Key Points
-
Biologic agents used for the treatment of rheumatic and immunological disorders can cause allergic adverse events (AEs), including standard infusion reactions and hypersensitivity reactions
-
Biologic agents are associated with an increased risk of severe infection, including reactivation of tuberculosis (high risk: infliximab, adalimumab; intermediate risk: etanercept, abatacept, tocilizumab; low risk: rituximab, anakinra)
-
Combination therapy using biologic agents and immunosuppressive drugs has a higher risk of serious infections than monotherapy, especially in the first 12 months of treatment
-
Organ-specific AEs are commonly hepatic, cutaneous or haematological AEs, whereas immunological syndromes and cardiovascular, noninfectious pulmonary, gastrointestinal and neurological AEs are rare
-
Whether patients receiving biologic therapy have an increased incidence of lymphomas and skin cancers, especially after combination therapy, is unknown
-
Biologic therapy decreases the immune response to primary (but not booster) vaccination; therefore, live vaccines (nasal flu; Bacillus Calmette–Guérin; measles, mumps and rubella; and yellow fever vaccines) during active biologic therapy are prohibited
Abstract
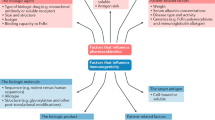
Biologic agents have substantially advanced the treatment of immunological disorders, including chronic inflammatory and autoimmune diseases. However, these drugs are often associated with adverse events (AEs), including allergic, immunological and other unwanted reactions. AEs can affect almost any organ or system in the body and can occur immediately, within minutes to hours, or with a delay of several days or more after initiation of biologic therapy. Although some AEs are a direct consequence of the functional inhibition of biologic-agent-targeted antigens, the pathogenesis of other AEs results from a drug-induced imbalance of the immune system, intermediary factors and cofactors, a complexity that complicates their prediction. Herein, we review the AEs associated with biologic therapy most relevant to rheumatic and immunological diseases, and discuss their underlying pathogenesis. We also include our recommendations for the medical management of such AEs. Increased understanding and improved risk management of AEs induced by biologic agents will enable better use of these versatile immune-response modifiers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pichler, W. J. Adverse side-effects to biological agents. Allergy 61, 912–920 (2006).
Lenz, H. J. Management and preparedness for infusion and hypersensitivity reactions. Oncologist 12, 601–609 (2007).
Johansson, S. G. et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 113, 832–836 (2004).
Matucci, A. et al. Allergological in vitro and in vivo evaluation of patients with hypersensitivity reactions to infliximab. Clin. Exp. Allergy 43, 659–664 (2013).
Dillman, R. O. & Hendrix, C. S. Unique aspects of supportive care using monoclonal antibodies in cancer treatment. Support Cancer Ther. 1, 38–48 (2003).
Coiffier, B. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 235–242 (2002).
Dillman, R. O. Infusion reactions associated with the therapeutic use of monoclonal antibodies in the treatment of malignancy. Cancer Metastasis Rev. 18, 465–471 (1999).
Chung, C. H. et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1, 3-galactose. N. Engl. J. Med. 358, 1109–1117 (2008).
Brennan, P. J., Rodriguez Bouza, T., Hsu, F. I., Sloane, D. E. & Castells, M. C. Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J. Allergy Clin. Immunol. 124, 1259–1266 (2009).
Vultaggio, A. et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 65, 657–661 (2010).
Vultaggio, A. et al. Drug-specific TH2 cells and IgE antibodies in a patient with anaphylaxis to rituximab. Int. Arch. Allergy Immunol. 159, 321–326 (2012).
Finkelman, F. D. Anaphylaxis: lessons from mouse models. J. Allergy Clin. Immunol. 120, 506–515 (2007).
Jönsson, F. et al. Mouse and human neutrophils induce anaphylaxis. J. Clin. Invest. 121, 1484–1496 (2011).
Vadas, P. et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N. Engl. J. Med. 358, 28–35 (2008).
Cheifetz, A. & Mayer, L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J. Med. 72, 250–256 (2005).
Svenson, M., Geborek, P., Saxne, T. & Bendtzen, K. Monitoring patients treated with anti-TNF-α biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology (Oxford) 46, 1828–1834 (2007).
Krintel, S. B. et al. The frequency of anti-infliximab antibodies in patients with rheumatoid arthritis treated in routine care and the associations with adverse drug reactions and treatment failure. Rheumatology (Oxford) 52, 1245–1253 (2013).
