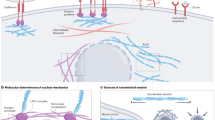
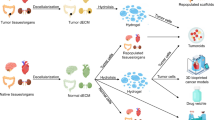
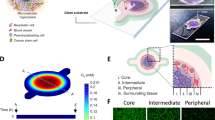
Abstract
Tissue engineering has produced innovative tools for cancer research. 3D cancer models based on molecularly designed biomaterials aim to harness the dimensionality and biomechanical and biochemical properties of tumour tissues. However, to date, despite the critical role that the extracellular matrix plays in cancer, only a minority of 3D cancer models are built on biomaterial-based matrices. Major reasons for avoiding this critical design feature are the difficulty in recreating the inherent complexity of the tumour microenvironment and the limited availability of practical analytical and validation techniques. Recent advances emerging at the interface of supramolecular chemistry, materials science and tumour biology are generating new approaches to overcome these boundaries and enable the design of physiologically relevant 3D models. Here, we discuss how these 3D systems are applied to deconstruct and engineer the tumour microenvironment, opening opportunities to model primary tumours, metastasis and responses to anticancer treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tomás-Bort, E., Kieler, M., Sharma, S., Candido, J. B. & Loessner, D. 3D approaches to model the tumor microenvironment of pancreatic cancer. Theranostics 10, 5074–5089 (2020).
Fischbach, C. et al. Engineering tumors with 3D scaffolds. Nat. Methods 4, 855–860 (2007).
Loessner, D. et al. A 3D tumor microenvironment regulates cell proliferation, peritoneal growth and expression patterns. Biomaterials 190–191, 63–75 (2019).
Loessner, D. et al. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31, 8494–8506 (2010).
Loessner, D. et al. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 11, 727–746 (2016).
Hedegaard, C. L. et al. Peptide-protein coassembling matrices as a biomimetic 3D model of ovarian cancer. Sci. Adv. 6, eabb3298 (2020).
Osuna de la Peña, D. et al. Bioengineered 3D models of human pancreatic cancer recapitulate in vivo tumour biology. Nat. Commun. 12, 5623 (2021).
Kast, V. et al. A tumor microenvironment model of pancreatic cancer to elucidate responses toward immunotherapy. Adv. Healthc. Mater. https://doi.org/10.1002/adhm.202201907 (2022).
Loessner, D. et al. A bioengineered 3D ovarian cancer model for the assessment of peptidase-mediated enhancement of spheroid growth and intraperitoneal spread. Biomaterials 34, 7389–7400 (2013).
Holzapfel, B. M. et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 35, 4108–4115 (2014).
DelNero, P. et al. 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials 55, 110–118 (2015).
Bray, L. J. et al. Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 53, 609–620 (2015).
Taubenberger, A. V. et al. 3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments. Acta Biomater. 36, 73–85 (2016).
Malacrida, B. et al. A human multi-cellular model shows how platelets drive production of diseased extracellular matrix and tissue invasion. iScience 24, 102676 (2021).
Colombo, M. V. et al. Engineering the early bone metastatic niche through human vascularized immuno bone minitissues. Biofabrication 13, 035036 (2021).
Sameni, M. et al. MAME models for 4D live-cell imaging of tumor: microenvironment interactions that impact malignant progression. J. Vis. Exp. https://doi.org/10.3791/3661 (2012).
Sameni, M. et al. Pathomimetic avatars reveal divergent roles of microenvironment in invasive transition of ductal carcinoma in situ. Breast Cancer Res. 19, 56 (2017).
Gjorevski, N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016).
Liu, A. P. et al. The living interface between synthetic biology and biomaterial design. Nat. Mater. 21, 390–397 (2022).
Hutmacher, D. W. et al. Convergence of regenerative medicine and synthetic biology to develop standardized and validated models of human diseases with clinical relevance. Curr. Opin. Biotechnol. 35, 127–132 (2015).
