Abstract
CD19 chimeric antigen receptor (CAR) T-cell therapy has shown great success against B-cell acute lymphoblastic leukemia (B-ALL). Tandem and sequential CD19/CD22 dual-target CAR T-cell therapies have been developed to reduce the possibility of CD19-negative relapse; however, the superior strategy is still uncertain. This study screened 219 patients with relapsed/refractory B-ALL who were enrolled in clinical trials of either CD19 (NCT03919240) or CD19/CD22 CAR T-cell therapy (NCT03614858). The complete remission (CR) rates in the single CD19, tandem CD19/CD22, and sequential CD19/CD22 groups were 83.0% (122/147), 98.0% (50/51), and 95.2% (20/21), respectively (single CD19 vs. tandem CD19/CD22, P = 0.006). Patients with high-risk factors achieved a higher rate of CR in the tandem CD19/CD22 group than in the single CD19 group (100.0% vs. 82.4%, P = 0.017). Tandem CD19/CD22 CAR T-cell therapy was one of the significant favorable factors in the multivariate analysis of the CR rate. The incidence of adverse events was similar among the three groups. Multivariable analysis in CR patients showed that a low frequency of relapse, a low tumor burden, minimal residual disease-negative CR and bridging to transplantation were independently associated with better leukemia-free survival. Our findings suggested that tandem CD19/CD22 CAR T-cell therapy obtains a better response than CD19 CAR T-cell therapy and a similar response to sequential CD19/CD22 CAR T-cell therapy.
Similar content being viewed by others
Introduction
Relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) is associated with a poor response to salvage therapies and a dismal prognosis [1,2,3,4,5]. Chimeric antigen receptor (CAR) T-cell therapy has achieved great advances in recent years. CD19 is a nearly ideal target antigen for B-ALL because of its homogeneous expression [6]. Multiple clinical trial results have shown a 68%–93% remission rate after CD19 CAR T-cell therapy in children and adults with R/R B-ALL [7,8,9,10,11,12]. However, it has not been able to maintain durable remission in most patients. A long-term follow-up study reported a median event-free survival (EFS) time of 6.1 months [11]. CD19-negative relapse is one of the major causes of therapeutic failure, occurring in 25%–42% of responding patients [10, 11, 13].
Dual-target CAR T-cells have been developed to reduce the possibility of CD19-negative relapse. CD22 is another member of the B-cell antigen family whose tissue distribution is similar to that of CD19 [14, 15]. Dual-target CAR T-cells can be applied using several combination strategies, including cocktail CD19/CD22 CAR T-cell therapy, sequential infusion and bispecific CAR T-cell products [13, 16,17,18,19,20,21]. The optimal combination strategy for CAR T-cells with different target antigens is still uncertain.
Therefore, we compared the efficacy and safety of single CD19, tandem CD19/CD22 and sequential CD19/CD22 CAR T-cell therapies in R/R B-ALL at our institution.
Methods
Patients
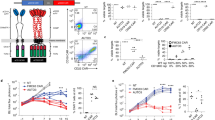
Between August 2018 and October 2021, a total of 219 patients with R/R B-ALL who successfully received CAR T-cell treatments were screened in this study (Fig. 1A). Among them, 147 patients received single CD19 CAR T-cell therapy, 51 patients received tandem CD19/CD22 CAR T-cell therapy, and 21 patients received sequential CD19/CD22 CAR T-cell therapy. All patients were enrolled in CD19 CAR T-cell clinical trials (NCT03919240) or CD19/CD22 CAR T-cell clinical trials (NCT03614858). This study was approved by the ethics committee of The First Affiliated Hospital of Soochow University, and all patients provided written informed consent.
A A total of 219 patients with R/R B-ALL successfully received CAR T-cell treatments (147 patients received single CD19 CAR T-cell therapy, 51 patients received tandem CD19/CD22 CAR T-cell therapy, and 21 patients received sequential CD19/CD22 CAR T-cell therapy) were screened in this study. Ninety-nine patients who achieved remission with CAR T-cell therapy bridged to allo-HSCT. B Schematic of CAR T-cell therapy regimen.
