Abstract
Rheumatoid arthritis, RA, is a chronic autoimmune disease characterized by joint pain, tenderness, swelling, and stiffness. This disease affects nearly 1% of the world population. RA predominates in females and typically develops between the ages of 30 and 50 years. Common therapeutics for the treatment of RA include immune system suppressants such as tumor necrosis factor, or TNF, inhibitors. There is growing concern related to multiple clinical cases reporting an unexpected onset of psoriasis following the use of TNF inhibitors. This adverse event is counterintuitive since some tumor necrosis factor inhibitors are indicated for the treatment of plaque psoriasis. In this study, we analyzed over 880 thousand postmarketing safety reports from patients being treated for RA with a single therapeutic and provided evidence for a statistically significant association of psoriasis adverse events with TNF inhibitor use as compared to methotrexate. Additionally, we quantified the reported odds ratios and their 95% confidence intervals between four individual TNF inhibitors and found that the degree of association with psoriasis was variable among the drugs studied, with certolizumab pegol exhibiting the highest reported risk.
Similar content being viewed by others
Introduction
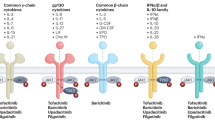
Rheumatoid arthritis (RA) is a common chronic autoimmune disorder primarily attacking the joints, affecting between 0.5 and 1% of the world population1. RA is characterized by joint pain, tenderness, swelling, and joint stiffness2. While the cause of RA is not certain, risk factors include genetics, smoking, obesity, sex, and age2. Similar to other autoimmune diseases, RA predominantly affects females, with a 2:1 to 3:1 female to male ratio1. While RA can develop at any age, typical onset occurs between 30 and 50 years3. RA is commonly treated with immunosuppressants such as methotrexate, hydroxychloroquine, sulfasalazine, or leflunomide, T-cell costimulatory inhibitors such as abatacept, interleukin-6 receptor (IL-6R) antibodies such as tocilizumab, Janus kinase (JAK) inhibitors, and tumor necrosis factor (TNF) inhibitors such as certolizumab pegol, adalimumab, golimumab, infliximab, and etanercept4.
T-cell costimulatory inhibitors prevent the activation of T-cells, which are believed to play a key role in the pathogenesis of RA. Abatacept, containing an extracellular domain of CTLA-4, accomplishes this by competitively binding the proteins CD80 and CD86, blocking their interaction with CD285. Another important class of therapeutics in treating RA are IL-6 pathway inhibitors, which either inhibit IL-6 directly or block its receptor IL-6R6. Another class of therapeutics, JAK inhibitors, suppress cytokine-mediated signals by the JAK-STAT pathway7.
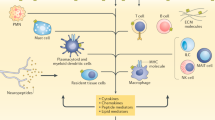
Anti-TNF treatments suppress the inflammatory response which results from the binding of TNF to its receptors8. Recently, there has been growing concern over TNF inhibitor-induced psoriasis, with many case reports detailing the onset of psoriasis in patients using TNF inhibitors, such as certolizumab pegol9,10,11. This relationship is seemingly paradoxical given that four of the five TNF inhibitors are indicated as a treatment for a type of psoriasis, plaque psoriasis: certolizumab pegol12, adalimumab13, infliximab14, and etanercept, which is a fusion of the TNF receptor and the IgG1-Fc part of an antibody15.
There have been postmarketing reports of incidents of new and worsening psoriasis in patients administered any of the four of the aforementioned TNF inhibitors12,13,14,15. These effects may be related to the cross-regulation of TNF and type-I interferons, including interferon alpha (IFN-α)16. TNF downregulates IRF7 and NF-κB pathways, which are responsible for IFN-α and TNF-α production in plasmacytoid dendritic cells17. Blocking the activity of TNF allows the production of IFN-α to increase10. Increased IFN-α has been associated with an increased risk of psoriasis18, possibly explaining the association between the use of TNF inhibitors and an increased risk of psoriasis. In this study, we analyzed over 880 thousand RA reports from FDA Adverse Event Reporting System (FAERS) and quantifed the evidence using the latest population scale dataset for a statistically significant association of psoriasis with TNF inhibitor use. Furthermore, we quantified the difference in the reported risk of developing psoriasis in RA patients between the TNF inhibitor cohorts.
