Abstract
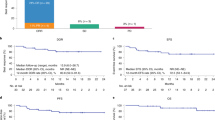
Outcomes are poor for patients with relapsed and/or refractory (R/R) large B-cell lymphoma (LBCL) post chimeric antigen receptor T-cell (CAR-T) therapy. Two CD19-directed therapies, tafasitamab- cxix plus lenalidomide (tafa-len) and loncastuximab tesirine (loncaT) are approved in R/R LBCL. The efficacy of these CD19 directed therapies in patients who relapse after CD19 directed CAR-T (CD19-CART) therapy is not well understood. We conducted a multi-center study of patients with R/R LBCL that received either tafa-len or loncaT at any timepoint for R/R disease after CD19-CART therapy. Fifty-three patients were included in this study with the median follow up of 56 (9.1–199) weeks from CAR-T infusion. Median number of systemic therapies pre–CAR-T therapy was 3 (range: 1–6); axicabtagene ciloleucel was the most utilized CAR-T product (n = 32,60%). Median time from CAR-T therapy to tafa-len or loncaT was 7.3 (1.2–38.2) months with median number of lines of therapy between CAR-T therapy and these regimens of 1 (0–5). Combined overall response rate and complete response rates were 27% and 10%, respectively. Median duration of response was 13.3 (2.1–56.7) weeks. In this real-world study, the use of currently approved CD19-directed therapies to treat R/R LBCL after CD19-CAR-T therapy showed limited clinical activity and duration of responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8.
Ellard R, Kenyon M, Hutt D, Aerts E, de Ruijter M, Chabannon C, et al. The EBMT immune effector cell nursing guidelines on CAR-T therapy: a framework for patient care and managing common toxicities. Clin Hematol Int. 2022;4:75–88.
Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol. 2020;38:3805–15.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42.
Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjani CS, et al. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol. 2019;94:E209–13.
Salles G, Duell J, González Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21:978–88.
Duell J, Maddocks KJ, González-Barca E, Jurczak W, Liberati AM, De Vos S, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106:2417–26.
Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22:790–800.
Furqan F, Hamadani M. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma: a review of clinical data. Ther Adv Hematol. 2022;13:20406207221087511.
Zammarchi F, Corbett S, Adams L, Tyrer PC, Kiakos K, Janghra N, et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood. 2018;131:1094–105.
Horvei P, Sakemura R, Cox MJ, Ruff MW, Hefazi M, Tapper EE, et al. Targeting of CD19 by tafasitamab does not impair CD19 directed chimeric antigen receptor T cell activity in vitro. Blood. 2019;134:2859.
Oliai C, de Vos S. Case report: sustained remission achieved from anti-CD19 CAR T cell therapy despite prior treatment with anti-CD19 antibody tafasitamab (MOR208) in a patient with relapsed and refractory diffuse large B-cell lymphoma. Blood. 2019;134:5360.
Sakemura RL, Manriquez-Roman C, Horvei P, Cox MJ, Huynh T, Girsch JH, et al. CD19 antigen occupancy on cancer cells with the CD19 monoclonal antibody tafasitamab improves the activation, antitumor efficacy, and safety profile of CART19 cell therapy. Blood. 2022;140:2362–4.
Thapa B, Caimi PF, Ardeshna KM, Solh M, Carlo-Stella C, Kahl BS, et al. CD19 antibody-drug conjugate therapy in DLBCL does not preclude subsequent responses to CD19-directed CAR T-cell therapy. Blood Adv. 2020;4:3850–2.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–308.
Qualls D, Buege MJ, Dao P, Caimi PF, Rutherford SC, Wehmeyer G, et al. Tafasitamab and lenalidomide in relapsed/refractory large B cell lymphoma (R/R LBCL): real world outcomes in a multicenter retrospective study. Blood. 2022;140:787–9.
Caimi PF, Ardeshna KM, Reid E, Ai W, Lunning M, Zain J, et al. The AntiCD19 antibody drug immunoconjugate loncastuximab achieves responses in DLBCL relapsing after antiCD19 CAR-T cell therapy. Clin Lymphoma Myeloma Leuk. 2022;22:e335–9.
Fischer J, Paret C, El Malki K, Alt F, Wingerter A, Neu MA, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40:187–95.
Plaks V, Rossi JM, Chou J, Wang L, Poddar S, Han G, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021;138:1081–5.
Shalabi H, Kraft IL, Wang HW, Yuan CM, Yates B, Delbrook C, et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica. 2018;103:e215–8.
Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38:155–65.
Gouni S, Rosenthal AC, Crombie JL, Ip A, Kamdar MK, Hess B, et al. A multicenter retrospective study of polatuzumab vedotin in patients with large B-cell lymphoma after CAR T-cell therapy. Blood Adv. 2022;6:2757–62.
Zurko J, Ramdial J, Shadman M, Ahmed S, Szabo A, Iovino L, et al. Allogeneic transplant following CAR T-cell therapy for large B-cell lymphoma. Haematologica. 2023;108:98–109.
Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022;387:2220–31.
Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9:e327–39.
Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol. 2023;41:2238–47.
Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41:2238–47.
Bezerra ED, Iqbal M, Munoz J, Khurana A, Wang Y, Maurer MJ, et al. Barriers to enrollment in clinical trials of patients with aggressive B-Cell NHL that progressed after CAR T-cell therapy. Blood Adv. 2023;7:1572–6.
Author information
Authors and Affiliations
Contributions
MI conceived the study, collected the data, wrote, and finalized the manuscript; DJ, JC, AK, AR, NE, II, FTA; BRD; JEM; JDS-S; RM; AS; KA; SRB; NS; AF; SAI; YL contributed data, reviewed, and provided feedback on the manuscript. EC; ZL provided statistical support. MA-KD conceived the study, reviewed, and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
AR: educational session honoraria for Curio Science and MJH Life Sciences; NE: Research funding: Beigene, Speakers Bureau for Incyte and Beigene, Honoraria/consulting/ad boards for Merck, ADC Therapeutics, Lilly, Novartis; FTA: consultancy to Genentech, Astrazeneca, Abbvie, Janssen, Pharmacyclics, Gilead sciences, Kite pharma, Celgene, Karyopharm, MEI Pharma, Verastem, Incyte, Beigene, Johnson and Johnson, Dava Oncology, BMS, Merck, Cardinal Health, ADCT therapeutics, Epizyme, Caribou Biosciences, Cellecter Bisosciences, Loxo Oncology, and received research funding from Pharmacyclics; BD: Institutional research funding: Janssen, Angiocrine, Pfizer, Poseida, MEI, Orcabio, Wugen, Allovir; Consultancy/Advisor: Jazz, Gamida Cell, MJH BioScience, Arivan Research, BEAM therapeutics, Janssen, Atheneum; JEM: My institution receives research funding on my behalf from Gilead, Atara, CRISPR, Precision Biosciences, Scripps, FATE Therapeutics; JDSS: Speaker’s bureau: Seagen; Consultant: Abbvie, BeiGene, Genmab, Janssen, Genentech, ADC therapeutics, MassiveBio; educational programs: Curio; MAK-D: research/clinical trial with Novartis and Bristol Myers Squibb. The remaining authors have no relevant conflicts of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iqbal, M., Jagadeesh, D., Chavez, J. et al. Efficacy of CD19 directed therapies in patients with relapsed or refractory large b-cell lymphoma relapsing after CD19 directed chimeric antigen receptor T-cell therapy. Bone Marrow Transplant 59, 211–216 (2024). https://doi.org/10.1038/s41409-023-02148-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02148-4