Treatment protocol
After chemotherapy to reduce the tumor burden, all patients received lymphodepletion with fludarabine (30 mg/m2/d) and cyclophosphamide (300 mg/m2/d) based conditioning regimens on day −5 to −3. CAR T-cells were then infused at day 0 (Fig. 1B). The median dose of CAR T-cells was 5 (5–20) × 106 cells/kg in the single CD19 group, 10 (5–20) × 106 cells/kg in the tandem CD19/CD22 group, and 10 × 106 cells/kg in the sequential CD19/CD22 group.
Definitions
Patients with high-risk factors were defined in accordance with the National Comprehensive Cancer Network guidelines, version 3.2020. Complete remission (CR) was defined as less than 5% blasts in bone marrow morphology and no extramedullary disease (EMD). Minimal residual disease (MRD)-negative status was defined as a leukemic cell count below the sensitivity threshold of 1 × 10−4 (0.01%) per bone marrow mononuclear cell (MNC) by multiparameter flow cytometry [22, 23]. Cytokine release syndrome (CRS) was defined as “a disorder characterized by fever, tachypnea, headache, tachycardia, hypotension, rash, and/or hypoxia caused by the release of cytokines” [24]. Immune effector cell-associated neurotoxicity syndrome (ICANS) was defined as “a disorder characterized by a pathologic process involving the central nervous system following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells. Symptoms or signs can be progressive and may include aphasia, altered level of consciousness, impairment of cognitive skills, motor weakness, seizures, and cerebral edema” [24]. CRS and ICANS were assessed according to the American Society for Transplantation and Cellular Therapy Consensus Grading [24]. Hemophagocytic histiocytosis (HLH) was diagnosed according to the HLH-2004 criteria [25]. The diagnosis of tumor lysis syndrome (TLS) was based on the Cairo-Bishop criteria [26]. Other organ toxicities were graded according to the Common Terminology Criteria for Adverse Events Version 5.0. Overall survival (OS) was the time from CAR T-cells infusion to death for any reason. Leukemia-free survival (LFS) was the duration from the day of CR after CAR T-cell therapy to leukemia relapse, death or the last follow-up. Patients alive in CR at the time of last follow-up were administratively censored.
Statistical analysis
The differences among the three groups were analyzed by one-way ANOVA for continuous variables, and by Chi-square test or Fisher’s exact test for categorical variables. The Bonferroni method was used for pairwise comparisons. Multivariate logistic regression was applied to analyze CR prognostic factors. The probabilities of OS and LFS were calculated by the Kaplan-Meier method. The cumulative incidence of relapse (CIR) was estimated using a competing risk model, with non-relapse mortality as a competing risk factor. Multivariate analyses of prognostic factors for LFS were conducted with Cox regression. P values (2-tailed) <0.05 were considered statistically significant. All statistical analyses were performed with SPSS version 22, GraphPad Prism version 8.3.0 and R version 3.6.2.
Results
Patient characteristics
A total of 219 R/R B-ALL patients who received CAR T-cell treatments were enrolled in our retrospective study, comprising 147 patients in the single CD19 group, 51 patients in the tandem CD19/CD22 group and 21 patients in the sequential CD19/CD22 group. The baseline characteristics of the three groups are summarized in Supplementary Table 1.
There were 106 males and 113 females. The median age of the patients was 31 years (range 6–72 years). Fifty patients (22.8%) had primary refractory disease at the time of enrollment, 120 patients (54.8%) were experiencing the first relapse, and 49 patients (22.4%) were experiencing the second or more relapse. A total of 118 patients (53.9%) had previously received more than 4 therapies, and 46 patients (21.0%) had previously undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT). Before lymphodepletion, 89 patients (40.6%) had a high tumor burden with over 20% blasts in bone marrow by morphology. Ninety patients (41.1%) had MRD with bone marrow blasts in the range of 0.01% to less than 5%. Twenty patients (9.1%) had EMD. Twenty-two patients (10.0%) harbored a complex karyotype. The BCR/ABL1 fusion gene was detected in 64 patients (29.2%), 30 of whom were accompanied by the T315I mutation. Ten patients (4.6%) were classified as Ph-like ALL, and 12 patients (5.5%) harbored a KMT2A rearrangement. TP53 mutation was detected in 17 patients (7.8%).