Methods
FDA adverse event reporting system (FAERS)
The United States Food and Drug Administration Adverse Event Reporting System (FAERS) is a data repository which collects voluntary drug-related reports from healthcare professionals, consumers, and legal representatives. In cases where the adverse event (AE) is reported to the manufacturer, the manufacturer is required to forward the report to FAERS. At the time of the study, FAERS contained 17,392,666 AE reports collected from the first quarter of 2004 (which included historical reports since 1982) to the second quarter of 2022. The reports are available online: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files.
Data preparation
FAERS reports are added and stored quarterly in a set of text files. Subsets of data are organized by specific report fields (demographics, drug, AE, outcome, etc.) and their respective case identifiers. The data format is not uniform and has been modified several times since its inception. Therefore, appropriate modifications have been introduced. Additionally, as the AE reports are collected from all over the world, the respective drug brand names are translated into the generic equivalents19,20,21.
Study outcomes
The MedDRA dictionary version 25.1 was searched to define the measured study outcomes by higher level terms such as “immune associated conditions not elsewhere classified (NEC)” and “psoriatic conditions”. All of the psoriasis-associated preferred terms (PT) were used in the query. In order to avoid any indication-related confounding effects, psoriatic conditions associated with RA, such as psoriatic arthropathy, were excluded from the MedDRA PT list. The following PTs were used to define psoriasis in the analysis: erythrodermic psoriasis, guttate psoriasis, nail psoriasis, dermatitis psoriasiform, pustular psoriasis, and psoriasis.
Cohort selection
Out of the total of 17,392,666 AE reports in FAERS, there were a total of 881,182 RA indication-containing reports, and for 663,922 of them, RA was listed as the only indication. Those records were further split by monotherapies and only reports by physicians, pharmacists and other healthcare professionals were included to avoid bias and increase clinical relevance. The final monoindication + monotherapy sets were the following: certolizumab pegol (n = 5168), adalimumab (n = 9221), golimumab (n = 2899), tocilizumab (n = 4819), abatacept (n = 7574), infliximab (n = 5579), rituximab (n = 2519), etanercept (n = 89543), tofacitinib (n = 10686), and methotrexate (n = 6142). Demographic analysis was performed for TNF inhibitors and methotrexate RA AE cohorts (Tables 1 and 2). The following psoriasis terms were included: erythrodermic psoriasis, guttate psoriasis, nail psoriasis, dermatitis psoriasiform, pustular psoriasis, and psoriasis. With these psoriasis type terms, the psoriasis AE report numbers were calculated for each drug cohort: certolizumab pegol (n = 98), adalimumab (n = 107), golimumab (n = 20), tocilizumab (n = 29), abatacept (n = 40), infliximab (n = 29), rituximab (n = 11), etanercept (n = 260), tofacitinib (n = 24), and methotrexate (n = 7). Disproportionality analysis was performed using reported AE frequencies to calculate reporting odds ratios (RORs). These numbers were used to calculate psoriasis reported frequencies. Methotrexate was selected as the control cohort due to its unique mechanism of action (MOA) as an immunosuppressant which inhibits the conversion of folic acid to folate cofactors, and common use as a monotherapy in RA.
Demographic analysis
Sex (Table 1).
Age (Table 2).
Statistical analysis
Descriptive statistics
Frequencies for each studied side effect (Figs. 1, 3) was calculated by the equation:
Frequency error:
Comparative statistics
Psoriasis report rates were compared via the Reporting Odds Ratio (ROR) analysis for Figs. 2, 4 and 5 and Tables 3, 4 and 5 using the following equations:
where Number of cases in exposed group with psoriasis, Number of cases in exposed group with no psoriasis, Number of cases in control group with psoriasis, Number of cases in control group with no psoriasis.