Wolbink, G. J. et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 54, 711–715 (2006).
Baert, F. et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N. Engl. J. Med. 348, 601–608 (2003).
Bartelds, G. M. et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305, 1460–1468 (2011).
Maneiro, J. R., Salgado, E. & Gomez-Reino, J. J. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated inflammatory conditions: systematic review and meta-analysis. JAMA Intern. Med. 173, 1416–1428 (2013).
de Vries, M. K. et al. Immunogenicity does not influence treatment with etanercept in patients with ankylosing spondylitis. Ann. Rheum. Dis. 68, 531–535 (2009).
Dore, R. K. et al. The immunogenicity, safety, and efficacy of etanercept liquid administered once weekly in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 25, 40–46 (2007).
van Schouwenburg, P. A., Rispens, T. & Wolbink, G. J. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 9, 164–172 (2013).
Haggerty, H. G. et al. Evaluation of immunogenicity of the T cell costimulation modulator abatacept in patients treated for rheumatoid arthritis. J. Rheumatol. 34, 2365–2373 (2007).
Thurlings, R. M. et al. Clinical response, pharmacokinetics, development of human anti-chimaeric antibodies, and synovial tissue response to rituximab treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 69, 409–412 (2010).
Zhou, H. et al. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-α monoclonal antibody, in subjects with rheumatoid arthritis. J. Clin. Pharmacol. 47, 383–396 (2007).
Stubenrauch, K. et al. Subset analysis of patients experiencing clinical events of a potentially immunogenic nature in the pivotal clinical trials of tocilizumab for rheumatoid arthritis: Evaluation of an antidrug antibody ELISA using clinical adverse event-driven immunogenicity testing. Clin. Ther. 32, 1597–1609 (2010).
Bingham, C. O. 3rd et al. Effectiveness and safety of etanercept in subjects with RA who have failed infliximab therapy: 16-week, open-label, observational study. Curr. Med. Res. Opin. 25, 1131–1142 (2009).
Chatzidionysiou, K., Askling, J., Eriksson, J., Kristensen, L. E. & van Vollenhoven, R. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2013-204714.
Furst, D. E. et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann. Rheum. Dis. 66, 893–899 (2007).
Karlsson, J. A. et al. Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology (Oxford) 47, 507–513 (2008).
Smolen, J. S. et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor α inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 374, 210–221 (2009).
van der Bijl, A. E. et al. An open-label pilot study of the effectiveness of adalimumab in patients with rheumatoid arthritis and previous infliximab treatment: relationship to reasons for failure and anti-infliximab antibody status. Clin. Rheumatol. 27, 1021–1028 (2008).
Hanekom, W. A., Abel, B. & Scriba, T. J. Immunological protection against tuberculosis. S. Afr. Med. J. 97, 973–977 (2007).
Harris, J. & Keane, J. How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin. Exp. Immunol. 161, 1–9 (2010).
Keane, J. et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N. Engl. J. Med. 345, 1098–1104 (2001).
Winthrop, K. L., Siegel, J. N., Jereb, J., Taylor, Z. & Iademarco, M. F. Tuberculosis associated with therapy against tumor necrosis factor α. Arthritis Rheum. 52, 2968–2974 (2005).
Furst, D. E. The risk of infections with biologic therapies for rheumatoid arthritis. Semin. Arthritis Rheum. 39, 327–346 (2010).
Tubach, F. et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 60, 1884–1894 (2009).
Winthrop, K. L. et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann. Rheum. Dis. 72, 37–42 (2013).
Curtis, J. R. et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann. Rheum. Dis. 70, 1401–1406 (2011).
Mohan, A. K. et al. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin. Infect. Dis. 39, 295–299 (2004).
Keane, J. & Bresnihan, B. Tuberculosis reactivation during immunosuppressive therapy in rheumatic diseases: diagnostic and therapeutic strategies. Curr. Opin. Rheumatol. 20, 443–449 (2008).
Fallahi-Sichani, M., Flynn, J. L., Linderman, J. J. & Kirschner, D. E. Differential risk of tuberculosis reactivation among anti-TNF therapies is due to drug binding kinetics and permeability. J. Immunol. 188, 3169–3178 (2012).
Takeuchi, T. et al. Golimumab monotherapy in Japanese patients with active rheumatoid arthritis despite prior treatment with disease-modifying antirheumatic drugs: results of the phase 2/3, multicentre, randomised, double-blind, placebo-controlled GO-MONO study through 24 weeks. Ann. Rheum. Dis. 72, 1488–1495 (2012).