The proportion of patients with high-risk cytogenetic or genetic characteristics was similar in the three groups (50.3%, 54.9% and 28.6%, respectively; P = 0.116), defined as complex karyotype, KMT2A rearranged, BCR-ABL1, Ph-like ALL, mutated TP53 or IKZF1. Among 64 patients with BCR/ABL1, 48 were in the single CD19 group, 16 were in the tandem CD19/CD22 group, and no patient was in the sequential CD19/CD22 group (P = 0.008). Otherwise, the baseline characteristics of the three groups were similar.
Tandem CD19/CD22 CAR T-cell therapy produced a better therapeutic response than single CD19 CAR T-cell therapy
The CR rate of patients in the tandem CD19/CD22 group was significantly higher than that in the single CD19 group (98.0% vs. 83.0%, P = 0.006), and was similar to that in the sequential CD19/CD22 group (98.0% vs. 95.2%, P = 0.501) (Fig. 2A). We further performed subgroup analyses to explore which patients benefited from dual CD19/CD22 CAR T-cell therapy (Table 1). Compared to the single CD19 group, the tandem CD19/CD22 group had an increased CR rate in patients with the following characteristics: no history of allo-HSCT (84.5% vs. 100.0%, P = 0.004), no EMD (88.0% vs. 100.0%, P = 0.013), a high tumor burden (78.2% vs. 100.0%, P = 0.014), and no complex karyotype (83.0% vs. 97.8%, P = 0.011). High-risk patients in the tandem CD19/CD22 group showed a higher CR rate than those in the single CD19 group (100.0% vs. 82.4%, P = 0.017) and a similar CR rate to those in the sequential CD19/CD22 group (100.0% vs. 83.3%, P = 0.176) (Fig. 2C). In patients without high-risk factors, there was no significant difference in the CR rate among the three groups (83.6%, 95.7% and 100.0%, respectively; P = 0.137) (Fig. 2E).
Furthermore, the MRD-negative CR rates were 65.3% (96/147) in patients who received CD19 CAR T-cell therapy, 86.3% (44/51) in patients who received tandem CD19/CD22 CAR T-cell therapy, and 76.2% (16/21) in patients who received sequential CD19/CD22 CAR T-cell therapy (P = 0.015) (Fig. 2B). Patients over the age of 35 years (56.7% vs. 100.0%, P = 0.001), patients without a history of allo-HSCT (65.5% vs. 85.7%, P = 0.014), patients with EMD (22.2% vs. 83.3%, P = 0.041), patients without a complex karyotype (65.2% vs. 86.7%, P = 0.006), and patients with BCR/ABL1 (60.4% vs. 100.0%, P = 0.002) had a greater chance of achieving MRD-negative CR with tandem CD19/CD22 CAR T-cell therapy than with single CD19 CAR T-cell therapy (Supplementary Table 2). Moreover, high-risk patients in the tandem CD19/CD22 group showed a significantly higher MRD-negative CR rate than those in the single CD19 group (92.9% vs. 64.9%, P = 0.005) (Fig. 2D). In patients without high-risk factors, there was no significant difference in the MRD-negative CR rate among the three groups (65.8%, 78.3% and 80.0%, respectively; P = 0.408) (Fig. 2F).
The univariate analyses revealed that lower frequencies of relapse before CAR T-cell therapy, a lack of evidence indicating EMD and tandem CD19/CD22 CAR T-cell therapy were significantly associated with a superior therapeutic response (Supplementary Table 3–4). In multivariate logistic regression analyses of CR and MRD-negative CR, tandem CD19/CD22 CAR T-cell therapy was also an independent prognostic factor (OR: 0.037, 95% CI: 0.003–0.485, P = 0.012; OR: 0.274, 95% CI: 0.108–0.691, P = 0.006; respectively) (Table 2). Moreover, EMD and tumor burden before CAR T-cell treatment remained significant independent predictive factors of the CR rate.