Standard Error of Log Reporting Odds Ratio;
95% Confidence Interval;
Results
Methotrexate-treated patient cases as a control set
Methotrexate, an immunosuppressant drug, was selected as the control RA treatment based on its unique MOA as a competitive dihydrofolate reductase inhibitor22. Methotrexate monotherapy exhibited the lowest reported frequency of psoriasis at 0.11% out of all of the monotherapies studied. Methotrexate is a first-line therapeutic for RA in a clinical setting, making it a suitable control choice.
TNF inhibitor-induced psoriasis
The reported frequencies of psoriasis AEs were calculated for each TNF inhibitor monotherapy. The frequencies of psoriasis reports between the TNF inhibitors ranged from 0.29 to 1.90%: certolizumab pegol 1.90%, adalimumab 1.16%, golimumab 0.69%, infliximab 0.52%, and etanercept 0.29% (Fig. 1).
The statistical significance of psoriasis association between the TNF inhibitor monotherapy cohorts and methotrexate cohort was calculated at 95% CI level: certolizumab pegol ROR 16.94 and 95% CI [7.86, 36.50], adalimumab 10.29 [4.79, 22.12], golimumab 6.09 [2.57, 14.42], infliximab 4.56 [1.99, 10.41], and etanercept 2.55 [1.20, 5.41]. The combined cohort of TNF inhibitors had an ROR 4.03 with a 95% CI of [1.91, 8.49] (Table 3, Fig. 2). The RORs and their 95% CIs demonstrate the risk of induced psoriasis in RA patients using TNF inhibitor monotherapies.
Reporting odds ratios were calculated by comparing the reported numbers of psoriasis reports and total reports between each TNF inhibitor monotherapy and the methotrexate cohort. The combined TNF inhibitors cohort exhibited a significant ROR of 4.03 with a 95% CI between 1.91 and 8.49. The ranges represent 95% CIs. The x-axis is shown in logarithmic scale.
Induced psoriasis using non-TNF inhibitor monotherapies
To further expand on the evidence of RA therapeutic-associated psoriasis, we quantified the frequencies of psoriasis reports for other RA monotherapy cohorts: tocilizumab 0.60%, abatacept 0.53%, rituximab 0.44%, tofacitinib 0.22%, and methotrexate 0.11% (Fig. 3). All of the non-TNF inhibitor monotherapy cohorts, except tofacitinib, were significantly associated with psoriasis when compared to methotrexate: tocilizumab ROR 5.31 and 95% CI [2.32–12.12], abatacept 4.63 [2.07–10.34], rituximab 3.83 [1.48–9.88], and tofacitinib 1.97 [0.85–4.58] (Table 4, Fig. 4). The combined non-TNF inhibitor therapeutic reports, when compared to the methotrexate cohort, had ROR 3.58 and 95% CI of [1.66–7.69]. The RORs and 95% CIs of the non-TNF inhibitor cohorts demonstrate the statistically significant risk of developing psoriasis for RA patients using any of the studied monotherapy cohorts, except tofacitinib.
Tofacitinib was the only monotherapy cohort studied with a 95% CI that was not statistically significant (the 95% CI range overlapped with the ROR value of 1.00), whereas all of the other monotherapy cohorts had 95% CIs that were greater than 1.00 in both the lower and upper bounds.
The small number of psoriasis reports in the methotrexate-treated patient cases allowed for overlapping 95% CIs between the individual TNF inhibitors. In order to clarify the difference between the TNF inhibitors, we derived the ROR + 95% CI values by doing a pairwise comparison without methotrexate as a common control.
Certolizumab pegol had a greater and statistically significant association with psoriasis AEs when compared directly with other RA drugs used as a control cohort instead of methotrexate. Certolizumab pegol compared to adalimumab yielded ROR 1.65 and 95% CI [1.25, 2.17], compared to golimumab 2.78 [1.72, 4.51], compared to tocilizumab 3.19 [2.11, 4.84], compared to abatacept 3.64 [2.52, 5.27], compared to infliximab 3.70 [2.44, 5.61], compared to rituximab 4.41 [2.36, 8.23], compared to etanercept 6.64 [5.25, 8.39], compared to tofacitinib 8.59 [5.49, 13.44], and compared to methotrexate 16.94 [7.86, 36.50] (Table 5, Fig. 5). It is noteworthy that the lower bound of the 95% CI range is greater than 1.00 for comparisons of certolizumab pegol with all of the other monotherapy cohorts.