Hsia, E. C. et al. Comprehensive tuberculosis screening program in patients with inflammatory arthritides treated with golimumab, a human anti-tumor necrosis factor antibody, in phase III clinical trials. Arthritis Care Res. (Hoboken) 65, 309–313 (2013).
Smolen, J. et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann. Rheum. Dis. 68, 797–804 (2009).
Campbell, L., Chen, C., Bhagat, S. S., Parker, R. A. & Östör, A. J. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford) 50, 552–562 (2011).
Smolen, J. S. et al. Consensus statement on blocking the effects of interleukin-6 and in particular by interleukin-6 receptor inhibition in rheumatoid arthritis and other inflammatory conditions. Ann. Rheum. Dis. 72, 482–492 (2013).
Mohrbacher, A. B cell non-Hodgkin's lymphoma: rituximab safety experience. Arthritis Res. Ther. 7 (Suppl. 3), S19–S25 (2005).
Burr, M. L., Malaviya, A. P., Gaston, J. H., Carmichael, A. J. & Östör, A. J. Rituximab in rheumatoid arthritis following anti-TNF-associated tuberculosis. Rheumatology (Oxford) 47, 738–739 (2008).
Chen, Y. M. et al. The effects of rituximab therapy on released interferon-gamma levels in the QuantiFERON assay among RA patients with different status of Mycobacterium tuberculosis infection. Rheumatology (Oxford) 52, 697–704 (2013).
Fleishmann, R. M. Safety of anakinra, a recombinant interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis and comparison to anti-TNF-α agents. Clin. Exp. Rheumatol. 20 (Suppl. 27), S35–S41 (2002).
Winthrop, K. L. Infections and biologic therapy in rheumatoid arthritis: our changing understanding of risk and prevention. Rheum. Dis. Clin. North Am. 38, 727–745 (2012).
Filler, S. G., Yeaman, M. R. & Sheppard, D. C. Tumor necrosis factor inhibition and invasive fungal infections. Clin. Infect. Dis. 41 (Suppl. 3), S208–S212 (2005).
Salmon-Ceron, D. et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann. Rheum. Dis. 70, 616–623 (2011).
Patel, D. D., Lee, D. M., Kolbinger, F. & Antoni, C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann. Rheum. Dis. 72 (Suppl. 2), ii116–ii123 (2013).
O'Quinn, D. B., Palmer, M. T., Lee, Y. K. & Weaver, C. T. Emergence of the TH17 pathway and its role in host defense. Adv. Immunol. 99, 115–163 (2008).
Singh, J. A. et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database of Systematic Reviews, Issue 16. Art No.: CD008794. http://dx.doi.org/10.1002/14651858.CD008794.pub2.
Salliot, C. et al. Infections during tumour necrosis factor-α blocker therapy for rheumatic diseases in daily practice: a systematic retrospective study of 709 patients. Rheumatology (Oxford) 46, 327–334 (2007).
Genovese, M. C. et al. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J. Rheumatol. 40, 768–780 (2013).
Schiff, M. H. et al. Integrated safety in tocilizumab clinical trials. Arthritis Res. Ther. 13, R141 (2011).
Fleischmann, R. M. et al. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann. Rheum. Dis. 65, 1006–1012 (2006).
Salliot, C., Dougados, M. & Gossec, L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann. Rheum. Dis. 68, 25–32 (2009).
Kremer, J. M. et al. Long-term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3-year results from the AIM trial. Ann. Rheum. Dis. 70, 1826–1830 (2011).
Weinblatt, M. E. et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J. Rheumatol. 40, 787–797 (2013).
Weinblatt, M. et al. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomized, placebo-controlled study. Arthritis Rheum. 54, 2807–2816 (2006).
Weinblatt, M. et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann. Rheum. Dis. 66, 228–234 (2007).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2793–2806 (2006).
Askling, J. et al. Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann. Rheum. Dis. 66, 1339–1344 (2007).
Dixon, W. G. et al. Serious infection following anti-tumor necrosis factor α therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 56, 2896–2904 (2007).
Galloway, J. B. et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50, 124–131 (2011).
Bongartz, T. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295, 2275–2285 (2006).
Curtis, J. R. et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor α antagonists. Arthritis Rheum. 56, 1125–1133 (2007).
Greenberg, J. D. et al. Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann. Rheum. Dis. 69, 380–386 (2010).