Tandem CD19/CD22 CAR T-cell therapy showed a similar incidence of adverse events to single CD19 and sequential CD19/CD22 CAR T-cell therapy
All adverse events that occurred during the course of CAR T-cell treatment were graded and are shown in Table 3. In our study, CRS occurred in 168 of 219 patients (76.7%), consisting of 54.3% grade 1–2 and 22.4% severe CRS (grade 3-4). Severe CRS occurred in 25.9% of patients in the single CD19 group, 13.7% of patients in the tandem CD19/CD22 group, and 19.0% in the sequential CD19/CD22 group (P = 0.196). A total of 15 patients with ICANS were observed, including 14 patients (9.5%) in the single CD19 group, and 1 patient (2.0%) in the tandem CD19/CD22 group (P = 0.108). Three patients harbored HLH, 1 in the single CD19 group, and 2 in the tandem CD19/CD22 group. TLS occurred in 3 patients.
Two hundred patients had their levels of serum cytokines monitored regularly from the day of CAR T-cell infusion. Patients in the sequential CD19/CD22 group showed a higher peak of interleukin (IL)-17A than the single CD19 group (P = 0.0394 and 0.0054, respectively) (Supplementary Figure 1). There was no significant difference in cytokine levels between the tandem CD19/CD22 group and the single CD19 group.
Regarding hematological toxicity, there was a significant difference in the incidence of severe thrombocytopenia (grade 3-4) among the single CD19, tandem CD19/CD22, and sequential CD19/CD22 groups (44.9%, 58.8% and 71.4%, respectively; P = 0.031) but no significant difference in the pairwise comparisons (Supplementary Table 5). Toxicity to other organs was similar among the three groups.
Allo-HSCT significantly improved the clinical outcomes of patients
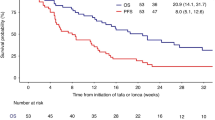
The median follow-up time across all patients was 25.1 months (range 1.2–50.3 months). The 2-year OS values in the single CD19, tandem CD19/CD22, and sequential CD19/CD22 groups were 59.2%, 76.3%, and 77.6%, respectively (P = 0.0187) (Fig. 3A). There was no significant difference in LFS or CIR among the three groups (1-year LFS: 61.7%, 71.1% and 52.2%, respectively, P = 0.2391; 1-year CIR: 33.0%, 23.3% and 43.8%, respectively, P = 0.1855) (Fig. 3B-C).
A Overall survival B leukemia-free survival and C cumulative incidence of relapse of patients in the single CD19 group, tandem CD19/CD22 group and sequential CD19/CD22 group. D Overall survival E leukemia-free survival and F cumulative incidence of relapse of patients who underwent allo-HSCT after CAR T-cell therapy among the three groups. G Overall survival H leukemia-free survival and I cumulative incidence of relapse of patients without allo-HSCT after CAR T-cell therapy among the three groups.
Ninety-nine patients who achieved remission with CAR T-cell therapy bridged to allo-HSCT. The allo-HSCT patients showed no significant difference in survival among the single CD19, tandem CD19/CD22, and sequential CD19/CD22 groups (2-year OS: 77.4%, 77.7% and 100.0%, respectively, P = 0.4906; 2-year LFS: 65.1%, 68.5% and 80.0%, respectively, P = 0.6980; 2-year CIR: 30.5%, 21.2% and 20.0%, respectively, P = 0.7073) (Fig. 3D–F). The three groups all had a similar prognosis for post-CAR T-cell therapy bridging to transplantation in patients with or without high-risk factors (Supplementary Fig. 2A–C, Supplementary Fig. 3A–C).
We initially performed univariable analyses to identify baseline and therapy-related factors that were associated with improved LFS in patients who achieved CR and could be included in subsequent multivariable analyses (Supplementary Table 6). Patients who underwent CAR T-cell treatment in refractory and first relapse showed a significantly better LFS than those in second or more relapses (2-year LFS: 61.4%, 57.9% and 32.1%, respectively; P = 0.0021) (Supplementary Fig. 4A). The 156 patients who achieved MRD-negative CR showed significantly better median LFS than the 36 patients who achieved MRD -positive CR after CAR T-cell treatment (not reached vs. 9.2 months, P = 0.0046) (Supplementary Fig. 4C). Patients who underwent allo-HSCT had better median LFS than patients without allo-HSCT (not reached vs. 10.9 months, P < 0.0001) (Supplementary Fig. 4D).