Reporting odds ratios were calculated for the association between certolizumab pegol and psoriasis by comparing the reported numbers of psoriasis reports and total reports between the certolizumab pegol cohort and each other RA monotherapy cohort. The ranges represent 95% CIs. The x-axis is shown in logarithmic scale.
Discussion
In summary, we arrived at three sets of conclusions: (i) all TNF inhibitors have a statistically significant association with psoriasis in RA treated patients compared to the single methotrexate control group, (ii) not all of the non-TNF inhibitor treatments were associated with psoriasis compared to the single methotrexate control group, with some overlapping with the TNF inhibitor 95% CI ranges, (iii) pairwise comparisons of certolizumab pegol with both other TNF inhibitors and non-TNF inhibitor therapeutics show a greater and statistically significant association with psoriasis.
Thus, certolizumab pegol was found to have a particularly high reported risk of psoriasis AE when compared to all the other RA monotherapy cohorts studied.
To our knowledge, this is the first study to analyze over 660 thousand AE reports for RA indication in postmarketing data to quantify the association between TNF inhibitor monotherapy use and psoriasis. In addition, we quantified the risk of psoriasis in RA patients undergoing other types of therapeutic treatments such as IL-6 inhibitors (tocilizumab) and JAK inhibitors (tofacitinib), among others. We discovered that all TNF inhibitor treatments have a statistically significant association with psoriasis in RA patients. We also discovered that all of the non-TNF inhibitor cohorts, except tofacitinib, were statistically significantly associated with psoriasis, though to a lesser extent than certolizumab pegol. Tofacitinib was the only non-TNF monotherapy that was not statistically significantly associated with psoriasis within the 95% CI.
While using methotrexate as a single control for all of the monotherapies was convenient, there was an overlap of their 95% CI ranges, making it difficult to differentiate between the propensity of developing psoriasis for particular pairs of drugs. To address this issue, pairwise ROR + 95% CI analyses were performed. These results revealed that certolizumab pegol exhibited the highest reported risk, with 95% CI statistically separating it from all of the other studied monotherapies (Table 5, Fig. 5).
Establishment of psoriasis AE dependencies on TNF inhibitor treatment
Given that RA and plaque psoriasis are both treated by many of the same TNF inhibitor therapeutics12,13,14,15, there may be co-occurrence of RA with psoriasis, independent of the treatment. However, this study excluded all non-RA indications during report selection to minimize any confounding effects. The large variability of reported psoriasis AEs between different RA monotherapy cohorts, ranging from certolizumab pegol 1.90% to methotrexate 0.11%, indicates that psoriasis AEs are treatment-dependent. These differences are further confirmed by more rigorous statistical comparison using 95% CIs between pairs of treatments. All of the TNF inhibitor monotherapy cohorts exhibited a significant association with psoriasis AEs, supporting the establishment of an association between TNF inhibitor therapeutic use and induced psoriasis.
Uniquely high ROR of psoriasis AEs with certolizumab pegol compared to all other treatments
Of the monotherapies studied, certolizumab pegol exhibited the highest reported frequency and ROR of psoriasis AEs when compared to the methotrexate cohort or any other treatment. Even when certolizumab pegol was compared to the second-highest psoriasis-risk associated RA drug, certolizumab pegol demonstrated a higher reported risk of psoriasis by 25% to 117% (Table 5). The higher frequency and ROR of certolizumab pegol may be due to the higher initial dosage12 recommended for certolizumab pegol compared to the other TNF inhibitors studied. In addition, certolizumab pegol is unique among the TNF inhibitors studied in that it is PEGylated, which is associated with a lower elimination rate12. A lower elimination rate, in addition to its higher initial dosage, may help to explain its higher risk of psoriasis AEs, even among the TNF inhibitor cohorts, given that TNF inhibition is correlated with psoriasis23. To determine whether more TNF inhibition may be associated with a higher frequency of psoriasis AEs, the nanomoles per recommended RA initial dose (nmols/(initial dose)) were calculated for each TNF inhibitor monotherapy cohort and compared with the respective cohort’s frequency of psoriasis reports (Table 6, Fig. 6).
