Abstract
Researchers have begun to characterize the subtle biological and cognitive processes that precede the clinical onset of Alzheimer disease (AD), and to set the stage for accelerated evaluation of experimental treatments to delay the onset, reduce the risk of, or completely prevent clinical decline. In this Review, we provide an overview of the experimental strategies, and brain imaging and cerebrospinal fluid biomarker measures that are used in early detection and tracking of AD, highlighting at-risk individuals who could be suitable for preclinical monitoring. We discuss how advances in the field have contributed to reconceptualization of AD as a sequence of biological changes that occur during progression from preclinical AD, to mild cognitive impairment and finally dementia, and we review recently proposed research criteria for preclinical AD. Advances in the study of preclinical AD have driven the recognition that efficacy of at least some AD therapies may depend on initiation of treatment before clinical manifestation of disease, leading to a new era of AD prevention research.
Key Points
-
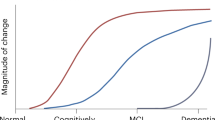
The pathogenic cascade of Alzheimer disease (AD) is thought to begin at least one to two decades prior to cognitive impairment
-
Disappointing results of several AD drugs in late-stage trials have suggested the need for early therapeutic intervention, calling for development of biomarkers and sensitive cognitive measures of preclinical disease
-
The best established measurements for detection and tracking of preclinical and clinical AD include MRI, fluorodeoxyglucose PET, amyloid PET, and cerebrospinal fluid measures of amyloid-β42, total tau, and phospho-tau
-
Studies of individuals with inherited AD can provide insights into cognitive and biomarker changes that precede clinical manifestation of AD, and are suitable candidates for ongoing monitoring and early-intervention strategies
-
We are entering an era of AD prevention research, with a number of preclinical AD treatment trials in the planning stages or under way for several at-risk, cognitively unimpaired populations
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
16 July 2013
In the version of this article initially published, the appropriate references to support the following sentence were omitted, and incorrect references were cited:"By contrast, possession of one copy of the ε4 allele, which is found in about 25% of the population and about 60% of patients with AD dementia, is associated with higher risk of late-onset AD and younger age at dementia onset, and individuals with two copies of this allele have an especially high risk of AD."The references that should have been cited are:Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921-923 (1993).Saunders, A. M. et al. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer's disease. Neurology 43, 1467-1472 (1993).The error has been corrected for the HTML and PDF versions of the article.
References
Alzheimer's Association. Alzheimer's Association 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 8, 131–168 (2012).
Corrada, M. M., Brookmeyer, R., Paganini-Hill, A., Berlau, D. & Kawas, C. H. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann. Neurol. 67, 114–121 (2010).
Brookmeyer, R. et al. National estimates of the prevalence of Alzheimer's disease in the United States. Alzheimers Dement. 7, 61–73 (2011).
Hebert, L. E., Beckett, L. A., Scherr, P. A. & Evans, D. A. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis. Assoc. Disord. 15, 169–173 (2001).
Reiman, E. M. & Langbaum, J. B. in Imaging the Aging Brain (eds Jagust, W. J. & D'Esposito, M.) 319–350 (Oxford University Press, Oxford, 2009).
Reiman, E. M., Langbaum, J. B. & Tariot, P. N. Alzheimer's Prevention Initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomark. Med. 4, 3–14 (2010).
Reiman, E. M. et al. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J. Alzheimers Dis. 26 (Suppl. 3), 321–329 (2011).
Bateman, R. J. et al. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res. Ther. 3, 1 (2011).
Aisen, P. S. et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology 76, 280–286 (2011).
Food and Drug Administration. Guidance for industry—Alzheimer's disease: developing drugs for the treatment of early stage disease. Food and Drug Administration [online], (2013).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Jack, C. R. Jr et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 9, 119–128 (2010).
Jack, C. R. Jr et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
Valla, J. et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE ε4 allele, the major late-onset Alzheimer's susceptibility gene. J. Alzheimers Dis. 22, 307–313 (2010).
Braak, H. & Del Tredici, K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 121, 171–181 (2011).