Listing, J. et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 52, 3403–3412 (2005).
Strangfeld, A. et al. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann. Rheum. Dis. 70, 1914–1920 (2011).
van Vollenhoven, R. F. et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J. Rheumatol. 37, 558–567 (2010).
Rigby, W. F., Mease, P. J., Olech, E., Ashby, M. & Tole, S. Safety of rituximab in combination with other biologic disease-modifying antirheumatic drugs in rheumatoid arthritis: an open-label study. J. Rheumatol. 40, 599–604 (2013).
Genovese, M. C. et al. Safety of biological therapies following rituximab treatment in rheumatoid arthritis patients. Ann. Rheum. Dis. 68, 1894–1897 (2009).
van Vollenhoven, R. F. et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann. Rheum. Dis. 72, 1496–1502 (2012).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Merrill, J. T. et al. Long-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosus. Arthritis Rheum. 64, 3364–3373 (2012).
Strangfeld, A. et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-α agents. JAMA 301, 737–744 (2009).
Dreiher, J., Kresch, F. S., Comaneshter, D. & Cohen, A. D. Risk of Herpes zoster in patients with psoriasis treated with biologic drugs. J. Eur. Acad. Dermatol. Venereol. 26, 1127–1132 (2012).
McDonald, J. R. et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin. Infect. Dis. 48, 1364–1371 (2009).
Wendling, D., Streit, G., Toussirot, E. & Prati, C. Herpes zoster in patients taking TNFα antagonists for chronic inflammatory joint disease. Joint Bone Spine 75, 540–543 (2008).
Failla, V., Jacques, J., Castronovo, C. & Nikkels, A. F. Herpes zoster in patients treated with biologicals. Dermatology 224, 251–256 (2012).
Koike, T. et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann. Rheum. Dis. 70, 2148–2151 (2011).
Terrier, B. & Cacoub, P. Cryoglobulinemia vasculitis: an update. Curr. Opin. Rheumatol. 25, 10–18 (2013).
Ferri, C. et al. Safety of anti-tumor necrosis factor-α therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J. Rheumatol. 35, 1944–1949 (2008).
Lee, Y. H., Bae, S. C. & Song, G. G. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin. Exp. Rheumatol. 31, 118–121 (2013).
Perez-Alvarez, R. et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 90, 359–371 (2011).
Sarrecchia, C., Cappelli, A. & Aiello, P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J. Infect. Chemother. 11, 189–191 (2005).
Westhoff, T. H. et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood 102, 1930 (2003).
Sneller, M. C., Hu, Z. & Langford, C. A. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. 64, 835–842 (2012).
Day, R. Adverse reactions to TNF-α inhibitors in rheumatoid arthritis. Lancet 359, 540–541 (2002).
Wetter, D. A. & Davis, M. D. Lupus-like syndrome attributable to anti-tumor necrosis factor α therapy in 14 patients during an 8-year period at Mayo Clinic. Mayo Clin. Proc. 84, 979–984 (2009).
Kerbleski, J. F. & Gottlieb, A. B. Dermatological complications and safety of anti-TNF treatments. Gut 58, 1033–1039 (2009).
Williams, V. L. & Cohen, P. R. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF α antagonists. Int. J. Dermatol. 50, 619–625 (2011).
De Bandt, M. et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor α therapy: a French national survey. Arthritis Res. Ther. 7, R545–R551 (2005).
Nosbaum, A. et al. Arterial thrombosis with anti-phospholipid antibodies induced by infliximab. Eur. J. Dermatol. 17, 546–547 (2007).
Ramos-Casals, M., Roberto Perez, A., Diaz-Lagares, C., Cuadrado, M. J. & Khamashta, M. A. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun. Rev. 9, 188–193 (2010).
Charles, P. J., Smeenk, R. J., De Jong, J., Feldmann, M. & Maini, R. N. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor α: findings in open-label and randomized placebo-controlled trials. Arthritis Rheum. 43, 2383–2390 (2000).
Ramos-Casals, M. et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine 86, 242–251 (2007).
Tan, E. M. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25, 1271–1277 (1982).
de Gannes, G. C. et al. Psoriasis and pustular dermatitis triggered by TNF-(α) inhibitors in patients with rheumatologic conditions. Arch. Dermatol. 143, 223–231 (2007).
Seneschal, J. et al. Cytokine imbalance with increased production of interferon-α in psoriasiform eruptions associated with antitumour necrosis factor-α treatments. Br. J. Dermatol. 161, 1081–1088 (2009).