We also performed Cox regression multivariable modeling with the identified variables that had P < 0.05 from univariable analyses and clinical factors that could impact survival, such as the number of prior therapies, tumor burden, and high-risk cytogenetics and genetic characteristics. The multivariate Cox model showed that MRD-negative CR (HR: 0.411, 95% CI: 0.240–0.704; P = 0.001) and bridging to HSCT (HR: 0.375, 95% CI: 0.234–0.600; P < 0.001) were independently associated with better LFS (Table 4). Second or more relapses (HR: 2.644, 95% CI: 1.199–5.832; P = 0.016) and a high tumor burden (HR: 1.785, 95% CI: 1.051–3.030; P = 0.032) were independent risk factors for LFS.
Tandem CD19/CD22 CAR T-cell therapy improved survival for patients without bridging to allo-HSCT
Among patients who did not receive allo-HSCT, 15 patients in the tandem CD19/CD22 group had a significantly longer median OS than the 90 patients in the single CD19 group (not reached vs. 23.5 months, P = 0.0311) (Fig. 3G). The median LFS was 12.4 months in the single CD19 group, not reached in the tandem CD19/CD22 group and 7.9 months in the sequential CD19/CD22 group (P = 0.4941) (Fig. 3H). Patients with high-risk factors in all three groups had a similar prognosis after CAR T-cell therapy without bridging to transplantation (Supplementary Fig. 2D–F). Patients without high-risk factors in the tandem CD19/CD22 group showed a significantly better OS than those in the single CD19 group (3-year OS: 75.0% vs. 21.2%, P = 0.0257) (Supplementary Fig. 3D). There was no difference in survival between the single CD19 and sequential CD19/CD22 CAR T-cell treatments. (Supplementary Fig. 3D–F).
Discussion
In this study, we compared the efficacy and safety of CD19 single-target and CD19/CD22 dual-target CAR T-cell therapies in a cohort of R/R B-ALL patients. We found that tandem CD19/CD22 CAR T-cell therapy produced a better therapeutic response than single CD19 CAR T-cell therapy, especially in patients with chemotherapy resistance. There was no evidence of increased toxicity associated with the tandem CD19/CD22 CAR T-cell dose. Bridging to transplantation after remission with CAR T-cell therapy significantly improved patient outcomes. Furthermore, tandem CD19/CD22 CAR T-cell therapy was associated with favorable survival for patients without allo-HSCT.
CD19 CAR T-cell therapy has a dramatic effect on R/R B-ALL patients achieving high CR rates, but approximately 20-50% of patients relapse at 6 months [8,9,10,11, 27]. To overcome the drawback of single-target CAR T-cell treatment, CD19 and CD22 cells have been applied in combination. Several studies have reported the efficacy of simultaneously targeting multiple antigens in preclinical models using a variety of CAR configurations to achieve recognition of multiple specific antigens [28,29,30,31,32,33]. Several small samples of clinical data showed that CAR T-cells targeting CD19 and CD22 were clinically active in B-ALL patients [13, 21].
Our data demonstrated that CR was achieved in 98.0% of patients 28 days after tandem CD19/CD22 CAR T-cell infusion. Patients in each high-risk subgroup (complex karyotype, BCR/ABL1, Ph-like and TP53 mutation) benefited from tandem CD19/CD22 CAR T-cell treatment achieving a 100% CR rate. Most importantly, 26 of 28 patients (92.9%) with poor-risk cytogenetic factors achieved MRD-negative CR. Compared with the single CD19 group, the tandem CD19/CD22 group showed an increased MRD-negative CR rate in patients over the age of 35 years, patients without a history of allo-HSCT and patients with EMD. Multivariate analysis confirmed that tandem CD19/CD22 dual-target CAR T-cell therapy was associated with a good treatment response in R/R B-ALL.