Elobeid, A., Soininen, H. & Alafuzoff, I. Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol. 123, 97–104 (2012).
Knickmeyer, R. C. et al. Common variants in psychiatric risk genes predict brain structure at birth. Cereb. Cortex. http://dx.doi.org/10.1093/cercor/bhs401.
Dubois, B. et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 9, 1118–1127 (2010).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging and Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 270–279 (2011).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 263–269 (2011).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 280–292 (2011).
Jack, C. R. Jr et al. An operational approach to National Institute on Aging—Alzheimer's Association criteria for preclinical Alzheimer disease. Ann. Neurol. 71, 765–775 (2012).
Knopman, D. S. et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 78, 1576–1582 (2012).
Reiman, E. M. & Jagust, W. J. Brain imaging in the study of Alzheimer's disease. Neuroimage 61, 505–516 (2012).
de Leon, M. J. et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann. N. Y. Acad. Sci. 1097, 114–145 (2007).
Dickerson, B. C. et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol. Aging 22, 747–754 (2001).
Johnson, K. A., Fox, N. C., Sperling, R. A. & Klunk, W. E. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006213 (2012).
Jack, C. R. Jr et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 52, 1397–1403 (1999).
Jack, C. R. Jr et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 65, 1227–1231 (2005).
Chetelat, G. et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage 27, 934–946 (2005).
McGeer, P. L. et al. Comparison of PET, MRI, and CT with pathology in a proven case of Alzheimer's disease. Neurology 36, 1569–1574 (1986).
Jack, C. R. Jr et al. Atrophy rates accelerate in amnestic mild cognitive impairment. Neurology 70, 1740–1752 (2008).
Langbaum, J. B. et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Neuroimage 45, 1107–1116 (2009).
Schwartz, W. J. et al. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science 205, 723–725 (1979).
Meguro, K. et al. Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non-human primate as shown by PET. Implications for Alzheimer's disease. Brain 122, 1519–1531 (1999).
Magistretti, P. J. & Pellerin, L. Cellular bases of brain energy metabolism and their relevance to functional brain imaging: evidence for a prominent role of astrocytes. Cereb. Cortex 6, 50–61 (1996).
Mark, R. J., Pang, Z., Geddes, J. W., Uchida, K. & Mattson, M. P. Amyloid β-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J. Neurosci. 17, 1046–1054 (1997).
Silverman, D. H. et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. J. Am. Med. Assoc. 286, 2120–2127 (2001).
Klunk, W. E. et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 55, 306–319 (2004).
Weiner, M. W. et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 8, S1–S68 (2012).
Clark, C. M. et al. Use of florbetapir-PET for imaging β-amyloid pathology. J. Am. Med. Assoc. 305, 275–283 (2011).
Clark, C. M. et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11, 669–678 (2012).
Buckner, R. L. & Vincent, J. L. Unrest at rest: default activity and spontaneous network correlations. Neuroimage 37, 1091–1096 (2007).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001).
Pihlajamaki, M., DePeau, K. M., Blacker, D. & Sperling, R. A. Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. Am. J. Geriatr. Psychiatry 16, 283–292 (2008).
Sorg, C. et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA 104, 18760–18765 (2007).
Buckner, R. L. et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 25, 7709–7717 (2005).
Andrews-Hanna, J. R. et al. Disruption of large-scale brain systems in advanced aging. Neuron 56, 924–935 (2007).
Lustig, C. et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc. Natl Acad. Sci. USA 100, 14504–14509 (2003).
Rombouts, S. A., Barkhof, F., Goekoop, R., Stam, C. J. & Scheltens, P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum. Brain Mapp. 26, 231–239 (2005).
Sperling, R. A. et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63, 178–188 (2009).
Hedden, T. et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 29, 12686–12694 (2009).
Drzezga, A. et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 134, 1635–1646 (2011).
Greicius, M. D., Supekar, K., Menon, V. & Dougherty, R. F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 19, 72–78 (2009).