Ingber, D. E. Reverse engineering human pathophysiology with organs-on-chips. Cell 164, 1105–1109 (2016).
Simian, M. & Bissell, M. J. Organoids: a historical perspective of thinking in three dimensions. J. Cell Biol. 216, 31–40 (2017).
Albritton, J. L. & Miller, J. S. 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis. Model. Mech. 10, 3–14 (2017).
Weiden, J., Tel, J. & Figdor, C. G. Synthetic immune niches for cancer immunotherapy. Nat. Rev. Immunol. 18, 212–219 (2018).
Liu, H.-Y., Korc, M. & Lin, C.-C. Biomimetic and enzyme-responsive dynamic hydrogels for studying cell–matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials 160, 24–36 (2018).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Labani-Motlagh, A., Ashja-Mahdavi, M. & Loskog, A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front. Immunol. 11, 940 (2020).
Cox, T. R. The matrix in cancer. Nat. Rev. Cancer 21, 217–238 (2021).
Pearce, O. M. T. et al. Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers. Cancer Discov. 8, 304–319 (2018).
Grünwald, B. T. et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 184, 5577–5592.e18 (2021).
Below, C. R. et al. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat. Mater. 21, 110–119 (2022).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e14 (2018).
Duan, Q., Zhang, H., Zheng, J. & Zhang, L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer 6, 605–618 (2020).
Tian, C. et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc. Natl Acad. Sci. USA 116, 19609 (2019).
Rice, A. J. et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6, e352 (2017).
Choi, S. et al. Intrafibrillar, bone-mimetic collagen mineralization regulates breast cancer cell adhesion and migration. Biomaterials 198, 95–106 (2019).
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J. & Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120 (2020).
Xu, K. et al. 3D porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials 217, 119311 (2019).
Yue, X., Nguyen, T. D., Zellmer, V., Zhang, S. & Zorlutuna, P. Stromal cell-laden 3D hydrogel microwell arrays as tumor microenvironment model for studying stiffness dependent stromal cell–cancer interactions. Biomaterials 170, 37–48 (2018).
Ling, L. et al. Obesity-associated adipose stromal cells promote breast cancer invasion through direct cell contact and ECM remodeling. Adv. Funct. Mater. 30, 1910650 (2020).
Srinivasan, S., Kryza, T., Batra, J. & Clements, J. Remodelling of the tumour microenvironment by the kallikrein-related peptidases. Nat. Rev. Cancer 22, 223–238 (2022).
Candido, J. B. et al. Kallikrein-related peptidase 6 is associated with the tumour microenvironment of pancreatic ductal adenocarcinoma. Cancers 13, 3969 (2021).
Loessner, D. et al. Kallikrein-related peptidases represent attractive therapeutic targets for ovarian cancer. Exp. Opin. Ther. Targets 22, 745–763 (2018).
Wang, Z. et al. 3D-organoid culture supports differentiation of human CAR(+) iPSCs into highly functional CAR T cells. Cell Stem Cell 29, 515–527.e18 (2022).
Steele, N. G. et al. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat. Cancer 1, 1097–1112 (2020).
Bear, A. S., Vonderheide, R. H. & O’Hara, M. H. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell 38, 788–802 (2020).
Ayuso Jose, M. et al. Microfluidic tumor-on-a-chip model to evaluate the role of tumor environmental stress on NK cell exhaustion. Sci. Adv. 7, eabc2331 (2021).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988.e16 (2018).
Liu, B. et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat. Cancer 3, 108–121 (2022).
Duraiswamy, J. et al. Myeloid antigen-presenting cell niches sustain antitumor T cells and license PD-1 blockade via CD28 costimulation. Cancer Cell 39, 1623–1642.e20 (2021).
Rebelo, S. P. et al. 3D-3-culture: a tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials 163, 185–197 (2018).
Cui, X. et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 161, 164–178 (2018).
Quail, D. F. & Dannenberg, A. J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15, 139–154 (2019).
