Abstract
Background:
Early-onset sepsis (EOS) is responsible for an important fraction of neonatal morbidity and mortality all over the world. The aim of this study was to assess whether presepsin (P-SEP) can be a more accurate biomarker of EOS compared with pro-calcitonin (PCT) and C-reactive protein (CRP).
Study design:
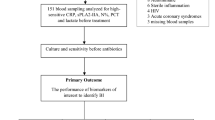
Consecutive preterm neonates (<34 wk gestational age, admitted to Neonatal Intensive Care Unit by 6 h of age and undergoing sepsis evaluation) were recruited as part of a case-matched control study. We determined CRP, PCT and P-SEP at admission, and then at 12, 24, and 48 h of age. Neonates recruited into the study were divided into the EOS group (n = 32) and the uninfected group (n =38) according to their infection screening.
Results:
P-SEP values were significantly higher in the EOS group than in the uninfected group at different time intervals. The highest accuracy was achieved by P-SEP at 24 h after birth. The AUC for P-SEP was 0.97. In our sample, P-SEP achieved the best accuracy for prediction of EOS at the cut-off of 788 ng/l with 93% sensitivity and 100% specificity.
Conclusions:
This study shows that P-SEP is significantly higher in preterm infants with EOS compared with uninfected infants.
Similar content being viewed by others
Main
Early-onset sepsis (EOS) is a much feared condition responsible for an important fraction of neonatal morbidity and mortality. The rate of EOS is currently estimated at 0.98 per 1,000 live births, with the highest rates among the most premature infants (1). Importantly, 13% of both well-appearing and ill-appearing infants are usually evaluated for EOS and 11% are treated empirically with antibiotics, although only 0.04% of them have blood-culture confirmed infection (1).
Although C-reactive protein (CRP) and pro-calcitonin (PCT) continue to be the most widely used biomarkers of neonatal sepsis, their accuracy is still debatable (2,3,4).
CRP has limited value as a diagnostic marker of early neonatal sepsis. In fact, a raised CRP is not necessarily diagnostic for sepsis and may occur due to a physiologic rise after birth or noninfection-associated conditions (5,6). Therefore, there are concerns about the reliability of CRP during EOS. The current evidence does not support a single CRP determination as an indicator to start or discontinue antibiotic therapy (3,7).
On the other hand, PCT response has the advantage of increasing rapidly after infection, approximately 1.5 to 2 h after contact with the bacterial endotoxin. In spite of this, its sensitivity and specificity in the diagnosis of EOS is 76% (range 68–82) and 76% (range 60–87) respectively (2).
For these reasons, the development of a rapid, sensitive diagnostic test with a strong negative predictive value for EOS is urgently needed in order to reduce unnecessary treatment in the newborn population.
There is emerging evidence concerning the ability of presepsin (P-SEP) to serve as a valuable marker in the diagnosis of sepsis in adults (8,9).
Furthermore, recent studies have also demonstrated that P-SEP is an accurate biomarker for the diagnosis of late-onset neonatal sepsis and that it can be considered as a reliable tool to monitor septic infants’ response to therapeutic interventions (9,10,11).
P-SEP is the cleaved truncated form of the soluble CD14 (sCD14). CD14 is a multifunctional cell surface glycoprotein, which is expressed on the cell-surface of different immune cell lines and represents a specific high-affinity receptor for complexes of lipopolysaccharides. In addition, it is implicated in the recognition of a wide variety of bacterial products, including peptidoglycans and the major cell wall component of Gram-positive bacteria (12,13). After its stimulation by pathogens, the CD14 complex is released by shedding from the cell-surface, yielding sCD14, which is then cleaved by the plasma protease activity generating sCD14 fragments. The 64-amino acid N-terminal fragments constitute the P-SEP (12,13).
The aim of this study was to assess whether P-SEP can be a more accurate biomarker of EOS in preterm infants, compared with other biomarkers of neonatal sepsis such as PCT and CRP.
Methods
Patient Population and Study Design
This single-center study was carried out between January 2013 and March 2016 at the level III Neonatal Intensive Care Unit of the Monaldi Hospital, in Naples, Italy. The study protocol was approved by Azienda Ospedaliera dei Colli ethics committee and written informed consent was obtained from parents or legal representatives of the infants.
