be used to recreate specific features of tumour microenvironments. We examine how biomaterials can be applied to capture circulating tumour cells in blood samples for the early detection of metastasis. We highlight biomaterial-based strategies to investigate local regions adjacent to the tumour and survey potential applications of biomaterial-based devices for diagnosis and prognosis, such as the detection of cellular deformability and the non-invasive surveillance of tumour-adjacent stroma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


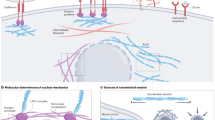
Figure is reproduced with permission from ref.3, Elsevier.

Similar content being viewed by others
References
Affo, S., Yu, L. X. & Schwabe, R. F. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu. Rev. Pathol. 12, 153–186 (2017).
Pankova, D. et al. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol. Cancer Res. 14, 287–295 (2016).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). This paper uses a hydrogel–Matrigel sandwich culture system to show that initial matrix stiffness can drive loss of mammary epithelial polarity.
Pickup, M. W., Mouw, J. K. & Weaver, V. M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253 (2014).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009). This paper demonstrates that stiffer tissues can drive tumour growth and metastasis in vivo.
Mak, I. W., Evaniew, N. & Ghert, M. Lost in translation: animal models and clinical trials in cancer treatment. Am. J. Transl Res. 6, 114–118 (2014).
Bissell, M. J., Hall, H. G. & Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 99, 31–68 (1982).
Kleinman, H. K. et al. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21, 6188–6193 (1982).
Orkin, R. W. et al. A murine tumor producing a matrix of basement membrane. J. Exp. Med. 145, 204–220 (1977).
Vukicevic, S. et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 202, 1–8 (1992).
Barcellos-Hoff, M. H., Aggeler, J., Ram, T. G. & Bissell, M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105, 223–235 (1989).
Kenny, P. A. et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 1, 84–96 (2007).
Petersen, O. W., Ronnovjessen, L., Howlett, A. R. & Bissell, M. J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl Acad. Sci. USA 89, 9064–9068 (1992).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006).
Ozdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Rhim, A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014).
Vennin, C. et al. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology 54, 820–838 (2018).
Nguyen-Ngoc, K. V. et al. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl Acad. Sci. USA 109, E2595–E2604 (2012).
Velez, D. O. et al. 3D collagen architecture induces a conserved migratory and transcriptional response linked to vasculogenic mimicry. Nat. Commun. 8, 1651 (2017).
Guzman, A., Ziperstein, M. J. & Kaufman, L. J. The effect of fibrillar matrix architecture on tumor cell invasion of physically challenging environments. Biomaterials 35, 6954–6963 (2014).
Mouw, J. K. et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med. 20, 360–367 (2014).
Denais, C. M. et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016). This manuscript is the first to describe the mechanisms that enable cancer cells to migrate through pores in matrix that are smaller than the diameter of the nucleus.
Sodek, K. L., Brown, T. J. & Ringuette, M. J. Collagen I but not Matrigel matrices provide an MMP-dependent barrier to ovarian cancer cell penetration. BMC Cancer 8, 223 (2008).
Wolf, K. et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 201, 1069–1084 (2013).
Carey, S. P., Martin, K. E. & Reinhart-King, C. A. Three-dimensional collagen matrix induces a mechanosensitive invasive epithelial phenotype. Sci. Rep. 7, 42088 (2017).
Friedl, P. & Wolf, K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188, 11–19 (2009).
Zaman, M. H. et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl Acad. Sci. USA 103, 10889–10894 (2006).
Mason, B. N., Starchenko, A., Williams, R. M., Bonassar, L. J. & Reinhart-King, C. A. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 9, 4635–4644 (2013).
Williams, C. M., Engler, A. J., Slone, R. D., Galante, L. L. & Schwarzbauer, J. E. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 68, 3185–3192 (2008).
Fraley, S. I. et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol. 12, 598–604 (2010).