Mukherjee, A. et al. Adipocyte-induced FABP4 expression in ovarian cancer cells promotes metastasis and mediates carboplatin resistance. Cancer Res. 80, 1748 (2020).
Guimarães, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Okesola, B. O. & Mata, A. Multicomponent self-assembly as a tool to harness new properties from peptides and proteins in material design. Chem. Soc. Rev. 47, 3721–3736 (2018).
Panja, S., Dietrich, B., Shebanova, O., Smith, A. J. & Adams, D. J. Programming gels over a wide pH range using multicomponent systems. Angew. Chem. Int. Ed. Engl. 60, 9973–9977 (2021).
Mendoza-Martinez, A. K., Loessner, D., Mata, A. & Azevedo, H. S. Modeling the tumor microenvironment of ovarian cancer: the application of self-assembling biomaterials. Cancers 13, 5745 (2021).
Redondo-Gomez, C., Abdouni, Y., Becer, C. R. & Mata, A. Self-assembling hydrogels based on a complementary host–guest peptide amphiphile pair. Biomacromolecules 20, 2276–2285 (2019).
Edelbrock, A. N. et al. Superstructured biomaterials formed by exchange dynamics and host–guest interactions in supramolecular polymers. Adv. Sci. 8, 2004042 (2021).
Blache, U., Stevens, M. M. & Gentleman, E. Harnessing the secreted extracellular matrix to engineer tissues. Nat. Biomed. Eng. 4, 357–363 (2020).
Freeman, R. et al. Reversible self-assembly of superstructured networks. Science 362, 808–813 (2018).
Redondo-Gomez, C., Padilla-Lopategui, S., Azevedo, H. S. & Mata, A. Host–guest-mediated epitope presentation on self-assembled peptide amphiphile hydrogels. ACS Biomater. Sci. Eng. 6, 4870–4880 (2020).
Okesola, B. O. et al. Supramolecular self-assembly to control structural and biological properties of multicomponent hydrogels. Chem. Mater. 31, 7883–7897 (2019).
Hermida, M. A. et al. Three dimensional in vitro models of cancer: bioprinting multilineage glioblastoma models. Adv. Biol. Regul. 75, 100658 (2020).
Curvello, R. et al. 3D collagen–nanocellulose matrices model the tumour microenvironment of pancreatic cancer. Front. Digit. Health 3, 704584 (2021).
Trujillo, S. et al. Engineered full-length fibronectin-hyaluronic acid hydrogels for stem cell engineering. Adv. Healthc. Mater. 9, e2000989 (2020).
Sarrigiannidis, S. O. et al. A tough act to follow: collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 10, 100098 (2021).
Seo, B. R. et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc. Natl Acad. Sci. USA 117, 11387 (2020).
Drost, J. & Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 18, 407–418 (2018).
Freudenberg, U., Liang, Y., Kiick, K. L. & Werner, C. Glycosaminoglycan-based biohybrid hydrogels: a sweet and smart choice for multifunctional biomaterials. Adv. Mater. 28, 8861–8891 (2016).
Anjum, F. et al. Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials 87, 104–117 (2016).
Binner, M. et al. Cell-instructive starPEG–heparin–collagen composite matrices. Acta Biomater. 53, 70–80 (2017).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Berger, A. J., Linsmeier, K. M., Kreeger, P. K. & Masters, K. S. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials 141, 125–135 (2017).
Guzman, A., Sanchez Alemany, V., Nguyen, Y., Zhang, C. R. & Kaufman, L. J. A novel 3D in vitro metastasis model elucidates differential invasive strategies during and after breaching basement membrane. Biomaterials 115, 19–29 (2017).
Nguyen, E. H. et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng. 1, 0096 (2017).
O’Melia, M. J. et al. Synthetic matrix scaffolds engineer the in vivo tumor immune microenvironment for immunotherapy screening. Adv. Mater. 34, e2108084 (2022).
