Abstract
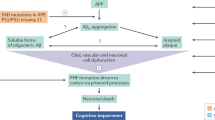
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with a complex pathogenesis. Senile plaques composed of the amyloid-β (Aβ) peptide in the brain are the core hallmarks of AD and a promising target for the development of disease-modifying therapies. However, over the past 20 years, the failures of clinical trials directed at Aβ clearance have fueled a debate as to whether Aβ is the principal pathogenic factor in AD and a valid therapeutic target. The success of the recent phase 3 trials of lecanemab (Clarity AD) and donanemab (Trailblazer Alz2), and lessons from previous Aβ clearance trials provide critical evidence to support the role of Aβ in AD pathogenesis and suggest that targeting Aβ clearance is heading in the right direction for AD treatment. Here, we analyze key questions relating to the efficacy of Aβ targeting therapies, and provide perspectives on early intervention, adequate Aβ removal, sufficient treatment period, and combinatory therapeutics, which may be required to achieve the best cognitive benefits in future trials in the real world.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66.
Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–5.
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–9.
Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol Psychiatry. 2021;26:5481–503.
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608.
Beyreuther K, Masters CL. Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease: precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1991;1:241–51.
Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–98.
Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharm Sci. 1991;12:383–8.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5.
Kumar M, Cohen D, Eisdorfer C. Serum IgG brain reactive antibodies in Alzheimer disease and Down syndrome. Alzheimer Dis Assoc Disord. 1988;2:50–55.
Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc Natl Acad Sci USA. 1996;93:452–5.
Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci USA. 1997;94:4109–12.
Schenk D. Hopes remain for an Alzheimer’s vaccine. Nature. 2004;431:398.
Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54.
Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, Holtzman DM. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25:6213–20.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl J Med. 2014;370:322–33.
Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2017;9:95.
Ostrowitzki S, Bittner T, Sink KM, Mackey H, Rabe C, Honig LS, et al. Evaluating the Safety and Efficacy of Crenezumab vs Placebo in Adults With Early Alzheimer Disease: Two Phase 3 Randomized Placebo-Controlled Trials. JAMA Neurol. 2022;79:1113–21.
Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer’s Dement. 2021;17:696–701.
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease. N. Engl J Med. 2014;370:311–21.
Kepp KP, Robakis NK, Høilund-Carlsen PF, Sensi SL, Vissel B. The amyloid cascade hypothesis: an updated critical review. Brain. 2023;146:3969–90.
Alexander GC, Knopman DS, Emerson SS, Ovbiagele B, Kryscio RJ, Perlmutter JS, et al. Revisiting FDA Approval of Aducanumab. N. Engl J Med. 2021;385:769–71.
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in Early Alzheimer’s Disease. N. Engl J Med. 2023;388:9–21.
Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023;330:512–27.
Rosenberg RN, Lambracht-Washington D, Yu G, Xia W. Genomics of Alzheimer Disease: A Review. JAMA Neurol. 2016;73:867–74.
Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive. Nature. 2012;488:96–99.
Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–18.
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl J Med. 2012;367:795–804.
Wang HF, Shen XN, Li JQ, Suckling J, Tan CC, Wang YJ, et al. Clinical and biomarker trajectories in sporadic Alzheimer’s disease: A longitudinal study. Alzheimers Dement. 2020;12:e12095.
Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–57.
CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS REVIEW(S). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761178Orig1s000ClinPharm_Redacted.pdf, 2021, Accessed Date Accessed 2021.
Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ. Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol. 2012;8:465–9.
Wang X. A Bridge Between the Innate Immunity System and Amyloid-β Production in Alzheimer’s Disease. Neurosci Bull. 2021;37:898–901.
Bertram L, Tanzi RE. Alzheimer disease risk genes: 29 and counting. Nat Rev Neurol. 2019;15:191–2.
Sarlus H, Heneka MT. Microglia in Alzheimer’s disease. J Clin Investig. 2017;127:3240–9.
Franco-Bocanegra DK, McAuley C, Nicoll JAR, Boche D. Molecular Mechanisms of Microglial Motility: Changes in Ageing and Alzheimer’s Disease. Cells. 2019;8:639.
Chen X, Holtzman DM. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity. 2022;55:2236–54.
Fan DY, Wang YJ. Early Intervention in Alzheimer’s Disease: How Early is Early Enough? Neurosci Bull. 2020;36:195–7.
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. 2021;397:1577–90.
Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl J Med. 2018;378:321–30.
Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimer’s Res Ther. 2021;13:80.
Rafii MS, Sperling RA, Donohue MC, Zhou J, Roberts C, Irizarry MC, et al. The AHEAD 3-45 Study: Design of a prevention trial for Alzheimer’s disease. Alzheimer’s Dement. 2023;19:1227–33.
Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69:203–13.
De Strooper B, Karran E. The Cellular Phase of Alzheimer’s Disease. Cell. 2016;164:603–15.