Consistent with previous findings, patients with a low tumor burden are more likely to achieve CR compared to patients with a high tumor burden [11, 34, 35]. Therefore, reducing tumor burden including with the use of chemotherapy or targeted therapy prior to CAR T-cell treatment or treating patients in their earlier disease course is important to consider. Furthermore, tandem CD19/CD22 CAR T-cell therapy may offer an additional therapeutic option for patients with chemotherapy resistance. In our study, 25 patients (100.0%) with a high tumor burden achieved CR in the tandem CD19/CD22 group.
Toxicities associated with CAR T-cells such as CRS remain a concern. In most clinical trials, the reported incidence of severe CRS was above 15%, with 13–63% of the patients experiencing neurotoxicity [36,37,38,39]. It has been shown that severe CRS is closely related to a high CAR T-cell dose [40]. In our cohort, patients in the tandem CD19/CD22 group received a higher dose of CAR T-cells than patients in the single CD19 group but showed a similar incidence of toxicities.
Despite the initial high CR from CAR T-cell treatment of R/R B-ALL, relapse is still a major problem [10, 11, 41]. No obvious leukemia-free survival advantage was found in patients who received tandem CD19/CD22 CAR T-cell therapy compared with those who received single CD19 or sequential CD19/CD22 CAR T-cell therapy. Multivariable analysis in patients who achieved CR showed that a low frequency of relapse before CAR T-cell therapy, low tumor burden, MRD-negative CR and bridging to HSCT were independently associated with better LFS.
Once patients relapse after allo-HSCT, currently available treatments are often unsatisfactory [42]. However, CAR T-cell therapy is now recognized as holding some promise [8, 9]. Zhang et al. reported that 34 of 43 patients (79.1%) with a history of transplantation achieved CR after CD19 CAR T-cell therapy, and 1-year EFS was 43% [43]. In our patients who relapsed after transplantation, the CR rates were 77.4% (24/31), 88.9% (8/9), and 100.0% (6/6) in the single CD19, tandem CD19/CD22, and sequential CD19/CD22 groups (P = 0.605). Among 38 patients who achieved CR, the LFS rate was 59.1% at 2-year. There was no significant difference in survival among the three groups. Overall, patients with a history of transplantation may benefit more from dual-target CAR T-cell therapy.
There is controversy about whether transplantation is necessary after CAR T-cell therapy. Multiple studies have reported that bridging to allo-HSCT after CAR T-cell therapy can improve LFS [35, 44]. However, a recent study suggested no survival benefit from allo-HSCT [11]. In their study, of the 17 patients who underwent transplantation, 6 subsequently relapsed, and another 6 died of transplant-related mortality. When allo-HSCT was incorporated into our LFS multivariable model as a time-dependent covariate, we observed better LFS in CR patients who proceeded to transplantation than in those who did not undergo transplantation.
In conclusion, tandem CD19/CD22 dual-target CAR T-cell therapy obtains a better response than single CD19 CAR T-cell therapy and a similar response to sequential CD19/CD22 CAR T-cell therapy. This provides an effective treatment option for R/R B-ALL patients with high-risk factors. However, our study is limited by the variability in tumor reduction chemotherapy regimens, the small sample size and short follow-up duration of the sequential CD19/CD22 CAR T-cell group, and the difference in CAR T-cell infusion doses among the three groups. Data from large prospective, randomized, and controlled clinical trials are required to verify the superiority of dual-target CAR T-cell therapy. Additionally, efforts should be made to improve the response duration.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–50.
Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–50.
Oriol A, Vives S, Hernández-Rivas JM, Tormo M, Heras I, Rivas C, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. 2010;95:589–96.
Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28:2339–47.
Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–7.
Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168:724–40.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl J Med. 2014;371:1507–17.
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–48.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378:449–59.
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Investig. 2016;126:2123–38.
Cordoba S, Onuoha S, Thomas S, Pignataro DS, Hough R, Ghorashian S, et al. CAR Tcells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med. 2021;27:1797–805.
Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–74.
Zhou Y, You MJ, Young KH, Lin P, Lu G, Medeiros LJ, et al. Advances in the molecular pathobiology of B-lymphoblastic leukemia. Hum Pathol. 2012;43:1347–62.
Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13:30.
Pan J, Zuo S, Deng B, Xu X, Li C, Zheng Q, et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood. 2020;135:387–91.