Aisenbrey, E. A. & Murphy, W. L. Synthetic alternatives to matrigel. Nat. Rev. Mater. 5, 539–551 (2020).
Place, E. S., Evans, N. D. & Stevens, M. M. Complexity in biomaterials for tissue engineering. Nat. Mater. 8, 457–470 (2009).
Limasale, Y. D. P., Atallah, P., Werner, C., Freudenberg, U. & Zimmermann, R. Tuning the local availability of VEGF within glycosaminoglycan-based hydrogels to modulate vascular endothelial cell morphogenesis. Adv. Funct. Mater. 30, 2000068 (2020).
Khademhosseini, A. & Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 11, 1775–1781 (2016).
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 (2009).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
LeSavage, B. L., Suhar, R. A., Broguiere, N., Lutolf, M. P. & Heilshorn, S. C. Next-generation cancer organoids. Nat. Mater. 21, 143–159 (2021).
Hofer, M. & Lutolf, M. P. Engineering organoids. Nat. Rev. Mater. 6, 402–420 (2021).
van Neerven, S. M. & Vermeulen, L. Cell competition in development, homeostasis and cancer. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-022-00538-y (2022).
Pothapragada, S. P., Gupta, P., Mukherjee, S. & Das, T. Matrix mechanics regulates epithelial defence against cancer by tuning dynamic localization of filamin. Nat. Commun. 13, 218 (2022).
Nam, S., Stowers, R., Lou, J., Xia, Y. & Chaudhuri, O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 200, 15–24 (2019).
Neves, M. I., Moroni, L. & Barrias, C. C. Modulating alginate hydrogels for improved biological performance as cellular 3D microenvironments. Front. Bioeng. Biotechnol. 8, 665 (2020).
Andersen, T., Auk-Emblem, P. & Dornish, M. 3D cell culture in alginate hydrogels. Microarrays 4, 133–161 (2015).
Ning, L. et al. 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications. Biofabrication 10, 035014 (2018).
Mosquera, M. J. et al. Extracellular matrix in synthetic hydrogel-based prostate cancer organoids regulate therapeutic response to EZH2 and DRD2 inhibitors. Adv. Mater. 34, e2100096 (2022).
Mahajan, V. et al. Mapping tumor spheroid mechanics in dependence of 3D microenvironment stiffness and degradability by brillouin microscopy. Cancers 13, 5549 (2021).
Prince, E. et al. Biomimetic hydrogel supports initiation and growth of patient-derived breast tumor organoids. Nat. Commun. 13, 1466 (2022).
Horst, E. N. et al. Injectable three-dimensional tumor microenvironments to study mechanobiology in ovarian cancer. Acta Biomater. 146, 222–234 (2022).
Elia, I. & Haigis, M. C. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat. Metab. 3, 21–32 (2021).
Raho, S. et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat. Metab. 2, 1373–1381 (2020).
Coassolo, S. et al. Citrullination of pyruvate kinase M2 by PADI1 and PADI3 regulates glycolysis and cancer cell proliferation. Nat. Commun. 12, 1718 (2021).
Park, M. K. et al. NEAT1 is essential for metabolic changes that promote breast cancer growth and metastasis. Cell Metab. 33, 2380–2397.e9 (2021).
Bertero, T. et al. Tumor–stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 29, 124–140.e10 (2019).
Biancur, D. E. et al. Functional genomics identifies metabolic vulnerabilities in pancreatic cancer. Cell Metab. 33, 199–210.e8 (2021).
Broekgaarden, M. et al. Modulation of redox metabolism negates cancer-associated fibroblasts-induced treatment resistance in a heterotypic 3D culture platform of pancreatic cancer. Biomaterials 222, 119421 (2019).
Sharma, N. S. et al. Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J. Clin. Invest. 130, 451–465 (2020).
Chen, D., Zhang, X., Li, Z. & Zhu, B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 11, 1016–1030 (2021).