Golde TE. Disease-Modifying Therapies for Alzheimer’s Disease: More Questions than Answers. NeuroTherapeutics. 2022;19:209–27.
Izuo N, Murakami K, Sato M, Iwasaki M, Izumi Y, Shimizu T, et al. Non-toxic conformer of amyloid β may suppress amyloid β-induced toxicity in rat primary neurons: implications for a novel therapeutic strategy for Alzheimer’s disease. Biochem Biophys Res Commun. 2013;438:1–5.
Ladiwala AR, Litt J, Kane RS, Aucoin DS, Smith SO, Ranjan S, et al. Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity. J Biol Chem. 2012;287:24765–73.
Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat Med. 1998;4:827–31.
Zhao Y, Sivaji S, Chiang MC, Ali H, Zukowski M, Ali S, et al. Amyloid Beta Peptides Block New Synapse Assembly by Nogo Receptor-Mediated Inhibition of T-Type Calcium Channels. Neuron. 2017;96:355–72.e356.
Zott B, Simon MM, Hong W, Unger F, Chen-Engerer HJ, Frosch MP, et al. A vicious cycle of β amyloid-dependent neuronal hyperactivation. Science. 2019;365:559–65.
Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73–88.
Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21:306–18.
Meilandt WJ, Maloney JA, Imperio J, Lalehzadeh G, Earr T, Crowell S, et al. Characterization of the selective in vitro and in vivo binding properties of crenezumab to oligomeric Aβ. Alzheimers Res Ther. 2019;11:97.
Salloway S, Farlow M, McDade E, Clifford DB, Wang G, Llibre-Guerra JJ, et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat Med. 2021;27:1187–96.
Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res Ther. 2021;13:80.
Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimer’s Dis. 2012;28:49–69.
Arndt JW, Qian F, Smith BA, Quan C, Kilambi KP, Bush MW, et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci Rep. 2018;8:6412.
Alexander GC, Emerson S, Kesselheim AS. Evaluation of Aducanumab for Alzheimer Disease: Scientific Evidence and Regulatory Review Involving Efficacy, Safety, and Futility. JAMA. 2021;325:1717–8.
Klein G, Delmar P, Voyle N, Rehal S, Hofmann C, Abi-Saab D, et al. Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer’s disease: a PET substudy interim analysis. Alzheimers Res Ther. 2019;11:101.
Söderberg L, Johannesson M, Nygren P, Laudon H, Eriksson F, Osswald G, et al. Lecanemab, Aducanumab, and Gantenerumab - Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics. 2023;20:195–206.
Tahami Monfared AA, Tafazzoli A, Ye W, Chavan A, Zhang Q. Long-Term Health Outcomes of Lecanemab in Patients with Early Alzheimer’s Disease Using Simulation Modeling. Neurol Ther. 2022;11:863–80.
Gantenerumab Falls Short in Phase 3. https://www.alzforum.org/news/research-news/gantenerumab-falls-short-phase-3-0, 2022, Accessed Date Accessed 2022 Accessed.
Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78.
La Joie R, Ayakta N, Seeley WW, Borys E, Boxer AL, DeCarli C, et al. Multisite study of the relationships between antemortem [(11)C]PIB-PET Centiloid values and postmortem measures of Alzheimer’s disease neuropathology. Alzheimer’s Dement. 2019;15:205–16.
Amadoru S, Doré V, McLean CA, Hinton F, Shepherd CE, Halliday GM, et al. Comparison of amyloid PET measured in Centiloid units with neuropathological findings in Alzheimer’s disease. Alzheimers Res Ther. 2020;12:22.
Knopman DS, Lundt ES, Therneau TM, Albertson SM, Gunter JL, Senjem ML, et al. Association of Initial β-Amyloid Levels With Subsequent Flortaucipir Positron Emission Tomography Changes in Persons Without Cognitive Impairment. JAMA Neurol. 2021;78:217–28.
Long JM, Holtzman DM. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell. 2019;179:312–39.
Delor I, Charoin JE, Gieschke R, Retout S, Jacqmin P. Modeling Alzheimer’s Disease Progression Using Disease Onset Time and Disease Trajectory Concepts Applied to CDR-SOB Scores From ADNI. CPT: Pharmacomet Syst Pharmacol. 2013;2:e78.
Could Benefit of Plaque Removal Grow in Time? https://www.alzforum.org/news/conference-coverage/could-benefit-plaque-removal-grow-time, 2022, Accessed Date Accessed 2022 Accessed.
Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimer’s Dement. 2019;5:354–63.
Liu KY, Schneider LS, Howard R. The need to show minimum clinically important differences in Alzheimer’s disease trials. lancet Psychiatry. 2021;8:1013–6.
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–81.
Liu F, Sun J, Wang X, Jin S, Sun F, Wang T, et al. Focal-type, but not Diffuse-type, Amyloid Beta Plaques are Correlated with Alzheimer’s Neuropathology, Cognitive Dysfunction, and Neuroinflammation in the Human Hippocampus. Neurosci Bull. 2022;38:1125–38.