Perez-Alvarez, R., Perez-de-Lis, M. & Ramos-Casals, M. Biologics-induced autoimmune diseases. Curr. Opin. Rheumatol. 25, 56–64 (2013).
Guillevin, L. & Mouthon, L. Tumor necrosis factor-α blockade and the risk of vasculitis. J. Rheumatol. 31, 1885–1887 (2004).
Giezen, T. J. et al. Rituximab-induced thrombocytopenia: a cohort study. Eur. J. Haematol. 89, 256–266 (2012).
El-Osta, H. & Nair, B. Rituximab-induced acute thrombocytopenia: an underappreciated entity. Leuk. Lymphoma (2013).
Szalay, B., Acs, L., Vásárhelyi, B., Kovács, L. & Balog, A. Successful use of tocilizumab in a patient with rheumatoid arthritis following severe pancytopenia during etanercept therapy. J. Clin. Rheumatol. 17, 377–379 (2011).
Martínez Santana, V., Izquierdo Navarro, M., Calleja Hernández, M. Á., Sánchez Sánchez, T. & Sainz Gil, M. Severe pancytopenia following etanercept administration in rheumatoid arthritis. Int. J. Rheum. Dis. 15, e78–e79 (2012).
Kuruvilla, J. et al. Aplastic anemia following administration of a tumor necrosis factor-α inhibitor. Eur. J. Haematol. 71, 396–398 (2003).
Korswagen, L. A. et al. Venous and arterial thromboembolic events in adalimumab-treated patients with antiadalimumab antibodies: a case series and cohort study. Arthritis Rheum. 63, 877–883 (2011).
Jones, G. et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann. Rheum. Dis. 69, 88–96 (2010).
Kwon, H. J., Coté, T. R., Cuffe, M. S., Kramer, J. M. & Braun, M. M. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann. Intern. Med. 138, 807–811 (2003).
Listing, J. et al. Does tumor necrosis factor α inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 58, 667–677 (2008).
Barnabe, C., Martin, B. J. & Ghali, W. A. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res. 63, 522–529 (2011).
Greenberg, J. D. et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 576–582 (2011).
Jacobsson, L. T. et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J. Rheumatol. 32, 1213–1218 (2005).
Carmona, L. et al. All-cause and cause-specific mortality in rheumatoid arthritis are not greater than expected when treated with tumour necrosis factor antagonists. Ann. Rheum. Dis. 66, 880–885 (2007).
Dixon, W. G. et al. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor α therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 56, 2905–2912 (2007).
Chung, E. S., Packer, M., Lo, K. H., Fasanmade, A. A. & Willerson, J. T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107, 3133–3140 (2003).
Mann, D. L. et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109, 1594–1602 (2004).
Petrou, I. Systemic medications for psoriasis therapy must be prescribed with caution. Dermatology Times: Modern Medicine [online]. (2014).
Ryan, C. et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA 306, 864–871 (2011).
Tzellos, T., Kyrgidis, A. & Zouboulis, C. C. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 27, 622–627 (2013).
Dommasch, E. D., Troxel, A. B. & Gelfand, J. M. Major cardiovascular events associated with anti-IL 12/23 agents: a tale of two meta-analyses. J. Am. Acad. Dermatol. 68, 863–865 (2013).
Myasoedova, E., Crowson, C. S., Turesson, C., Gabriel, S. E. & Matteson, E. L. Incidence of extraarticular rheumatoid arthritis in Olmsted County, Minnesota, in 1995–2007 versus 1985–1994: a population-based study. J. Rheumatol. 38, 983–989 (2011).
Hadjinicolaou, A. V. et al. Non-infectious pulmonary complications of newer biological agents for rheumatic diseases—a systematic literature review. Rheumatology 50, 2297–2305 (2011).
Jani, M., Hirani, N., Matteson, E. L. & Dixon, W. G. The safety of biologic therapies in RA-associated interstitial lung disease. Nat. Rev. Rheumatol. 10, 284–294 (2013).
Lateef, O., Shakoor, N. & Balk, R. A. Methotrexate pulmonary toxicity. Expert Opin. Drug Saf. 4, 723–730 (2005).
Kim, D. S. Interstitial lung disease in rheumatoid arthritis: recent advances. Curr. Opin. Pulm. Med. 12, 346–353 (2006).