Vlassenko, A. G. et al. Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ) deposition. Proc. Natl Acad. Sci. USA 107, 17763–17767 (2010).
Holtzman, D. M. CSF biomarkers for Alzheimer's disease: current utility and potential future use. Neurobiol. Aging 32 (Suppl. 1), S4–S9 (2011).
Thal, L. J. et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis. Assoc. Disord. 20, 6–15 (2006).
Fagan, A. M. et al. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 64, 343–349 (2007).
Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N. Engl. J. Med. 367, 795–804 (2012).
Fagan, A. M. et al. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann. Neurol. 65, 176–183 (2009).
Fleisher, A. S. et al. Florbetapir PET analysis of amyloid-β deposition in presenilin 1 E280A autosomal-dominant Alzheimer's disease kindred: a cross-sectional study. Lancet Neurol. 11, 1057–1065 (2012).
Sunderland, T. et al. Decreased β-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 289, 2094–2103 (2003).
Fagan, A. M. et al. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 64, 343–349 (2007).
Sunderland, T. et al. Longitudinal stability of CSF tau levels in Alzheimer patients. Biol. Psychiatry 46, 750–755 (1999).
Reiman, E. M. et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the ε4 allele for apolipoprotein E. N. Engl. J. Med. 334, 752–758 (1996).
Beacher, F. et al. Brain anatomy and ageing in non-demented adults with Down's syndrome: an in vivo MRI study. Psychol. Med. 40, 611–619 (2010).
Jack, C. R. Jr et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 65, 1227–1231 (2005).
Morris, J. C. et al. Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch. Neurol. 66, 1469–1475 (2009).
Rowe, C. C. et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging 31, 1275–1283 (2010).
Roses, A. D. et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer's disease. Pharmacogenomics J. 10, 375–384 (2010).
Naj, A. C. et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat. Genet. 43, 436–441 (2011).
Hollingworth, P. et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat. Genet. 43, 429–435 (2011).
Guerreiro, R. et al. TREM2 variants in Alzheimer's disease. N. Engl. J. Med. 368, 117–127 (2013).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N. Engl. J. Med. 368, 107–116 (2013).
Espeseth, T. et al. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E ε4. Neurobiol. Aging 29, 329–340 (2008).
Wishart, H. A. et al. Regional brain atrophy in cognitively intact adults with a single APOE ε4 allele. Neurology 67, 1221–1224 (2006).
Chen, K. et al. Correlations between apolipoprotein E ε4 gene dose and whole brain atrophy rates. Am. J. Psychiatry 164, 916–921 (2007).
Reiman, E. M. et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the ε4 allele for apolipoprotein E. N. Engl. J. Med. 334, 752–758 (1996).
Small, G. W. et al. Early detection of Alzheimer's disease by combining apolipoprotein E and neuroimaging. Ann. N. Y. Acad. Sci. 802, 70–78 (1996).
de Leon, M. J. et al. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET). Proc. Natl Acad. Sci. USA 98, 10966–10971 (2001).
Langbaum, J. B. et al. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E ε4 allele. Arch. Neurol. 67, 462–468 (2010).
Small, G. W. et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA 97, 6037–6042 (2000).
Lo, R. Y. et al. Longitudinal change of biomarkers in cognitive decline. Arch. Neurol. 68, 1257–1266 (2011).
Reiman, E. M. et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc. Natl Acad. Sci. USA 101, 284–289 (2004).
Reiman, E. M. et al. Declining brain activity in cognitively normal apolipoprotein E ε4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc. Natl Acad. Sci. USA 98, 3334–3339 (2001).
Reiman, E. M. et al. Correlations between apolipoprotein E ε4 gene dose and brain-imaging measurements of regional hypometabolism. Proc. Natl Acad. Sci. USA 102, 8299–8302 (2005).
Protas, H. D. et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle age persons at three levels of genetic risk for Alzheimer's disease. JAMA Neurol. 70, 320–325 (2013).
Cohen, A. D. et al. Basal cerebral metabolism may modulate the cognitive effects of Aβ in mild cognitive impairment: an example of brain reserve. J. Neurosci. 29, 14770–14778 (2009).