All preterm neonates (< 34 wk gestational age) admitted to the Neonatal Intensive Care Unit by 6 h of age and undergoing sepsis evaluation were enrolled in the study.
Exclusion criteria were the presence of major congenital malformations, fetal hydrops, confirmed intrauterine infection (toxoplasmosis, rubella, cytomegalovirus, syphilis and herpes), and lack of parental consent.
Blood was sampled at admission (T0) before starting antibiotic therapy, for culture, complete blood count and for measuring P-SEP, PCT, and CRP. These biomarkers were measured again at 12 (T12), 24 (T24), and 48 (T48) hours of age.
Blood samples for cultures were obtained after aseptic nontouch venipuncture; 1.0 ml whole blood was inoculated into a single pediatric blood culture bottle (BACTEC Peds Plus, Becton Dickinson, Sparks, MD). P-SEP was determined in whole blood samples collected in a 150-µl Ethylenediaminetetraacetic acid-containing syringe from venipuncture, each requiring 50 µl of whole blood (20,22). P-SEP was measured at the bedside point of care by chemiluminescent enzyme immunoassay with the automated analyzer PATHFAST (Mitsubishi Chemical Medience Corporation, Tokyo, Japan). PCT and CRP were measured from serum samples in our central laboratory (BRAHMS PCT KRYPTOR; Thermo Scientific, Hennigsdorf, Germany and LX20; Beckman Coulter, Brea, CA respectively).
Patient Classification
The neonates recruited into the study were divided into three groups according to their infection screening and clinical findings: the septic group, the clinical sepsis group and the uninfected group. Clinical sepsis was considered in the presence of signs of infection (23) and if antibiotics were started on the day of birth and continued for greater than 48 h despite negative culture. For the analysis of the study, only the infants part of the septic group, and of the uninfected group were considered.
The septic group included those neonates with proven sepsis. Proven sepsis was diagnosed in case of growth of a recognized pathogen in pure culture or—in case of a mixed growth or growth of a skin commensal—the acute onset of >3 predefined clinical signs was considered (23).
The considered signs of infection were: acute onset of increased oxygen requirement or ventilatory support; increased apnea/bradycardia; hypotension; glucose intolerance; impaired peripheral perfusion (capillary refill time > 3 s/pallor/mottling/core-peripheral temperature gap > 2C); lethargy/irritability/poor handling; temperature instability; ileus/onset of feed intolerance; increase in serum bilirubin; fall in urine output; metabolic acidosis/ base deficit > −10 mmol/l; and anticonvulsant therapy (23).
The uninfected group included those neonates who did not show any clinical, radiographic, or laboratory findings attributable to sepsis, or showed findings fully explained by another diagnosis. The uninfected infants enrolled into the study were retrospectively matched with the septic infants, according to the gestational age and birth weight.
Classification of infants according to infection definitions was made by two clinicians unaware of P-SEP measurement (PM and RR) and samples were included as septic or uninfected only if both clinicians agreed. The treating physicians were blinded to the P-SEP results.
Treatment of EOS
According to our Neonatal Intensive Care Unit policy, the newborns admitted to the unit received antibiotics (amoxicillin clavulanate plus tobramycin) based on the presence of risk factors and clinical indicators suggestive of sepsis. Those strongly suggestive of sepsis were defined “red flags” ( Table 1 ) (17).
The other clinical indicators and risk factors were classified as “non-red flag”. These included: maternal Group B streptococcus colonization, bacteriuria, or infection in the current pregnancy, preterm birth following spontaneous labor, prelabor rupture of membranes, confirmed rupture of membranes for more than 18 h, intrapartum fever higher than 38 °C or confirmed or suspected chorioamnionitis as well as feed intolerance, jaundice in the baby within 24 h of birth and signs of respiratory distress. Antibiotic treatment was commenced if one “red flag” or more than one “non-red flag” risk factor or clinical indicator was present (17).
The antibiotic therapy was stopped after 2 d of treatment if bacterial cultures remained negative, CRP remained low and the neonate was clinically stable. This empirical antibiotic combination was chosen according to the most frequent pathogens isolated in our Neonatal Intensive Care Unit and eventually adjusted according to the results of blood culture and antibiogram. All patients with EOS were treated for 10 consecutive days.