Khasnis, A. A. & Calabrese, L. H. Tumor necrosis factor inhibitors and lung disease: a paradox of efficacy and risk. Semin. Arthritis Rheum. 40, 147–163 (2010).
Wada, T. et al. A case of rheumatoid arthritis complicated with deteriorated interstitial pneumonia after the administration of abatacept [Japanese]. Nihon Rinsho Men'eki Gakkai Kaishi 35, 433–438 (2012).
Curtis, J. R. et al. The incidence of gastrointestinal perforations among rheumatoid arthritis patients. Arthritis Rheum. 63, 346–351 (2011).
Glund, S. & Krook, A. Role of interleukin-6 signalling in glucose and lipid metabolism. Acta Physiol. 192, 37–48 (2008).
Furst, D. E. et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2011. Ann. Rheum. Dis. 71 (Suppl. 2), i2–i45 (2012).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation http://dx.doi.org/10.1016/j.jacc.2013.11.002.
Collamer, A. N. & Battafarano, D. F. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin. Arthritis Rheum. 40, 233–240 (2010).
Wollina, U. et al. Tumor necrosis factor-α inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am. J. Clin. Dermatol. 9, 1–14 (2008).
Harrison, M. J. et al. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor α therapy: results from the British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 68, 209–215 (2009).
Shmidt, E., Wetter, D. A., Ferguson, S. B. & Pittelkow, M. R. Psoriasis and palmoplantar pustulosis associated with tumor necrosis factor-α inhibitors: the Mayo Clinic experience, 1998 to 2010. J. Am. Acad. Dermatol. 67, e179–e185 (2012).
Dass, S., Vital, E. M. & Emery, P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum. 56, 2715–2718 (2007).
Mielke, F., Schneider-Obermeyer, J. & Dörner, T. Onset of psoriasis with psoriatic arthropathy during rituximab treatment of non-Hodgkin lymphoma. Ann. Rheum. Dis. 67, 1056–1057 (2008).
Laurent, S., Le Parc, J. M., Clérici, T., Bréban, M. & Mahé, E. Onset of psoriasis following treatment with tocilizumab. Br. J. Dermatol. 163, 1364–1365 (2010).
Hawryluk, E. B., Linskey, K. R., Duncan, L. M. & Nazarian, R. M. Broad range of adverse cutaneous eruptions in patients on TNF-α antagonists. J. Cutan Pathol. 39, 481–492 (2012).
Nakamura, M. & Tokura, Y. Tocilizumab-induced erythroderma. Eur. J. Dermatol. 19, 273–274 (2009).
Brunasso, A. M., Laimer, M. & Massone, C. Paradoxical reactions to targeted biological treatments: A way to treat and trigger? Acta Derm. Venereol. 90, 183–185 (2010).
Mohan, N. et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-α blocking agents. J. Rheumatol. 31, 1955–1958 (2004).
Dereure, O., Navarro, R., Rossi, J. F. & Guilhou, J. J. Rituximab-induced vasculitis. Dermatology 203, 83–84 (2001).
Kandula, P. & Kouides, P. A. Rituximab-induced leukocytoclastic vasculitis: a case report. Arch. Dermatol. 142, 246–247 (2006).
Kim, M. J., Kim, H. O., Kim, H. Y. & Park, Y. M. Rituximab-induced vasculitis: a case report and review of the medical published work. J. Dermatol. 36, 284–287 (2009).
RITUXAN (rituximab) - Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome - for health professionals. Government of Canada, Healthy Canadians [online]. (2014).
Seror, R. et al. Pattern of demyelination occurring during anti-TNF-α therapy: a French national survey. Rheumatology (Oxford) 52, 868–874 (2013).
Cruz Fernández-Espartero, M. et al. Demyelinating disease in patients treated with TNF antagonists in rheumatology: data from BIOBADASER, a pharmacovigilance database, and a systematic review. Semin. Arthritis Rheum. 41, 524–533 (2011).
Bernatsky, S., Renoux, C. & Suissa, S. Demyelinating events in rheumatoid arthritis after drug exposures. Ann. Rheum. Dis. 69, 1691–1693 (2010).
Fromont, A., De Seze, J., Fleury, M. C., Maillefert, J. F. & Moreau, T. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine 45, 55–57 (2009).
Matsumoto, T., Nakamura, I., Miura, A., Momoyama, G. & Ito, K. New-onset multiple sclerosis associated with adalimumab treatment in rheumatoid arthritis: a case report and literature review. Clin. Rheumatol. 32, 271–275 (2013).