Cho, H. et al. Cancer-stimulated CAFs enhance monocyte differentiation and protumoral TAM activation via IL6 and GM-CSF secretion. Clin. Cancer Res. 24, 5407 (2018).
Lin, W. et al. Function of CSF1 and IL34 in macrophage homeostasis, inflammation, and cancer. Front. Immunol. 10, 2019 (2019).
House, I. G. et al. Macrophage-derived CXCL9 and CXCL10 are required for antitumor immune responses following immune checkpoint blockade. Clin. Cancer Res. 26, 487 (2020).
Unterleuthner, D. et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 23, 159–177 (2020).
Stuelten, C. H., Parent, C. A. & Montell, D. J. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat. Rev. Cancer 18, 296–312 (2018).
Marcelin, G., Silveira, A. L. M., Martins, L. B., Ferreira, A. V. M. & Clément, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Invest. 129, 4032–4040 (2019).
Zhang, W. et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184, 2471–2486.e20 (2021).
Monteran, L. et al. Bone metastasis is associated with acquisition of mesenchymal phenotype and immune suppression in a model of spontaneous breast cancer metastasis. Sci. Rep. 10, 13838 (2020).
González Díaz, E. C., Sinha, S., Avedian, R. S. & Yang, F. Tissue-engineered 3D models for elucidating primary and metastatic bone cancer progression. Acta Biomater. 99, 18–32 (2019).
Hofbauer, L. C. et al. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 18, 488–505 (2021).
Han, W. et al. In vitro bone metastasis dwelling in a 3D bioengineered niche. Biomaterials 269, 120624 (2021).
Chen, L. et al. Tumor-secreted GRP78 promotes the establishment of a pre-metastatic niche in the liver microenvironment. Front. Immunol. 11, 2544 (2020).
Chen, C. et al. Dahuang Zhechong Pill suppresses colorectal cancer liver metastasis via ameliorating exosomal CCL2 primed pre-metastatic niche. J. Ethnopharmacol. 238, 111878 (2019).
Zheng, Y. et al. XIAOPI formula inhibits the pre-metastatic niche formation in breast cancer via suppressing TAMs/CXCL1 signaling. Cell Commun. Signal. 18, 48 (2020).
Chen, X. W. et al. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene 36, 5045–5057 (2017).
Anlaş, A. A. & Nelson, C. M. Soft microenvironments induce chemoresistance by increasing autophagy downstream of integrin-linked kinase. Cancer Res. 80, 4103 (2020).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Naba, A. et al. Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J. Proteome Res. 16, 3083–3091 (2017).
Ravichandran, A., Meinert, C., Bas, O., Hutmacher, D. W. & Bock, N. Engineering a 3D bone marrow adipose composite tissue loading model suitable for studying mechanobiological questions. Mater. Sci. Eng. C 128, 112313 (2021).
Cui, Z.-K. et al. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 10, 3523 (2019).
Romero-Moreno, R. et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat. Commun. 10, 4404 (2019).
Massagué, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Wang, Y. et al. Metastasis-on-a-chip mimicking the progression of kidney cancer in the liver for predicting treatment efficacy. Theranostics 10, 300–311 (2020).
Rafaeva, M. et al. Modeling metastatic colonization in a decellularized organ scaffold-based perfusion bioreactor. Adv. Healthc. Mater. 11, e2100684 (2022).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27, 152–164 (2021).
Marturano-Kruik, A. et al. Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc. Natl Acad. Sci. USA 115, 1256 (2018).
Brylka, L. J. & Schinke, T. Chemokines in physiological and pathological bone remodeling. Front. Immunol. 10, 2182 (2019).
Bouchet, M. et al. ERRα expression in bone metastases leads to an exacerbated antitumor immune response. Cancer Res. 80, 2914 (2020).
Yao, Y. et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 26, 17–26.e6 (2020).
Driehuis, E., Kretzschmar, K. & Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 15, 3380–3409 (2020).