Statistical Analysis
We estimated a sample size of 30 patients for each group, based on the following assumptions: (i) the difference in P-SEP serum concentration between the EOS and uninfected group would be at least 220 ng/l (11, ii) SD for P-SEP in uninfected newborns is ~300 ng/l (normal reference values 643.1 ± 303.8 ng/l) (20,21, iii) a two-sided test of statistical significance, (iv) a probability of 0.05 for a type I error associated with the two-sided test, and (v) a probability of 0.2 for a type II error associated with the test (i.e., the power of the test is 80%).
The infants’ characteristics are presented as numbers and proportions for categorical variables, means, and SDs for continuous variables. Infection markers are given as median and interquartile range. Statistical analysis was performed by using two-sided Student t-test for parametric continuous variables, Fisher exact test for categorical variables, and Mann-Whitney U-test for continuous nonparametric variables. The ROC curves were analyzed for P-SEP, CRP, and PCT values at different time intervals, calculating the AUC and the most accurate cut-off values. These were then used for calculations of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), LR+, and LR- for all the study parameters to predict sepsis. As far as CRP is concerned, we also used a fixed cut-off value of 10 mg/l (15,17) for calculations of sensitivity, specificity, PPV, NPV, LR+, and LR-. Exact 95% CI were calculated for all the parameters. A P value of < 0.05 was considered statistically significant. All analyses were conducted using Stratigraphics Centurion XV.II (Warrenton, VA).
Results
Seventy patients (32 in the EOS group and 38 in the uninfected group), were enrolled in the study. The first blood sample (T0) was collected at 2 ± 0.9 h of age.
Infants in the EOS group had a higher rate of mechanical ventilation, clinical chorioamnionitis, death, and a greater need for surfactant and inotropic support than infants in the uninfected group ( Table 2 ). Death occurred in three septic infants. Two of them died at 2 d and one at 3 d of life.
The microorganisms isolated from blood cultures were Coagulase-negative staphylococcus (n =2), Streptococcus group B (n = 7), Klebsiella pneumoniae (n = 7), Escherichia coli (n = 16).
The sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR-), PPV, NPV, and the AUC for identifying septic patients are shown in Table 3 for the various parameters.
The ROC curves of all the study parameters to differentiate between uninfected and septic neonates at different time intervals are shown in Figure 1 .
Comparison of the receiver operative curves of all the study parameters (presepsin: black solid line, C-reactive protein: dashed black line, pro-calcitonin: gray solid line) between the noninfected and infected neonates at different time intervals. (a) Admission; (b) 12 h; (c) 24 h; (d) 48 h.
In our population, P-SEP achieved the best accuracy for prediction of sepsis at the cut-off of 788 ng/l (AUC 0.97; 95% CI 0.94–1.00) with 93% sensitivity (95% CI 82–98) and 100% specificity (95% CI 83–100). The PPV and NPV were 100% (95% CI 86–100) and 94% (95% CI 82–100). The LR- was 0.04 (95% CI 0.02–0.2), and the LR+ was infinity ( Table 3 ).
The median and IQR of all the study parameters in the septic and uninfected groups at different time intervals are shown in Table 4 .
P-SEP values were higher in the EOS group than in the uninfected group at T0 (598 (457–787) vs. 328 (311–527) ng/l; P = 0.01), T12 (802 (511–1,005) vs. 385 (280–587) ng/l; P = 0.004), T24 (1,228 (738–1,546) vs. 504 (212–646) ng/l; P < 0.001), T48 (979 (588–1,031) vs. 476 (227–602) ng/l; P = 0.002). PCT and CRP levels were significantly higher in EOS-group when compared with uninfected infants at T12, T24, T48 (P < 0.05) but not at T0 (P > 0.05) ( Table 4 ).
Discussion
Our study highlights that P-SEP levels are significantly higher in infants with EOS compared with uninfected infants, suggesting its potential utility in the early diagnosis of sepsis. Furthermore, P-SEP showed a better diagnostic accuracy in discriminating bacterial sepsis from controls when compared with CRP and PCT, as highlighted by the ROC analysis.