Anguiano, M. et al. Characterization of three-dimensional cancer cell migration in mixed collagen-Matrigel scaffolds using microfluidics and image analysis. PLOS One 12, e0171417 (2017).
Steinwachs, J. et al. Three-dimensional force microscopy of cells in biopolymer networks. Nat. Methods 13, 171–176 (2016).
Koch, T. M., Munster, S., Bonakdar, N., Butler, J. P. & Fabry, B. 3D Traction Forces in Cancer Cell Invasion. PLOS One 7, e33476 (2012).
Miller, J. P. et al. Clinical doses of radiation reduce collagen matrix stiffness. APL Bioeng. 2, 031901 (2018).
Beck, J. N., Singh, A., Rothenberg, A. R., Elisseeff, J. H. & Ewald, A. J. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials 34, 9486–9495 (2013).
Wen, J. H. et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 13, 979–987 (2014).
Taubenberger, A. V. et al. 3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments. Acta Biomater. 36, 73–85 (2016).
Loessner, D. et al. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31, 8494–8506 (2010).
Alcaraz, J. et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 27, 2829–2838 (2008).
Reticker-Flynn, N. E. et al. A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nat. Commun. 3, 1122 (2012). This paper highlights how non-additive matrix properties, specifically the combination of multiple matrix proteins, can drive metastatic behaviour.
Kraning-Rush, C. M., Califano, J. P. & Reinhart-King, C. A. Cellular traction stresses increase with increasing metastatic potential. PLOS One 7, e32572 (2012).
Leight, J. L., Wozniak, M. A., Chen, S., Lynch, M. L. & Chen, C. S. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol. Biol. Cell 23, 781–791 (2012).
Stowers, R. S., Allen, S. C. & Suggs, L. J. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl Acad. Sci. USA 112, 1953–1958 (2015).
Wei, S. C. et al. Matrix stiffness drives epithelial–mesenchymal transition and tumor metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat. Cell Biol. 17, 678–688 (2015).
Fenner, J. et al. Macroscopic stiffness of breast tumors predicts metastasis. Sci. Rep. 4, 5512 (2014).
Tse, J. R. & Engler, A. J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol 47, 10.16.1–10.16.16 (2010).
Emerman, J. T. & Pitelka, D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 13, 316–328 (1977).
Bissell, M. J. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int. Rev. Cytol. 70, 27–100 (1981).
Pang, M. F. et al. Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Res. 76, 5277–5287 (2016).
Jabbari, E., Sarvestani, S. K., Daneshian, L. & Moeinzadeh, S. Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells. PLOS One 10, e0132377 (2015).
Shu, X. Z., Ahmad, S., Liu, Y. & Prestwich, G. D. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J. Biomed. Mater. Res. A 79, 902–912 (2006).
Young, J. L. & Engler, A. J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32, 1002–1009 (2011).
Guvendiren, M. & Burdick, J. A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 3, 792 (2012).
Ondeck, M. G. & Engler, A. J. Mechanical characterization of a dynamic and tunable methacrylated hyaluronic acid hydrogel. J. Biomech. Eng. 138, 021003 (2016).
Stowers, R. S. et al. Extracellular matrix stiffening induces a malignant phenotypic transition in breast epithelial cells. Cell. Mol. Bioeng. 10, 114–123 (2017). Temporal changes in matrix stiffness can modulate mammary epithelial cell responses in a different way than static materials, which when stiff, always induce phenotype transitions.
Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009).
Maghdouri-White, Y., Elmore, L. W., Bowlin, G. L. & Dreau, D. Breast epithelial cell infiltration in enhanced electrospun silk scaffolds. J. Tissue Eng. Regen Med. 10, E121–131 (2016).
Chen, Z. et al. Electrospun nanofibers for cancer diagnosis and therapy. Biomater. Sci. 4, 922–932 (2016).