Ganesh, K. et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 25, 1607–1614 (2019).
Norkin, M., Ordonez-Moran, P. & Huelsken, J. High-content, targeted RNA-seq screening in organoids for drug discovery in colorectal cancer. Cell Rep. 35, 109026 (2021).
Brandenberg, N. et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 4, 863–874 (2020).
Jung, Y. H. et al. Drug screening by uniform patient derived colorectal cancer hydro-organoids. Biomaterials 276, 121004 (2021).
Monteiro, C. F., Custódio, C. A. & Mano, J. F. Bioengineering a humanized 3D tri-culture osteosarcoma model to assess tumor invasiveness and therapy response. Acta Biomater. 134, 204–214 (2021).
Antunes, J. et al. In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening. Acta Biomater. 94, 392–409 (2019).
Labat, B. et al. Biomimetic matrix for the study of neuroblastoma cells: a promising combination of stiffness and retinoic acid. Acta Biomater. 135, 383–392 (2021).
Prince, E. et al. Microfluidic arrays of breast tumor spheroids for drug screening and personalized cancer therapies. Adv. Healthc. Mater. 11, e2101085 (2022).
Eggermont, A. M. M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 22, 643–654 (2021).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Horn, L. et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379, 2220–2229 (2018).
Nevala-Plagemann, C., Hidalgo, M. & Garrido-Laguna, I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat. Rev. Clin. Oncol. 17, 108–123 (2020).
Mollica, H. et al. A 3D pancreatic tumor model to study T cell infiltration. Biomater. Sci. 9, 7420–7431 (2021).
Sontheimer-Phelps, A., Hassell, B. A. & Ingber, D. E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 19, 65–81 (2019).
Kim, D. et al. Vascularized lung cancer model for evaluating the promoted transport of anticancer drugs and immune cells in an engineered tumor microenvironment. Adv. Healthc. Mater. 11, e2102581 (2022).
Huinen, Z. R., Huijbers, E. J. M., van Beijnum, J. R., Nowak-Sliwinska, P. & Griffioen, A. W. Anti-angiogenic agents — overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 18, 527–540 (2021).
Wunner, F. M. et al. Melt electrospinning writing of highly ordered large volume scaffold architectures. Adv. Mater. 30, e1706570 (2018).
Wagner, F. et al. Humanization of bone and bone marrow in an orthotopic site reveals new potential therapeutic targets in osteosarcoma. Biomaterials 171, 230–246 (2018).
Gavin, C. et al. Neuroblastoma invasion strategies are regulated by the extracellular matrix. Cancers 13, 736 (2021).
Villasante, A. et al. Recapitulating the size and cargo of tumor exosomes in a tissue-engineered model. Theranostics 6, 1119–1130 (2016).
Moroni, L. et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 3, 21–37 (2018).
Gaspar, V. M., Lavrador, P., Borges, J., Oliveira, M. B. & Mano, J. F. Advanced bottom-up engineering of living architectures. Adv. Mater. 32, e1903975 (2020).
Hedegaard, C. L. & Mata, A. Integrating self-assembly and biofabrication for the development of structures with enhanced complexity and hierarchical control. Biofabrication 12, 032002 (2020).
Humphries, B. A. et al. Ultrasound-induced mechanical compaction in acoustically responsive scaffolds promotes spatiotemporally modulated signaling in triple negative breast cancer. Adv. Healthc. Mater. 11, e2101672 (2022).
Tibbits, S. 4D printing: multi-material shape change. Archit. Des. 84, 116–121 (2014).
Kirillova, A., Maxson, R., Stoychev, G., Gomillion, C. T. & Ionov, L. 4D biofabrication using shape-morphing hydrogels. Adv. Mater. https://doi.org/10.1002/adma.201703443 (2017).
Vinson, B. T. et al. Laser direct-write based fabrication of a spatially-defined, biomimetic construct as a potential model for breast cancer cell invasion into adipose tissue. Biofabrication 9, 025013 (2017).