Haier, R. J. et al. Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology 61, 1673–1679 (2003).
Oh, H., Habeck, C., Madison, C. & Jagust, W. Covarying alterations in Aβ deposition, glucose metabolism, and gray matter volume in cognitively normal elderly. Hum. Brain Mapp. http://dx.doi.org/10.1002/hbm.22173.
Persson, J. et al. Altered deactivation in individuals with genetic risk for Alzheimer's disease. Neuropsychologia 46, 1679–1687 (2008).
Fleisher, A. S. et al. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage 47, 1678–1690 (2009).
Filippini, N. et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl Acad. Sci. USA 106, 7209–7214 (2009).
Reiman, E. M. et al. Fibrillar amyloid-β burden in cognitively normal people at three levels of genetic risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA 106, 6820–6825 (2009).
Pike, K. E. et al. Cognition and β-amyloid in preclinical Alzheimer's disease: data from the AIBL study. Neuropsychologia 49, 2384–2390 (2011).
Villemagne, V. L. et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann. Neurol. 69, 181–192 (2011).
Mielke, M. M. et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 79, 1570–1577 (2012).
Fleisher, A. S. et al. Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol. Aging 34, 1–12 (2013).
Morris, J. C. et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 67, 122–131 (2010).
Kantarci, K. et al. APOE modifies the association between Aβ load and cognition in cognitively normal older adults. Neurology 78, 232–240 (2012).
Lim, Y. Y. et al. Aβ amyloid, cognition, and APOE genotype in healthy older adults. Alzheimers Dement. http://dx.doi.org/10.1016/j.jalz.2012.07.004.
Peskind, E. R. et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid β-amyloid 42 in adults with normal cognition. Arch. Neurol. 63, 936–939 (2006).
Popp, J. et al. Cerebrospinal fluid markers for Alzheimer's disease over the lifespan: effects of age and the APOE ε4 genotype. J. Alzheimers Dis. 22, 459–468 (2010).
Kester, M. I. et al. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology 73, 1353–1358 (2009).
Fagan, A. M. et al. Differences in the Aβ40/Aβ42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann. Neurol. 48, 201–210 (2000).
Glodzik-Sobanska, L. et al. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer's disease. Neurobiol. Aging 30, 672–681 (2009).
Mosconi, L. et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol. Psychiatry 63, 609–618 (2008).
Ihle, A., Bunce, D. & Kliegel, M. APOE ε4 and cognitive function in early life: a meta-analysis. Neuropsychology 26, 267–277 (2012).
Baxter, L. C., Caselli, R. J., Johnson, S. C., Reiman, E. & Osborne, D. Apolipoprotein E ε4 affects new learning in cognitively normal individuals at risk for Alzheimer's disease. Neurobiol. Aging 24, 947–952 (2003).
Caselli, R. J. et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE ε4 allele. Neurology 62, 1990–1995 (2004).
Lind, J. et al. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E ε4: relation to chronological age and recognition memory. Neurosci. Lett. 396, 23–27 (2006).
Caselli, R. J. et al. Cognitive domain decline in healthy apolipoprotein E ε4 homozygotes before the diagnosis of mild cognitive impairment. Arch. Neurol. 64, 1306–1311 (2007).
Caselli, R. J. et al. Longitudinal modeling of age-related memory decline and the APOE ε4 effect. N. Engl. J. Med. 361, 255–263 (2009).
Caselli, R. J. et al. Longitudinal modeling of frontal cognition in APOE ε4 homozygotes, heterozygotes, and noncarriers. Neurology 76, 1383–1388 (2011).
Human Genome Variation Society. Alzheimer's Disease and Frontotemporal Dementia Mutation Database [online], (2013).
Campion, D. et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 65, 664–670 (1999).
Cirrito, J. R. et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-β deposition in an Alzheimer disease mouse model. J. Clin. Invest. 115, 3285–3290 (2005).
Castellano, J. M. et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3, 89ra57 (2011).