Kushiro, K., Yaginuma, T., Ryo, A. & Takai, M. Differences in three-dimensional geometric recognition by non-cancerous and cancerous epithelial cells on microgroove-based topography. Sci. Rep. 7, 4244 (2017).
Ning, D. et al. Mechanical and morphological analysis of cancer cells on nanostructured substrates. Langmuir 32, 2718–2723 (2016).
Chaudhuri, P. K., Pan, C. Q., Low, B. C. & Lim, C. T. Topography induces differential sensitivity on cancer cell proliferation via Rho-ROCK-Myosin contractility. Sci. Rep. 6, 19672 (2016).
Ulrich, T. A., Jaim, A., Tanner, K., MacKay, J. L. & Kumar, S. Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials 31, 1875–1884 (2010).
Pathak, A. & Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl Acad. Sci. USA 109, 10334–10339 (2012).
Attieh, Y. & Vignjevic, D. M. The hallmarks of CAFs in cancer invasion. Eur. J. Cell Biol. 95, 493–502 (2016).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Gaggioli, C. et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392–1400 (2007).
Goetz, J. G. et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163 (2011).
Glentis, A. et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 8, 924 (2017).
Wang, K. et al. Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials 54, 63–71 (2015).
Labernadie, A. et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19, 224–237 (2017).
Calvo, F. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637–646 (2013).
Chen, M. B. et al. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat. Protoc. 12, 865–880 (2017).
Lee, H., Park, W., Ryu, H. & Jeon, N. L. A microfluidic platform for quantitative analysis of cancer angiogenesis and intravasation. Biomicrofluidics 8, 054102 (2014).
Zervantonakis, I. K. et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl Acad. Sci. USA 109, 13515–13520 (2012).
Chen, M. B., Whisler, J. A., Jeon, J. S. & Kamm, R. D. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr. Biol. 5, 1262–1271 (2013).
Bersini, S. et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 35, 2454–2461 (2014).
Saquib, N., Saquib, J. & Ioannidis, J. P. Does screening for disease save lives in asymptomatic adults? Systematic review of meta-analyses and randomized trials. Int. J. Epidemiol. 44, 264–277 (2015).
Zhang, Z. et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget 5, 12383–12397 (2014).
Mehta, S. et al. Predictive and prognostic molecular markers for cancer medicine. Ther. Adv. Med. Oncol. 2, 125–148 (2010).
Qian, W. Y., Zhang, Y. & Chen, W. Q. Capturing cancer: emerging microfluidic technologies for the capture and characterization of circulating tumor cells. Small 11, 3850–3872 (2015).
Chen, W. Q. et al. Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies. ACS Nano 7, 566–575 (2013).
Park, G. S. et al. Full surface embedding of gold clusters on silicon nanowires for efficient capture and photothermal therapy of circulating tumor cells Nano Lett. 12, 2176–2176 (2012).
Zhai, T. T., Ye, D. K., Zhang, Q. W., Wu, Z. Q. & Xia, X. H. Highly efficient capture and electrochemical release of circulating tumor cells by using aptamers modified gold nanowire arrays. ACS Appl. Mater. Interfaces 9, 34706–34714 (2017).
Zhang, W., Zhao, K., Banks, C. E. & Zhang, Y. Antibody-modified hydroxyapatite surfaces for the efficient capture of bladder cancer cells in a patient’s urine without recourse to any sample pre-treatment. J. Mater. Chem. B 5, 8125–8132 (2017).
Xu, H. W. et al. Three-dimensional inverse opal photonic crystal substrates toward efficient capture of circulating tumor cells. ACS Appl. Mater. Interfaces 9, 30510–30518 (2017).
Zhang, J. et al. Surface chemistry induces mitochondria-mediated apoptosis of breast cancer cells via PTEN/PI3K/AKT signaling pathway. Biochim. Biophys. Acta Mol. Cell Res. 1865, 172–185 (2018).
Jan, Y. J. et al. NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv. Drug Deliv. Rev. 125, 78–93 (2018).