Brancato, V. et al. Bioengineered tumoral microtissues recapitulate desmoplastic reaction of pancreatic cancer. Acta Biomater. 49, 152–166 (2017).
Vennin, C. et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci. Transl. Med. 9, eaai8504 (2017).
Graham, A. D. et al. High-resolution patterned cellular constructs by droplet-based 3D printing. Sci. Rep. 7, 7004 (2017).
Pradhan, S., Clary, J. M., Seliktar, D. & Lipke, E. A. A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres. Biomaterials 115, 141–154 (2017).
Valdez, J. et al. On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial–stromal communication networks. Biomaterials 130, 90–103 (2017).
Laronda, M. M. et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 8, 15261 (2017).
Sawicki, L. A. et al. Tunable synthetic extracellular matrices to investigate breast cancer response to biophysical and biochemical cues. APL Bioeng. 3, 016101 (2019).
Bray, L. J. et al. Three-dimensional in vitro hydro- and cryogel-based cell-culture models for the study of breast-cancer metastasis to bone. Cancers 10, 292 (2018).
Ovadia, E. M. et al. Understanding ER+ breast cancer dormancy using bioinspired synthetic matrices for long-term 3D culture and insights into late recurrence. Adv. Biosyst. 4, 2000119 (2020).
Zhou, N. et al. Effect of RGD content in poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-alt-maleic acid) hydrogels on the expansion of ovarian cancer stem-like cells. Mater. Sci. Eng. C 118, 111477 (2021).
Solano, A. G. et al. An alginate-based macroporous hydrogel matrix to trap cancer cells. Carbohydr. Polym. 266, 118115 (2021).
Wolf, K. J. et al. A mode of cell adhesion and migration facilitated by CD44-dependent microtentacles. Proc. Natl Acad. Sci. USA 117, 11432 (2020).
Chia, J. Y., Miki, T., Mihara, H. & Tsutsumi, H. Biofunctional supramolecular hydrogels fabricated from a short self-assembling peptide modified with bioactive sequences for the 3D culture of breast cancer MCF-7 cells. Bioorg. Med. Chem. 46, 116345 (2021).
Weiss, M. S. et al. The impact of adhesion peptides within hydrogels on the phenotype and signaling of normal and cancerous mammary epithelial cells. Biomaterials 33, 3548–3559 (2012).
Reis, E. M. D., Berti, F. V., Colla, G. & Porto, L. M. Bacterial nanocellulose-IKVAV hydrogel matrix modulates melanoma tumor cell adhesion and proliferation and induces vasculogenic mimicry in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 106, 2741–2749 (2018).
Hughes, C. S., Postovit, L. M. & Lajoie, G. A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 (2010).
Passaniti, A., Kleinman, H. K. & Martin, G. R. Matrigel: history/background, uses, and future applications. J. Cell Commun. Signal. 16, 621–626 (2022).
Curvello, R. et al. Engineered plant-based nanocellulose hydrogel for small intestinal organoid growth. Adv. Sci. 8, 2002135 (2021).
Rubiano, A. et al. Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta Biomater. 67, 331–340 (2018).
De Stefano, P. et al. Bioprinting of matrigel scaffolds for cancer research. Polymers 13, 2026 (2021).
Alave Reyes-Furrer, A. et al. Matrigel 3D bioprinting of contractile human skeletal muscle models recapitulating exercise and pharmacological responses. Commun. Biol. 4, 1183 (2021).
Hedegaard, C. L. et al. Hydrodynamically guided hierarchical self-assembly of peptide–protein bioinks. Adv. Funct. Mater. https://doi.org/10.1002/adfm.201703716 (2018).
Mao, S. et al. Bioprinting of in vitro tumor models for personalized cancer treatment: a review. Biofabrication 12, 042001 (2020).