Gomez-Gallego, M., Meca-Lallana, J. & Fernandez-Barreiro, A. Multiple sclerosis onset during etanercept treatment. Eur. Neurol. 59, 91–93 (2008).
Bensouda-Grimaldi, L., Mulleman, D., Valat, J. P. & Autret-Leca, E. Adalimumab-associated multiple sclerosis. J. Rheumatol. 34, 239–240 (2007).
Alvarez-Lario, B., Prieto-Tejedo, R., Colazo-Burlato, M. & Macarrón-Vicente, J. Severe Guillain-Barre syndrome in a patient receiving anti-TNF therapy. Consequence or coincidence. A case-based review. Clin. Rheumatol. 32, 1407–1412 (2013).
Silburn, S., McIvor, E., McEntegart, A. & Wilson, H. Guillain-Barre syndrome in a patient receiving anti-tumour necrosis factor α for rheumatoid arthritis: a case report and discussion of literature. Ann. Rheum. Dis. 67, 575–576 (2008).
de Masson, A. et al. Optic neuritis associated with tumor necrosis factor-α antagonists for the treatment of psoriasis. J. Am. Acad. Dermatol. 67, e280–e282 (2012).
Simsek, I., Erdem, H., Pay, S., Sobaci, G. & Dinc, A. Optic neuritis occurring with anti-tumour necrosis factor α therapy. Ann. Rheum. Dis. 66, 1255–1258 (2007).
Kleinschmidt-DeMasters, B. K. & Tyler, K. L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon β-1a for multiple sclerosis. N. Engl. J. Med. 353, 369–374 (2005).
Langer-Gould, A., Atlas, S. W., Green, A. J., Bollen, A. W. & Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353, 375–381 (2005).
Van Assche, G. et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353, 362–368 (2005).
Palazzo, E. & Yahia, S. A. Progressive multifocal leukoencephalopathy in autoimmune diseases. Joint Bone Spine 79, 351–355 (2012).
Molloy, E. S. & Calabrese, L. H. Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies. Arthritis Rheum. 64, 3043–3051 (2012).
Bharat, A. et al. Incidence and risk factors for progressive multifocal leukoencephalopathy among patients with selected rheumatic diseases. Arthritis Care Res. (Hoboken) 64, 612–615 (2012).
Clifford, D. B. et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch. Neurol. 68, 1156–1164 (2011).
Kumar, D., Bouldin, T. W. & Berger, R. G. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 62, 3191–3195 (2010).
Fleischmann, R. M. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis Rheum. 60, 3225–3228 (2009).
Lopez-Olivo, M. A. et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA 308, 898–908 (2012).
Rennard, S. I. et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 175, 926–934 (2007).
Stone, J. H. et al. Solid malignancies among patients in the Wegener's Granulomatosis Etanercept Trial. Arthritis Rheum. 54, 1608–1618 (2006).
Diak, P. et al. Tumor necrosis factor α blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 62, 2517–2524 (2010).
Wolfe, F. & Michaud, K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 50, 1740–1751 (2004).
Wong, A. K., Kerkoutian, S., Said, J., Rashidi, H. & Pullarkat, S. T. Risk of lymphoma in patients receiving antitumor necrosis factor therapy: a meta-analysis of published randomized controlled studies. Clin. Rheumatol. 31, 631–636 (2012).
Mariette, X. et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann. Rheum. Dis. 70, 1895–1904 (2011).
Ramiro, S. et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 73, 529–535 (2014).
Adler, S. et al. Protective effect of A/H1N1 vaccination in immune-mediated disease—a prospectively controlled vaccination study. Rheumatology (Oxford) 51, 695–700 (2012).
Bingham, C. O. 3rd. et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 62, 64–74 (2010).
Tay, L., Leon, F., Vratsanos, G., Raymond, R. & Corbo, M. Vaccination response to tetanus toxoid and 23-valent pneumococcal vaccines following administration of a single dose of abatacept: a randomized, open-label, parallel group study in healthy subjects. Arthritis Res. Ther. 9, R38 (2007).
Eisenberg, R. A. et al. Rituximab-treated patients have a poor response to influenza vaccination. J. Clin. Immunol. 33, 388–396 (2013).
van Assen, S. et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 62, 75–81 (2010).
Mori, S. et al. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann. Rheum. Dis. 71, 2006–2010 (2012).
Ribeiro, A. C. et al. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res. (Hoboken) 65, 476–80 (2013).