Consortium AB, Jia YJ, Wang J, Ren JR, Chan P, Chen S, et al. A framework of biomarkers for brain aging: a consensus statement by the Aging Biomarker Consortium. Life Med. 2023;2:lnad017.
Sato C, Barthélemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018;97:1284–98.e1287.
Ying Y, Wang JZ. Illuminating Neural Circuits in Alzheimer’s Disease. Neurosci Bull. 2021;37:1203–17.
Lu MH, Zhao XY, Yao PP, Xu DE, Ma QH. The Mitochondrion: A Potential Therapeutic Target for Alzheimer’s Disease. Neurosci Bull. 2018;34:1127–30.
Hou X, Zhang X, Zou H, Guan M, Fu C, Wang W, et al. Differential and substrate-specific inhibition of γ-secretase by the C-terminal region of ApoE2, ApoE3, and ApoE4. Neuron. 2023;111:1898–913.e1895.
Tsai RM, Miller Z, Koestler M, Rojas JC, Ljubenkov PA, Rosen HJ, et al. Reactions to Multiple Ascending Doses of the Microtubule Stabilizer TPI-287 in Patients With Alzheimer Disease, Progressive Supranuclear Palsy, and Corticobasal Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020;77:215–24.
Vaz M, Silvestre S. Alzheimer’s disease: Recent treatment strategies. Eur J Pharmacol. 2020;887:173554.
Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73–88.
Busche MA, Grienberger C, Keskin AD, Song B, Neumann U, Staufenbiel M, et al. Decreased amyloid-β and increased neuronal hyperactivity by immunotherapy in Alzheimer’s models. Nat Neurosci. 2015;18:1725–7.
Liu J, van Beusekom H, Bu XL, Chen G, Henrique Rosado de Castro P, Chen X, et al. Preserving cognitive function in patients with Alzheimer’s disease: The Alzheimer’s disease neuroprotection research initiative (ADNRI). Neuroprotection. 2023.in press.
Bu XL, Jiao SS, Lian Y, Wang YJ. Perspectives on the Tertiary Prevention Strategy for Alzheimer’s Disease. Curr Alzheimer Res. 2016;13:307–16.
Zhang Q, Xie C. Apolipoprotein E Drives Early Blood-Brain Barrier Damage in Alzheimer’s Disease. Neurosci Bull. 2021;37:281–3.
Tolar M, Abushakra S, Hey JA, Porsteinsson A, Sabbagh M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res Ther. 2020;12:95.
Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022;79:13–21.
Gueorguieva I, Willis BA, Chua L, Chow K, Ernest CS, Shcherbinin S, et al. Donanemab Population Pharmacokinetics, Amyloid Plaque Reduction, and Safety in Participants with Alzheimer’s Disease. Clin Pharmacol Therap. 2023;113:1258–67.
Winkler DT, Biedermann L, Tolnay M, Allegrini PR, Staufenbiel M, Wiessner C, et al. Thrombolysis induces cerebral hemorrhage in a mouse model of cerebral amyloid angiopathy. Ann Neurol. 2002;51:790–3.
Alves F, Kalinowski P, Ayton S. Accelerated Brain Volume Loss Caused by Anti-β-Amyloid Drugs: A Systematic Review and Meta-analysis. Neurology. 2023;100:e2114–24.
Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid-Related Imaging Abnormalities and β-Amyloid-Targeting Antibodies: A Systematic Review. JAMA Neurol. 2022;79:291–304.
Safety and Amyloid Plaque Reduction Effects of Remternetug in Patients with Alzheimer’s Disease: Interim Analysis from a Phase 1 Study. https://assets.ctfassets.net/mpejy6umgthp/51Sv0wOrFxfiHJNqcyuNYu/0c5df804e8a23256262fcb40489ae181/REMIPT3_ADPD2023_JIN_SAFETY_PLAQUE_REDUCTION_Ph1LAKB.pdf, 2023, Accessed Date Accessed 2023 Accessed.
Vandenberghe R, Rinne JO, Boada M, Katayama S, Scheltens P, Vellas B, et al. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res Ther. 2016;8:18.
Sperling RA, Donohue MC, Raman R, Rafii MS, Johnson K, Masters CL, et al. Trial of Solanezumab in Preclinical Alzheimer’s Disease. N Engl J Med. 2023;389:1096–107.
Acknowledgements
This study is supported by Natural Science Foundation of China (Grant No. 82120108010 and 81930028).
Author information
Authors and Affiliations
Contributions
All authors contributed to conceptualisation, drafting, review and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
YJW received research funding from the Natural Science Foundation of China (Grant No. 82120108010 and 81930028). The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lian, Y., Jia, YJ., Wong, J. et al. Clarity on the blazing trail: clearing the way for amyloid-removing therapies for Alzheimer’s disease. Mol Psychiatry (2023). https://doi.org/10.1038/s41380-023-02324-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-023-02324-4