Wang, L. X., Asghar, W., Demirci, U. & Wan, Y. Nanostructured substrates for isolation of circulating tumor cells. Nano Today 8, 374–387 (2013).
Wang, S. T. et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew. Chem. Int. Ed. 48, 8970–8973 (2009).
Wang, S. T. et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew. Chem. Int. Ed. 50, 3084–3088 (2011).
Guo, S. et al. Degradable zinc-phosphate-based hierarchical nanosubstrates for capture and release of circulating tumor cells. ACS Appl. Mater. Interfaces 8, 15917–15925 (2016).
Lou, H. Y. et al. Dual-functional lipid coating for the nanopillar-based capture of circulating tumor cells with high purity and efficiency. Langmuir 33, 1097–1104 (2017).
Sun, N. et al. Chitosan nanofibers for specific capture and nondestructive release of CTCs assisted by pCBMA brushes. Small 12, 5090–5097 (2016).
Rao, S. S. et al. Enhanced survival with implantable scaffolds that capture metastatic breast cancer cells in vivo. Cancer Res. 76, 5209–5218 (2016).
Azarin, S. M. et al. In vivo capture and label-free detection of early metastatic cells. Nat. Commun. 6, 8094 (2015). This paper describes an implantable hydrogel-based method to capture cells that have intravasated from tumours.
Aguado, B. A. et al. Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche. Acta Biomater. 33, 13–24 (2016).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–U129 (2011).
Kitamura, T., Qian, B. Z. & Pollard, J. W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86 (2015).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–U1331 (2006).
Mauro, N., Scialabba, C., Pitarresi, G. & Giammona, G. Enhanced adhesion and in situ photothermal ablation of cancer cells in surface-functionalized electrospun microfiber scaffold with graphene oxide. Int. J. Pharm. 526, 167–177 (2017).
Ma, H. S. et al. A bifunctional biomaterial with photothermal effect for tumor therapy and bone regeneration. Adv. Funct. Mater. 26, 1197–1208 (2016).
Aguado, B. A. et al. Secretome identification of immune cell factors mediating metastatic cell homing. Sci. Rep. 5, 17566 (2015).
de la Fuente, A. et al. M-trap: exosome-based capture of tumor cells as a new technology in peritoneal metastasis. J. Natl Cancer Inst. 107, djv184 (2015).
Seib, F. P., Berry, J. E., Shiozawa, Y., Taichman, R. S. & Kaplan, D. L. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials 51, 313–319 (2015).
Aguado, B. A., Bushnell, G. G., Rao, S. S., Jeruss, J. S. & Shea, L. D. Engineering the pre-metastatic niche. Nat. Biomed. Eng. 1, 0077 (2017).
Davidson, P. M., Denais, C., Bakshi, M. C. & Lammerding, J. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell. Mol. Bioeng. 7, 293–306 (2014).
Yao, N. et al. Structure and function analysis in circulating tumor cells: using nanotechnology to study nuclear size in prostate cancer. Am. J. Clin. Exp. Urol. 6, 43–54 (2018).
Chen, J. F. et al. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer 121, 3240–3251 (2015).
Alibert, C., Goud, B. & Manneville, J. B. Are cancer cells really softer than normal cells? Biol. Cell 109, 167–189 (2017).
Guo, Q., Park, S. & Ma, H. S. Microfluidic micropipette aspiration for measuring the deformability of single cells. Lab. Chip 12, 2687–2695 (2012).
Mak, M. & Erickson, D. A serial micropipette microfluidic device with applications to cancer cell repeated deformation studies. Integr. Biol. 5, 1374–1384 (2013).
Malboubi, M., Jayo, A., Parsons, M. & Charras, G. An open access microfiuidic device for the study of the physical limits of cancer cell deformation during migration in confined environments. Microelectron. Eng. 144, 42–45 (2015).