Fukumoto, H., Cheung, B. S., Hyman, B. T. & Irizarry, M. C. β-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 59, 1381–1389 (2002).
Godbolt, A. K. et al. Sporadic and familial dementia with ubiquitin-positive tau-negative inclusions: clinical features of one histopathological abnormality underlying frontotemporal lobar degeneration. Arch. Neurol. 62, 1097–1101 (2005).
Lleo, A., Berezovska, O., Growdon, J. H. & Hyman, B. T. Clinical, pathological, and biochemical spectrum of Alzheimer disease associated with PS-1 mutations. Am. J. Geriatr. Psychiatry 12, 146–156 (2004).
Quiroz, Y. et al. Cortical signature of Alzheimer's disease-related thinning in presymptomatic presenilin-1 mutation carriers. Alzheimers Dement. 7, S220 (2011).
Reiman, E. M. et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 11, 1048–1056 (2012).
Fox, N. C., Warrington, E. K., Stevens, J. M. & Rossor, M. N. Atrophy of the hippocampal formation in early familial Alzheimer's disease. A longitudinal MRI study of at-risk members of a family with an amyloid precursor protein 717Val-Gly mutation. Ann. N. Y. Acad. Sci. 777, 226–232 (1996).
Fox, N. C. et al. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain 119, 2001–2007 (1996).
Kennedy, A. M. et al. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer's disease. Neurosci. Lett. 186, 17–20 (1995).
Mosconi, L. et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J. Nucl. Med. 47, 1778–1786 (2006).
Schöll, M. et al. Glucose metabolism and PIB binding in carriers of a His163Tyr presenilin 1 mutation. Neurobiol. Aging 32, 1388–1399 (2011).
Klunk, W. E. et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J. Neurosci. 27, 6174–6184 (2007).
Villemagne, V. L. et al. High striatal amyloid β-peptide deposition across different autosomal Alzheimer disease mutation types. Arch. Neurol. 66, 1537–1544 (2009).
Ringman, J. M. et al. Cerebrospinal fluid biomarkers and proximity to diagnosis in preclinical familial Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 33, 1–5 (2012).
Parra, M. A. et al. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain 133, 2702–2713 (2010).
Arango-Lasprilla, J. C., Cuetos, F., Valencia, C., Uribe, C. & Lopera, F. Cognitive changes in the preclinical phase of familial Alzheimer's disease. J. Clin. Exp. Neuropsychol. 29, 892–900 (2007).
Newman, S. K., Warrington, E. K., Kennedy, A. M. & Rossor, M. N. The earliest cognitive change in a person with familial Alzheimer's disease: presymptomatic neuropsychological features in a pedigree with familial Alzheimer's disease confirmed at necropsy. J. Neurol. Neurosurg. Psychiatry 57, 967–972 (1994).
Ringman, J. M. et al. Neuropsychological function in nondemented carriers of presenilin-1 mutations. Neurology 65, 552–558 (2005).
Acosta-Baena, N. et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer's disease: a retrospective cohort study. Lancet Neurol. 10, 213–220 (2011).
Pike, K. E. et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain 130, 2837–2844 (2007).
Johnson, K. A. et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. http://dx.doi.org/10.1016/j.jalz.2012.10.007.
Mintun, M. A. et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67, 446–452 (2006).
Vlassenko, A. G. et al. Amyloid-β plaque growth in cognitively normal adults: longitudinal [11C]Pittsburgh compound B data. Ann. Neurol. 70, 857–861 (2011).
Sperling, R. A. et al. Amyloid deposition detected with florbetapir F 18 (18F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol. Aging 34, 822–831 (2013).
Aizenstein, H. J. et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 65, 1509–1517 (2008).
Rentz, D. M. et al. Cognition, reserve, and amyloid deposition in normal aging. Ann. Neurol. 67, 353–364 (2010).
Resnick, S. M. et al. Longitudinal cognitive decline is associated with fibrillar amyloid- β measured by [11C]PiB. Neurology 74, 807–815 (2010).