The statistically significant associations of TNF inhibitors with psoriasis AEs were in line with current understanding of TNF inhibitors’ risk of psoriasis, as stated in the FDA package inserts of four of the five TNF inhibitors: certolizumab pegol12, adalimumab13, infliximab14, and etanercept15. However, given that golimumab’s package insert lacks a warning for psoriasis24, we underscore its statistically significant association with psoriasis as an adverse event.
Here we established and quantified an association of the use of TNF inhibitors, as well as the non-TNF inhibitors studied with the exception of tofacitinib, with the risk of psoriasis AEs.
Conclusion
We established and quantified an association of TNF inhibitor monotherapies use by RA patients with psoriasis as an AE. All of the TNF inhibitor therapies approved for the treatment of RA and three out of the four non-TNF inhibitor drugs studied had a statistically significant association with psoriasis when compared to methotrexate. Out of the TNF inhibitors studied, certolizumab pegol exhibited the highest reported frequency of psoriasis.
Limitations of this study
Due to the voluntary reporting nature of the FAERS system, the sample of reports in the FAERS database may be incomplete compared to real population data. FAERS reporting exhibits biases related to its newsworthiness and legal and scientific variables. Due to potential underreporting, some real-world data may be missing from the FAERS database which this study used to conduct its analyses. The changing criteria for AE diagnosis may lead to under or overreporting and affect the reporting frequencies. The frequencies and reporting odds ratios calculated are based on the sample of reports in the FAERS database and may not correspond to real population frequencies25,26. The individual cases were not adjudicated for causality assessment. Further studies may be required to establish the molecular mechanism behind this counterintuitive adverse event to better understand the uses and risks of TNF inhibitor therapies as it relates to RA and psoriasis. Most individual reports do not contain details on the progression of the AEs over time.
Data availability
The data sets available online to the public are de-identified. Institutional Review Board requirements do not apply under 45 CFR 46.102. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-latest-quarterly-data-files. Both FAERS and AERS datasets are de-identified and are made available online at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm. Institutional Review Board Requirements do not apply under 45 CFR 46.102. There was no direct human participation in the study. All experiments were performed in accordance with relevant guidelines and regulations.
References
Firestein, G. S., Budd, R., Gabriel, S. E., McInnes, I. B. & O’Dell, J. R. Kelley’s Textbook of Rheumatology E-Book: Expert Consult Premium Edition: Enhanced Online Features (Elsevier, 2014).
National Institute of Arthritis and Musculoskeletal and Skin Diseases. Rheumatoid Arthritis, https://www.niams.nih.gov/health-topics/rheumatoid-arthritis (2019).
Stoll, J. G. & Yasothan, U. Rheumatoid arthritis market. Nat. Rev. Drug Discov. 8, 693–694. https://doi.org/10.1038/nrd2947 (2009).
Fraenkel, L. et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 73, 924–939. https://doi.org/10.1002/acr.24596 (2021).
Blair, H. A. & Deeks, E. D. Abatacept: A review in rheumatoid arthritis. Drugs 77, 1221–1233. https://doi.org/10.1007/s40265-017-0775-4 (2017).
Scott, L. J. Tocilizumab: A review in rheumatoid arthritis. Drugs 77, 1865–1879. https://doi.org/10.1007/s40265-017-0829-7 (2017).
Kaur, K., Kalra, S. & Kaushal, S. Systematic review of tofacitinib: A new drug for the management of rheumatoid arthritis. Clin. Ther. 36, 1074–1086. https://doi.org/10.1016/j.clinthera.2014.06.018 (2014).
Lis, K., Kuzawińska, O. & Bałkowiec-Iskra, E. Tumor necrosis factor inhibitors - state of knowledge. Arch. Med. Sci. AMS 10, 1175–1185 (2014).
Melo, F. J. & Magina, S. Clinical management of Anti-TNF-alpha-induced psoriasis or psoriasiform lesions in inflammatory bowel disease patients: a systematic review. Int. J. Dermatol. 57, 1521–1532 (2018).