Gossett, D. R. et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl Acad. Sci. USA 109, 7630–7635 (2012). This paper demonstrates a high-throughput single cell microfluidic-based deformability cytometry assay showing that mechanical properties of cells can be used to separate populations with different metastatic potentials.
Dudani, J. S., Gossett, D. R., Tse, H. T. K. & Di Carlo, D. Pinched-flow hydrodynamic stretching of single-cells. Lab. Chip 13, 3728–3734 (2013).
Qi, D. et al. Screening cell mechanotype by parallel microfiltration. Sci. Rep. 5, 17595 (2015).
Nyberg, K. D. et al. The physical origins of transit time measurements for rapid, single cell mechanotyping. Lab. Chip 16, 3330–3339 (2016).
Palmer, C. P. et al. Single cell adhesion measuring apparatus (SCAMA): application to cancer cell lines of different metastatic potential and voltage-gated Na+ channel expression. Eur. Biophys. J. 37, 359–368 (2008).
Veiseh, M. et al. Cellular heterogeneity profiling by hyaluronan probes reveals an invasive but slow-growing breast tumor subset. Proc. Natl Acad. Sci. USA 111, E1731–E1739 (2014).
Bijian, K. et al. Targeting focal adhesion turnover in invasive breast cancer cells by the purine derivative reversine. Br. J. Cancer 109, 2810–2818 (2013).
Fuhrmann, A., Banisadr, A., Beri, P., Tlsty, T. D. & Engler, A. J. Metastatic state of cancer cells may be indicated by adhesion strength. Biophys. J. 112, 736–745 (2017).
Seltzer, M. H., Rosato, F. E. & Fletcher, M. J. Serum and tissue magnesium levels in human breast carcinoma. J. Surg. Res. 10, 159–162 (1970).
Seltzer, M. H., Rosato, F. E. & Fletcher, M. J. Serum and tissue calcium in human breast carcinoma. Cancer Res. 30, 615–616 (1970).
Zhou, Z. X. & Lu, Z. R. Molecular imaging of the tumor microenvironment. Adv. Drug Deliv. Rev. 113, 24–48 (2017).
Chaabane, L. et al. In vivo MR imaging of fibrin in a neuroblastoma tumor model by means of a targeting Gd-containing peptide. Mol. Imag. Biol. 17, 819–828 (2015).
Starmans, L. W. E. et al. Noninvasive visualization of tumoral fibrin deposition using a peptidic fibrin-binding single photon emission computed tomography tracer. Mol. Pharm. 12, 1921–1928 (2015).
Obonai, T. et al. Tumour imaging by the detection of fibrin clots in tumour stroma using an anti-fibrin Fab fragment. Sci. Rep. 6, 23613 (2016).
van Mourik, T. R., Claesener, M., Nicolay, K. & Grull, H. Development of a novel, fibrin-specific PET tracer. J. Labelled Comp. Radiopharm. 60, 286–293 (2017).
Zhou, Z. X. et al. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat. Commun. 6, 7984 (2015).
Li, C. L. et al. Fibronectin induces epithelial-mesenchymal transition in human breast cancer MCF-7 cells via activation of calpain. Oncol. Lett. 13, 3889–3895 (2017).
Han, Z. & Lu, Z. R. Targeting fibronectin for cancer imaging and therapy. J. Mater. Chem. B 5, 639–654 (2017).
Heidari, P. et al. Imaging of secreted extracellular periostin, an important marker of invasion in the tumor microenvironment in esophageal cancer. J. Nuclear Med. 56, 1246–1251 (2015).
Khawar, I. A., Kim, J. H. & Kuh, H. J. Improving drug delivery to solid tumors: priming the tumor microenvironment. J. Control. Release 201, 78–89 (2015).
Grossman, M. et al. Tumor cell invasion can be blocked by modulators of collagen fibril alignment that control assembly of the extracellular matrix. Cancer Res. 76, 4249–4258 (2016).