Storandt, M., Mintun, M. A., Head, D. & Morris, J. C. Cognitive decline and brain volume loss as signatures of cerebral amyloid-β peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Aβ deposition. Arch. Neurol. 66, 1476–1481 (2009).
Ellis, K. A. et al. Decline in cognitive function over 18 months in healthy older adults with high amyloid-β. J. Alzheimers Dis. 34, 861–871 (2013).
Lim, Y. Y. et al. Rapid decline in episodic memory in healthy older adults with high amyloid-β. J. Alzheimers Dis. 33, 675–679 (2013).
Elias, M. F. et al. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch. Neurol. 57, 808–813 (2000).
Saxton, J. et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology 63, 2341–2347 (2004).
Wilson, R. S., Leurgans, S. E., Boyle, P. A. & Bennett, D. A. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch. Neurol. 68, 351–356 (2011).
Sperling, R., Donohue, M. & Aisen, P. The A4 trial: anti-amyloid treatment of asymptomatic Alzheimer's disease. Alzheimers Dement. 8, 425–426 (2012).
Langbaum, J. B. et al. Composite cognitive endpoints with improved power to detect presymptomatic Alzheimer's disease treatment effects in APOE4 carriers: findings from the Alzheimer's prevention initiative. Alzheimers Dement. 7, S502 (2011).
Ayutyanont, N. et al. Composite cognitive endpoints with improved power to detect presymptomatic Alzheimer's disease treatment effects: findings in the Colombian kindred with the E280A Presenilin 1 mutation and the Alzheimer's Prevention Initiative. Alzheimers Dement. 7, S608 (2011).
Mosconi, L. et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology 63, 2332–2340 (2004).
Drzezga, A. et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur. J. Nucl. Med. Mol. Imaging 30, 1104–1113 (2003).
de Leon, M. J. et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol. Aging 27, 394–401 (2006).
Jack, C. R. Jr et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 132, 1355–1365 (2009).
Dickerson, B. C. et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 56, 27–35 (2004).
Celone, K. A. et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J. Neurosci. 26, 10222–10231 (2006).
Palop, J. J. et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron 55, 697–711 (2007).
Jagust, W. J. & Mormino, E. C. Lifespan brain activity, β-amyloid, and Alzheimer's disease. Trends Cogn. Sci. 15, 520–526 (2011).
Klunk, W. E., Mathis, C. A., Price, J. C., Lopresti, B. J. & DeKosky, S. T. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain 129, 2805–2807 (2006).
Li, G. et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology 69, 631–639 (2007).
Forsberg, A. et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol. Aging 29, 1456–1465 (2008).
Mattsson, N. et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393 (2009).
Visser, P. J. et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 8, 619–627 (2009).
Wolk, D. A. et al. Amyloid imaging in mild cognitive impairment subtypes. Ann. Neurol. 65, 557–568 (2009).
Vemuri, P. et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology 73, 287–293 (2009).
Vemuri, P. et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology 73, 294–301 (2009).
van Rossum, I. A. et al. Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology 79, 1809–1816 (2012).
Prestia, A. et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology 80, 1048–1056 (2013).
Roe, C. M. et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 80, 1784–1791 (2013).
Andrews, K. A. et al. Atrophy rates in asymptomatic amyloidosis: implications for Alzheimer prevention trials. PLoS ONE 8, e58816 (2013).
Fleisher, A. S. et al. Using positron emission tomography and florbetapir F 18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch. Neurol. 68, 1404–1411 (2011).
Fox, N. C. et al. Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64, 1563–1572 (2005).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 (1993).
Saunders, A. M. et al. Association of apolipoprotein E allele ε4 with late‐onset familial and sporadic Alzheimer's disease. Neurology 43, 1467–1472 (1993).