Despite significant advances in the care of newborn infants, EOS continues to be one of the major causes of morbidity and mortality in neonates. The diagnosis of neonatal sepsis is still extremely challenging due to nonspecific signs and symptoms with a number needed to treat between 18 and 38 in order to potentially benefit one septic infant (14). For these reasons, identification of rapid diagnostic biomarkers of sepsis to differentiate septic from uninfected neonates is a major priority in neonatal research. In particular, Polin et al. have recently pointed out that a common effort should be focused on developing a rapid, sensitive diagnostic test with a strong negative predictive value for EOS so as to avoid unnecessary treatment of symptomatic infants who are at low risk (14).
Our results indicate that P-SEP could be included as a marker of EOS screening and, if these findings were validated on a larger scale, its use might help to decrease false-positive diagnoses and the exposure of preterm infants to adverse drug effects and invasive procedures. This is particularly important in this population where administration of antibiotics in the case of suspected EOS (negative blood cultures) can cause the development and spread of antibiotic-resistant bacteria in neonatal units.
Our data are in agreement with recent studies showing a high accuracy of P-SEP as a marker of neonatal bacterial infection (10,11). In fact, Poggi et al. have reported 100% specificity and 94% sensitivity with a cut-off value of 885 ng/l in infants with late-onset sepsis (11).
Meanwhile Mussap et al. have recently found that with a cut-off of 600 ng/l, P-SEP had a sensitivity of 97.5% and specificity of 100% in bacterial sepsis (10). Unfortunately, there was no systematic timed measurement of P-SEP, and both newborns with EOS and with late-onset sepsis were included in the bacterial sepsis group (10).
Our findings point out that CRP and PCT, which are usually assessed as part of the clinical management of septic newborns, have a suboptimal accuracy to identify newborns who are likely to be septic as their AUC at 24 h was 0.74 (95% CI 0.70–0.89) and 0.83 (95% CI 0.72–0.92) respectively. However, the use of these biomarkers can still represent a useful tool to support an early discontinuation of antibiotic therapy, as suggested by the American Academy of Pediatrics, which recommends that the duration of antibiotic therapy should be guided according to serial CRP determinations (15).
So far, the literature has shown conflicting data as to CRP cut-off points, ranging from 1.5 to 20 mg/l and has reported wide-ranging sensitivities and specificities (7,16). Currently, the most used cut-off value is 10 mg/l (15,17). However, because of the physiologic changes of CRP during the first days of life and the influence of gestational age on its response to infection, different studies have assessed the possible advantages of using dynamic reference values (6,18).
In our study, we therefore assessed CRP diagnostic utility both using a fixed threshold of 10 mg/l and determining the optimal cut-off values at different time points using ROC curve analysis. Our results confirm that CRP has a good NPV and they suggest that its clinical use for discontinuing antibiotics might be a successful strategy.
In our sample, there was a relatively modest CRP response in the infected neonates compared with P-SEP, although both are driven by IL-6 production (19). This might probably reflect the multifunctional role of CD14, which is not only released by the liver as a minor acute-phase protein but also by immunocompetent cells. In fact, in addition to being a receptor to LPS and other bacterial structures, CD14 may modulate LPS-triggered apoptosis and regulate T and B lymphocyte activation and function (13).
The main limitation of this study is the sample size, which is relatively small, thus not allowing any stratification of P-SEP according to gestational age. However, previous evidence suggests that P-SEP is not affected by gestational age in healthy infants (20,21).
In conclusion, our study shows that P-SEP is significantly higher in preterm infants with EOS when compared with uninfected infants.
Furthermore, this study provides a direct comparison of P-SEP with other biomarkers such as CRP and PCT at different time intervals and highlights that P-SEP has a higher accuracy.
Our data support the use of P-SEP measurement at the bedside, and highlight its promise as a useful biomarker for the early diagnosis of EOS. Nevertheless, subsequent larger studies are required to confirm and ascertain the additional value of this biomarker.
Statement of Financial Support
No financial assistance was received to support this study.
Disclosure
There were no conflicts of interest to declare when completing this study.
References
Escobar GJ, Puopolo KM, Wi S, et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics 2014;133:30–6.
Vouloumanou EK, Plessa E, Karageorgopoulos DE, Mantadakis E, Falagas ME. Serum procalcitonin as a diagnostic marker for neonatal sepsis: a systematic review and meta-analysis. Intensive Care Med 2011;37:747–62.
Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics 1998;102:E41.
Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta 2015;451(Pt A):46–64.
Chiesa C, Natale F, Pascone R, et al. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chim Acta 2011;412:1053–9.