Wang N, Hu X, Cao W, Li C, Xiao Y, Cao Y, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135:17–27.
Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, et al. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-Targeted CAR-T Cell Therapy for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia. Clin Cancer Res. 2021;27:2764–72.
Liu S, Deng B, Yin Z, Lin Y, An L, Liu D, et al. Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol. 2021;96:671–9.
Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27:1419–31.
Gaipa G, Cazzaniga G, Valsecchi MG, Panzer-Grümayer R, Buldini B, Silvestri D, et al. Time point-dependent concordance of flow cytometry and real-time quantitative polymerase chain reaction for minimal residual disease detection in childhood acute lymphoblastic leukemia. Haematologica. 2012;97:1582–93.
Denys B, van der Sluijs-Gelling AJ, Homburg C, van der Schoot CE, de Haas V, Philippé J, et al. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia. 2013;27:635–41.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2019;25:625–38.
Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31.
Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11.
Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X, Lin YH, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia. 2017;31:2587–93.
He X, Feng Z, Ma J, Ling S, Cao Y, Gurung B, et al. Bispecific and split CAR T cells targeting CD13 and TIM3 eradicate acute myeloid leukemia. Blood. 2020;135:713–23.
Schmidts A, Ormhøj M, Choi BD, Taylor AO, Bouffard AA, Scarfò I, et al. Rational design of a trimeric APRIL-based CAR-binding domain enables efficient targeting of multiple myeloma. Blood Adv. 2019;3:3248–60.
Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Investig. 2016;126:3036–52.
Zah E, Nam E, Bhuvan V, Tran U, Ji BY, Gosliner SB, et al. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat Commun. 2020;11:2283.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–28.
Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20:506–18.
Zhang X, Lu XA, Yang J, Zhang G, Li J, Song L, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020;4:2325–38.
Zhang X, Yang J, Li J, Li W, Song D, Lu XA, et al. Factors associated with treatment response to CD19 CAR-T therapy among a large cohort of B cell acute lymphoblastic leukemia. Cancer immunology, immunotherapy: CII. 2022;71:689–703.
Curran KJ, Margossian SP, Kernan NA, Silverman LB, Williams DA, Shukla N, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134:2361–8.
Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med. 2018;66:50–52. May
Abbasi J. Amid FDA Approval Filings, Another CAR-T Therapy Patient Death. Jama. 2017;317:2271.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62.
Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306.
Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–31.
Pavletic SZ, Kumar S, Mohty M, de Lima M, Foran JM, Pasquini M, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2010;16:871–90.
Zhang C, Wang XQ, Zhang RL, Liu F, Wang Y, Yan ZL, et al. Donor-derived CD19 CAR-T cell therapy of relapse of CD19-positive B-ALL post allotransplant. Leukemia. 2021;35:1563–70. Jun
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133:1652–63.
Acknowledgements
This work was supported by research grants from National Natural Science Foundation of China (82070162), The Key Science Research Project of Jiangsu Commission of Health (K2019022), Translational Research Grant of NCRCH (2020ZKZC04) and Natural Science Foundation of Jiangsu Province (BK20201169), Bethune Charitable Foundation (BCF-IBW-XY-20220930-13), Suzhou diagnosis and treatment project of Clinical Key Diseases (LCZX202201), Frontier Clinical Technical Project of Suzhou Science and Technology plan (SKY2022001), China International Medical Foundation (Z-2018-31-2102-4), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
SNL and XYZ analyzed data, and wrote the manuscript. XWT, DPW, HPD, QYC, WC, JY, ZL, XY, CXY, SLX, HYQ, MM, SNC, ZMJ, CCF, CXL, ANS, YH and YW recruited the patients and collected clinical data. LY designed and prepared the CAR T-cells. XWT, QYC and DPW revised the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Zhang, X., Dai, H. et al. Which one is better for refractory/relapsed acute B-cell lymphoblastic leukemia: Single-target (CD19) or dual-target (tandem or sequential CD19/CD22) CAR T-cell therapy?. Blood Cancer J. 13, 60 (2023). https://doi.org/10.1038/s41408-023-00819-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-023-00819-5