Fischer, R., DaCunha, M. & Rajpara, A. Certolizumab-induced guttate psoriasiform dermatitis. Dermatol. Online J. 23(1), 13030/qt0197d9gk (2017).
Li, S. J., Perez-Chada, L. M. & Merola, J. F. TNF inhibitor-induced psoriasis: Proposed algorithm for treatment and management. J. Psoriasis Psoriatic Arthritis 4, 70–80 (2019).
Food and Drug Administration. Certolizumab Pegol Label, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125160s283lbl.pdf (2018).
Food and Drug Administration. Adalimumab Label, https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125057s0276lbl.pdf (2011).
Food and Drug Administration. Infliximab Label, https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103772s5401lbl.pdf (2021).
Food and Drug Administration. Etanercept Label, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103795s5503lbl.pdf (2012).
Cantaert, T., Baeten, D., Tak, P. P. & van Baarsen, L. G. M. Type I IFN and TNFα cross-regulation in immune-mediated inflammatory disease: Basic concepts and clinical relevance. Arthritis Res. Ther. 12, 219. https://doi.org/10.1186/ar3150 (2010).
Psarras, A. et al. TNF-α regulates human plasmacytoid dendritic cells by suppressing IFN-α production and enhancing T cell activation. J. Immunol. Baltim. Md. 1950 1950(206), 785–796 (2021).
Funk, J., Langeland, T., Schrumpf, E. & Hanssen, L. E. Psoriasis induced by interferon-alpha. Br. J. Dermatol. 125, 463–465 (1991).
Cohen, I. V., Makunts, T., Abagyan, R. & Thomas, K. Concomitant drugs associated with increased mortality for MDMA users reported in a drug safety surveillance database. Sci. Rep. 11, 5997. https://doi.org/10.1038/s41598-021-85389-x (2021).
Keshishi, D., Makunts, T. & Abagyan, R. Common osteoporosis drug associated with increased rates of depression and anxiety. Sci. Rep. 11, 23956. https://doi.org/10.1038/s41598-021-03214-x (2021).
Wollmer, M. A., Makunts, T., Krüger, T. H. C. & Abagyan, R. Postmarketing safety surveillance data reveals protective effects of botulinum toxin injections against incident anxiety. Sci. Rep. 11, 24173. https://doi.org/10.1038/s41598-021-03713-x (2021).
Chan, E. S. L. & Cronstein, B. N. Methotrexate—How does it really work?. Nat. Rev. Rheumatol. 6, 175–178. https://doi.org/10.1038/nrrheum.2010.5 (2010).
Mazloom, S. E. et al. TNF-α inhibitor-induced psoriasis: A decade of experience at the Cleveland Clinic. J. Am. Acad. Dermatol. 83, 1590–1598 (2020).
Food and Drug Administration. Golimumab Label, https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125433s014lbl.pdf (2015).
Maciejewski, M. et al. Reverse translation of adverse event reports paves the way for de-risking preclinical off-targets. Elife 6, e25818. https://doi.org/10.7554/eLife.25818 (2017).
Alatawi, Y. M. & Hansen, R. A. Empirical estimation of under-reporting in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin. Drug Saf. 16, 761–767. https://doi.org/10.1080/14740338.2017.1323867 (2017).
Acknowledgements
We thank Dr. Da Shi for contributions to processing the FAERS/AERS data files and supporting the computer environment. We are also thankful to Dr. Isaac V. Cohen for helpful discussions. This work was funded in part by NIH R35GM131881.
Author information
Authors and Affiliations
Contributions
H.J. performed the experiments, R.A. and T.M. designed the study and, H.J., R.A., and T.M. drafted the manuscript and reviewed the final version. R.A. processed the data set.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joulfayan, H., Makunts, T. & Abagyan, R. Anti-TNF-α therapy induced psoriasis in rheumatoid arthritis patients according to FDA postmarketing surveillance data. Sci Rep 13, 10448 (2023). https://doi.org/10.1038/s41598-023-37010-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37010-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.