Chiesa C, Signore F, Assumma M, et al. Serial measurements of C-reactive protein and interleukin-6 in the immediate postnatal period: reference intervals and analysis of maternal and perinatal confounders. Clin Chem 2001;47:1016–22.
Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology 2012;102:25–36.
Behnes M, Bertsch T, Lepiorz D, et al. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care 2014;18:507.
Ulla M, Pizzolato E, Lucchiari M, et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care 2013;17:R168.
Mussap M, Puxeddu E, Puddu M, et al. Soluble CD14 subtype (sCD14-ST) presepsin in premature and full term critically ill newborns with sepsis and SIRS. Clin Chim Acta 2015;451(Pt A):65–70.
Poggi C, Bianconi T, Gozzini E, Generoso M, Dani C. Presepsin for the detection of late-onset sepsis in preterm newborns. Pediatrics 2015;135:68–75.
Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens YE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta 2015;450:97–103.
Mussap M, Noto A, Fravega M, Fanos V. Soluble CD14 subtype presepsin (sCD14-ST) and lipopolysaccharide binding protein (LBP) in neonatal sepsis: new clinical and analytical perspectives for two old biomarkers. J Matern Fetal Neonatal Med 2011;24 Suppl 2:12–4.
Benitz WE, Wynn JL, Polin RA. Reappraisal of guidelines for management of neonates with suspected early-onset sepsis. J Pediatr 2015;166:1070–4.
Polin RA ; Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129:1006–15.
Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem 2004;50:279–87.
Caffrey Osvald E, Prentice P. NICE clinical guideline: antibiotics for the prevention and treatment of early-onset neonatal infection. Arch Dis Child Educ Pract Ed 2014;99:98–100.
Ishibashi M, Takemura Y, Ishida H, Watanabe K, Kawai T. C-reactive protein kinetics in newborns: application of a high-sensitivity analytic method in its determination. Clin Chem 2002;48:1103–6.
Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol 2004;172:4470–9.
Mussap M, Puxeddu E, Burrai P, et al. Soluble CD14 subtype (sCD14-ST) presepsin in critically ill preterm newborns: preliminary reference ranges. J Matern Fetal Neonatal Med 2012;25(Suppl 5):51–3.
Pugni L, Pietrasanta C, Milani S, et al. Presepsin (soluble CD14 subtype): reference ranges of a new sepsis marker in term and preterm neonates. PLoS One 2015;10:e0146020.
Okamura Y, Yokoi H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin Chim Acta 2011;412:2157–61.
Modi N, Dore CJ, Saraswatula A, et al. A case definition for national and international neonatal bloodstream infection surveillance. Arch Dis Child Fetal Neonatal Ed 2009;94:F8–12.
Romero R, Chaiworapongsa T, Savasan ZA, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med 2012;25:558–67.
United Kingdom NICE guidelines 149. Antibiotics for the prevention and treatment of early onset neonatal infection. https://www.nice.org.uk/guidance/cg149/resources/neonatal-infection-early-onset-antibiotics-for-prevention-and-treatment-35109579233221.
Seri I, Evans J. Controversies in the diagnosis and management of hypotension in the newborn infant. Curr Opin Pediatr 2001;13:116–23.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Montaldo, P., Rosso, R., Santantonio, A. et al. Presepsin for the detection of early-onset sepsis in preterm newborns. Pediatr Res 81, 329–334 (2017). https://doi.org/10.1038/pr.2016.217
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.217
This article is cited by
-
Comparison of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for the diagnosis of neonatal sepsis: a systematic review and meta-analysis
BMC Pediatrics (2023)
-
Cord blood presepsin as a predictor of early-onset neonatal sepsis in term and preterm newborns
Italian Journal of Pediatrics (2023)
-
Comparison of presepsin and Mid-regional pro-adrenomedullin in the diagnosis of sepsis or septic shock: a systematic review and meta-analysis
BMC Infectious Diseases (2023)
-
Soluble CD14 subtype (sCD14-ST) as biomarker in neonatal early-onset sepsis and late-onset sepsis: a systematic review and meta-analysis
BMC Immunology (2019)
-
Presepsin and fetuin-A dyad for the diagnosis of proven sepsis in preterm neonates
BMC Infectious Diseases (2019)