Murphy, R. J., Browning, A. P., Gunasingh, G., Haass, N. K. & Simpson, M. J. Designing and interpreting 4D tumour spheroid experiments. Commun. Biol. 5, 91 (2022).
Meng, F. et al. 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv. Mater. 31, 1806899 (2019).
Cao, X. et al. A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair. Adv. Funct. Mater. 29, 1807173 (2019).
Nashimoto, Y. et al. Vascularized cancer on a chip: the effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 229, 119547 (2020).
Chi, C.-W. et al. High-throughput tumor-on-a-chip platform to study tumor–stroma interactions and drug pharmacokinetics. Adv. Healthc. Mater. 9, 2000880 (2020).
Gilardi, M. et al. The driving role of the Cdk5/Tln1/FAKS732 axis in cancer cell extravasation dissected by human vascularized microfluidic models. Biomaterials 276, 120975 (2021).
Hutton, C. et al. Single-cell analysis defines a pancreatic fibroblast lineage that supports anti-tumor immunity. Cancer Cell 39, 1227–1244.e20 (2021).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
DePeaux, K. & Delgoffe, G. M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 21, 785–797 (2021).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Acknowledgements
This work was supported by the Medical Research Council (UK Regenerative Medicine Platform Acellular Smart Materials – 3D Architecture, MR/R015651/1) to A.M. and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Program (grant agreement number 864253) to D.L.
Author information
Authors and Affiliations
Contributions
D.L. conceived the article. All authors researched the data and drafted the article. R.C., V.K. and D.L. conceived and illustrated the figures and tables. All authors made substantial contributions to the discussion of content and reviewed and edited the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Biomimetic
-
Replica of the properties or elements of natural tissues or systems.
- Bioscaffolds
-
Decellularized tissues that preserve the structural and molecular integrity of the organ-specific native extracellular matrix.
- Cell-instructive
-
Short peptide sequences in biomaterials that present insoluble adhesion ligands and proteolytically degradable motifs and enable binding or release of soluble factors.
- Desmoplastic tumour stroma
-
Deposition of a dense and crosslinked extracellular matrix resulting in a fibrotic tumour tissue.
- Epithelial-to-mesenchymal transition
-
When cells become more motile, migratory and invasive through the loss of epithelial features and the gain of mesenchymal ones.
- Fluid shear stress
-
Force created by the fluid flow parallel to a surface of a material or tissue.
- Hydrogel
-
Water-swollen polymer network.
- Interstitial flow
-
Movement of fluid that is transported through the extracellular matrix.
- Metabolic vulnerabilities
-
Abnormal metabolism of cancer cells targeted by anticancer therapies.
- Organoids
-
Self-organizing and self-renewing clusters of cells derived from tissue fragments or stem cells that mimic the functionality and behaviour of tissues.
- Personalized medicines
-
Therapies designed for a specific patient based on its genetic or individual signature.
- Scaffold
-
3D structure with defined geometry and mechanical properties.
- Spheroids
-
Cell aggregates or clusters that resemble some features of tissues.
- Stiffness
-
Resistance of a material, or tissue, to deformation and alteration of its original shape when a force is applied.
- Tumorigenesis
-
Formation of a tumour whereby normal cells transform into cancer cells following a disrupted differentiation.
- Tumour microenvironment
-
Dynamic network of malignant and non-malignant cells, extracellular and cell-secreted elements and soluble factors that promote tumour development, growth and metastasis.
- Viscoelasticity
-
A time-dependent mechanical property of materials or tissues that exhibit viscous and elastic responses when forces are applied, causing temporary or permanent deformation, respectively.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Curvello, R., Kast, V., Ordóñez-Morán, P. et al. Biomaterial-based platforms for tumour tissue engineering. Nat Rev Mater 8, 314–330 (2023). https://doi.org/10.1038/s41578-023-00535-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-023-00535-3
This article is cited by
-
Responsive biomaterials: optimizing control of cancer immunotherapy
Nature Reviews Materials (2023)