Acknowledgements
This article was supported by grants from the National Institute on Aging (R01AG031581 and P30AG19610 to E. M. Reiman, and RF1AG041705 to E. M. Reiman, P. N. Tariot and F. Lopera), the National Institute of Neurological Disorders and Stroke (F31-NS078786 to Y. T. Quiroz), Colciencias (1115-493-26133, 1115-545-31651 and 1115-519-29028 to F. Lopera), the Banner Alzheimer's Foundation, and the state of Arizona. The authors acknowledge research support from the Geoffrey Benne Gives Back Alzheimer's Initiative (to J. B. Langbaum), the Anonymous Foundation (to E. M. Reiman) and the Nomis Foundations (to P. N. Tariot, F. Lopera and E. M. Reiman). We thank H. Protas for her assistance in creating the figures prior to submission, and N. Fox, C. Rowe, M. Weiner and their colleagues for permission to use their images in Figure 2. We thank our valued research participants for their invaluable dedication and inspiration.
Author information
Authors and Affiliations
Contributions
J. B. Langbaum, K. Chen, N. Ayutyanont, F. Lopera, Y. T. Quiroz, R. J. Caselli and E. M. Reiman researched data for the article. A. S. Fleisher, P. N. Tariot and E. M. Reiman made substantial contributions to discussion of the content. J. B. Langbaum, P. N. Tariot and E. M. Reiman wrote the article. J. B. Langbaum, A. S. Fleisher, P. N. Tariot and E. M. Reiman contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. B. Langbaum has received consulting fees from Janssen Alzheimer Immunotherapy. A. S. Fleisher has received consulting fees from Eli Lilly, Avid, Merck, Grifols, Quintiles; has been an invited speaker for Siemens, Quintiles, Avid; has Data and Safety Monitoring Board membership with Merck, Pfizer; has received grant funding from Lilly; has been involved in studies sponsored by Merck, Roche, Genentech, Pfizer, Avanir, Takeda, Lilly, BMS, Baxter, Neuroptix, Wyeth. Y. T. Quiroz serves as a consultant to Medavante. P. N. Tariot has received consulting fees from Abbott Laboratories, AC Immune, Adamas, Boehringer-Ingelheim, California Pacific Medical Center, Chase Pharmaceuticals, Chiesi, CME, Eisai, Elan, Medavante, Merz, Otsuka, Sanofi-Aventis; has received consulting fees and research support from AstraZeneca, Avanir, Avid, Bristol Myers Squibb, Cognoptix, Genentech, GlaxoSmithKline, Janssen, Eli Lilly, Medivation, Merck and Company, Pfizer, Roche; has received research support only from Baxter Healthcare Corp., Functional Neuromodulation, GE Healthcare, Medavante, Targacept, Toyama; has stock options in Adamas; and is listed as a contributor to a patent owned by the University of Rochester, 'Biomarkers of Alzheimer's Disease.' E. M. Reiman has been a Scientific Advisor for AstraZeneca, Baxter, Bayer, Eisai, Elan, Eli Lilly, GlaxoSmithKline, Intellect, Novartis, Siemens, Takeda; has research contracts with Avid/Eli Lilly and Genentech Research Grants; and has a patent pending for a biomarker strategy for the evaluation of presymptomatic AD treatments (through Banner Health). All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Langbaum, J., Fleisher, A., Chen, K. et al. Ushering in the study and treatment of preclinical Alzheimer disease. Nat Rev Neurol 9, 371–381 (2013). https://doi.org/10.1038/nrneurol.2013.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2013.107
This article is cited by
-
Cognitive training and brain stimulation in patients with cognitive impairment: a randomized controlled trial
Alzheimer's Research & Therapy (2024)
-
Brain structural alterations and clinical features of cognitive frailty in Japanese community-dwelling older adults: the Arao study (JPSC-AD)
Scientific Reports (2022)
-
Computing Univariate Neurodegenerative Biomarkers with Volumetric Optimal Transportation: A Pilot Study
Neuroinformatics (2020)
-
A novel kit for early diagnosis of Alzheimer’s disease using a fluorescent nanoparticle imaging
Scientific Reports (2019)
-
Neuroimaging of Alzheimer’s disease: focus on amyloid and tau PET
Japanese Journal of Radiology (2019)