Schiff, M., Saewert, M. & Bahrt, K. Response to influenza vaccine in rheumatoid arthritis patients with an inadequate response to anti-TNF therapy treated with abatacept in the ARRIVE trial. Arthritis Rheum. 56, S392 (2007).
Elkayam, O. et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin. Arthritis Rheum. 39, 442–447 (2010).
Yri, O. E. et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood 118, 6769–6771 (2011).
Kapetanovic, M. C. et al. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum. 63, 3723–3732 (2011).
Kapetanovic, M. C. et al. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology (Oxford) 45, 106–111 (2006).
Gelinck, L. B. et al. Synergistic immunosuppressive effect of anti-TNF combined with methotrexate on antibody responses to the 23 valent pneumococcal polysaccharide vaccine. Vaccine 26, 3528–3533 (2008).
Bijl, M. et al. Vaccination of patients with auto-immune inflammatory rheumatic diseases requires careful benefit-risk assessment. Autoimmun. Rev. 11, 572–576 (2012).
Winthrop, K. L. & Furst, D. E. Rheumatoid arthritis and herpes zoster: risk and prevention in those treated with anti-tumour necrosis factor therapy. Ann. Rheum. Dis. 69, 1735–1737 (2010).
Simons, F. E. et al. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 12, 389–399 (2012).
Keating, G. M. Shingles (herpes zoster) vaccine (zostavax®): a review of its use in the prevention of herpes zoster and postherpetic neuralgia in adults aged >50 years. Drugs 73, 1227–1244 (2013).
Pollak, A. W. & McBane, R. D. 2nd. Succinct review of the new VTE prevention and management guidelines. Mayo Clin. Proc. 89, 394–408 (2014).
Boyman, O. et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199, 731–736 (2004).
Mease, P. J. et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 356, 385–390 (2000).
Chaudhari, U. et al. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 357, 1842–1847 (2001).
Bouchaud, G. et al. Epidermal IL-15Rα acts as an endogenous antagonist of psoriasiform inflammation in mouse and man. J. Exp. Med. 210, 2105–2117 (2013).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202, 135–143 (2005).
Sancho-Serra Mdel, C., Simarro, M. & Castells, M. Rapid IgE desensitization is antigen specific and impairs early and late mast cell responses targeting FcεRI internalization. Eur. J. Immunol. 41, 1004–1013 (2011).
Hong, D. I., Bankova, L., Cahill, K. N., Kyin, T. & Castells, M. C. Allergy to monoclonal antibodies: cutting-edge desensitization methods for cutting-edge therapies. Expert Rev. Clin. Immunol. 8, 43–52 (2012).
Quercia, O., Emiliani, F., Foschi, F. G. & Stefanini, G. F. Adalimumab desensitization after anaphylactic reaction. Ann. Allergy Asthma Immunol. 106, 547–548 (2011).
Rodriguez-Jiménez, B. et al. Successful adalimumab desensitization after generalized urticaria and rhinitis. J. Investig. Allergol. Clin. Immunol. 19, 246–247 (2009).
Acknowledgements
O.B. is funded by Swiss National Science Foundation grants PP00P3-128421 and PP00P3-150751, Stiftung für wissenschaftliche Forschung from the University of Zurich, National Psoriasis Foundation, and a Novartis Foundation grant. D.C. is funded by a SICPA Foundation grant.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching the data for the article, provided a substantial contribution to discussions of the content, and contributed to writing the article and to review and/or editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Box 1
Screening and prophylaxis of latent TB (DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Boyman, O., Comte, D. & Spertini, F. Adverse reactions to biologic agents and their medical management. Nat Rev Rheumatol 10, 612–627 (2014). https://doi.org/10.1038/nrrheum.2014.123
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.123
This article is cited by
-
Assessment of safety profile of secukinumab in real-world scenario using United States food and drug administration adverse event reporting system database
Scientific Reports (2024)
-
TNFα inhibitor biosimilars associated with alopecia areata. Case-based review
Rheumatology International (2022)
-
Non-immediate drug hypersensitivity reactions secondary to intravitreal anti-vascular endothelial growth factors
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Adverse Reactions to Biologic Medications Used in Allergy and Immunology Diseases
Current Allergy and Asthma Reports (2022)
-
Incidence of infection other than tuberculosis in patients with autoimmune rheumatic diseases treated with bDMARDs: a real-time clinical experience from India
Rheumatology International (2019)