Theocharis, A. D., Skandalis, S. S., Gialeli, C. & Karamanos, N. K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 97, 4–27 (2016).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Jacobetz, M. A. et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013).
Hingorani, S. R. et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin. Cancer Res. 22, 2848–2854 (2016).
Lee, S. et al. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J. Control. Release 263, 68–78 (2017).
Baronzio, G., Parmar, G. & Baronzio, M. Overview of methods for overcoming hindrance to drug delivery to tumors, with special attention to tumor interstitial fluid. Front. Oncol. 5, 165 (2015).
Yhee, J. Y. et al. Effects of tumor microenvironments on targeted delivery of glycol chitosan nanoparticles. J. Control. Release 267, 223–231 (2017).
Nicolas-Boluda, A., Silva, A. K. A., Fournel, S. & Gazeau, F. Physical oncology: new targets for nanomedicine. Biomaterials 150, 87–99 (2018).
Marangon, I. et al. Tumor stiffening, a key determinant of tumor progression, is reversed by nanomaterial-induced photothermal therapy. Theranostics 7, 329–343 (2017).
Raeesi, V. & Chan, W. C. W. Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods. Nanoscale 8, 12524–12530 (2016).
Schuh, J. C. Trials, tribulations, and trends in tumor modeling in mice. Toxicol. Pathol. 32, 53–66 (2004).
Day, C. P., Merlino, G. & Van Dyke, T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 163, 39–53 (2015).
Quintana, E. et al. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 (2008).
Tzvetkova-Chevolleau, T. et al. The motility of normal and cancer cells in response to the combined influence of the substrate rigidity and anisotropic microstructure. Biomaterials 29, 1541–1551 (2008).
Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31, 4639–4656 (2010).
Lewis, K. J. R. et al. Epithelial-mesenchymal crosstalk influences cellular behavior in a 3D alveolus-fibroblast model system. Biomaterials 155, 124–134 (2018).
Fischbach, C. et al. Engineering tumors with 3D scaffolds. Nat. Methods 4, 855–860 (2007).
Makadia, H. K. & Siegel, S. J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397 (2011).
Choi, Y. S. et al. The alignment and fusion assembly of adipose-derived stem cells on mechanically patterned matrices. Biomaterials 33, 6943–6951 (2012).
Nasrollahi, S. et al. Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials 146, 146–155 (2017).
Zustiak, S., Nossal, R. & Sackett, D. L. Multiwell stiffness assay for the study of cell responsiveness to cytotoxic drugs. Biotechnol. Bioeng. 111, 396–403 (2014).
Chaudhuri, O. et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 13, 970–978 (2014).
Fang, J. Y., Tan, S. J., Yang, Z., Tayag, C. & Han, B. Tumor bioengineering using a transglutaminase crosslinked hydrogel. PLOS One 9, e105616 (2014).
Acknowledgements
Funding for this work was provided by US National Institutes of Health grants R01CA206880 (A.J.E. and J.Y.) and R21CA217735 (A.J.E.), a US National Science Foundation grant 1463689 (A.J.E.) and the Graduate Research Fellowship programme (P.B.). Additional fellowship support was provided by Brazilian Federal Agency for Support and Evaluation of Graduate Education award 88881.135357/2016-01 (B.F.M.).
Author information
Authors and Affiliations
Contributions
P.B., J.Y. and A.J.E. organized the manuscript content. The manuscript was written by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beri, P., Matte, B.F., Fattet, L. et al. Biomaterials to model and measure epithelial cancers. Nat Rev Mater 3, 418–430 (2018). https://doi.org/10.1038/s41578-018-0051-6
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-018-0051-6
This article is cited by
-
Measuring and modelling tumour heterogeneity across scales
Nature Reviews Bioengineering (2023)
-
Additive manufacturing of bio-based hydrogel composites: recent advances
Journal of Polymers and the Environment (2022)
-
The epithelial-mesenchymal transition and the cytoskeleton in bioengineered systems
Cell Communication and Signaling (2021)