Key Points
-
Post-transplantation diabetes mellitus (PTDM) is a frequent complication among renal transplant recipients and is associated with a reduced life expectancy
-
Pretransplantation risk factors for PTDM include family history of diabetes mellitus, increasing age, obesity, a sedentary lifestyle, and impaired glucose tolerance before transplantation
-
Diagnosis of PTDM should be made on the basis of fasting glucose levels, supplemented with an oral glucose tolerance test if needed; the glycated haemoglobin test is not a well validated diagnostic tool
-
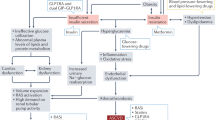
Drugs used to treat PTDM should not interact with immunosuppressive agents, elicit weight gain or hypoglycaemic events, or reduce glomerular filtration rate
-
Short-term use of the DPP-4 inhibitors sitagliptin and vildagliptin, as well as glinides have been shown to be safe and effective in small clinical trials
-
The use of GLP-1 analogues, SGLT2 inhibitors, and metformin at GFR <60 ml/min/1.73 m2 needs to be explored among patients with PTDM
Abstract
Post-transplantation diabetes mellitus (PTDM), also known as new-onset diabetes mellitus (NODM), occurs in 10–15% of renal transplant recipients and is associated with cardiovascular disease and reduced lifespan. In the majority of cases, PTDM is characterized by β-cell dysfunction, as well as reduced insulin sensitivity in liver, muscle and adipose tissue. Glucose-lowering therapy must be compatible with immunosuppressant agents, reduced glomerular filtration rate (GFR) and severe arteriosclerosis. Such therapy should not place the patient at risk by inducing hypoglycaemic episodes or exacerbating renal function owing to adverse gastrointestinal effects with hypovolaemia. First-generation and second-generation sulphonylureas are generally avoided, and caution is currently advocated for the use of metformin in patients with GFR <60 ml/min/1.73 m2. DPP-4 inhibitors do not interact with immunosuppressant drugs and have demonstrated safety in small clinical trials. Other therapeutic options include glinides and glitazones. Evidence-based treatment regimens used in patients with type 2 diabetes mellitus cannot be directly implemented in patients with PTDM. Studies investigating the latest drugs are required to direct the development of improved treatment strategies for patients with PTDM. This Review outlines the modern principles of glucose-lowering treatment in PTDM with specific reference to renal transplant recipients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Davidson, J. et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 75, SS3–SS24 (2003).
Wilkinson, A. et al. Guidelines for the treatment and management of new-onset diabetes after transplantation. Clin. Transplant. 19, 291–298 (2005).
Sharif, A. et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am. J. Transplant. 14, 1992–2000 (2014).
Cosio, F. G. et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 67, 2415–2421 (2005).
Kasiske, B. L., Snyder, J. J., Gilbertson, D. & Matas, A. J. Diabetes mellitus after kidney transplantation in the United States. Am. J. Transplant. 3, 178–185 (2003).
Valderhaug, T. G. et al. The association of early post-transplant glucose levels with long-term mortality. Diabetologia 54, 1341–1349 (2011).
Hecking, M. et al. Glucose metabolism after renal transplantation. Diabetes Care 36, 2763–2771 (2013).
Hjelmesaeth, J. et al. The impact of impaired insulin release and insulin resistance on glucose intolerance after renal transplantation. Clin. Transplant. 16, 389–396 (2002).
Vincenti, F. et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am. J. Transplant. 7, 1506–1514 (2007).
Chakkera, H. A., Weil, E. J., Pham, P. T., Pomeroy, J. & Knowler, W. C. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care 36, 1406–1412 (2013).
Hjelmesaeth, J., Midtvedt, K., Jenssen, T. & Hartmann, A. Insulin resistance after renal transplantation: impact of immunosuppressive and antihypertensive therapy. Diabetes Care 24, 2121–2126 (2001).
Hornum, M. et al. New-onset diabetes mellitus after kidney transplantation in Denmark. Clin. J. Am. Soc. Nephrol. 5, 709–716 (2010).
Voytovich, M. H. et al. Short-term treatment with rosiglitazone improves glucose tolerance, insulin sensitivity and endothelial function in renal transplant recipients. Nephrol. Dial. Transplant. 20, 413–418 (2005).
Werzowa, J. et al. Vildagliptin and pioglitazone in patients with impaired glucose tolerance after kidney transplantation: a randomized, placebo-controlled clinical trial. Transplantation 95, 456–462 (2013).
Delaunay, F. et al. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J. Clin. Invest. 100, 2094–2098 (1997).
Andrews, R. C. & Walker, B. R. Glucocorticoids and insulin resistance: old hormones, new targets. Clin. Sci. (Lond.) 96, 513–523 (1999).
Hjelmesaeth, J. et al. The impact of short-term ciclosporin A treatment on insulin secretion and insulin sensitivity in man. Nephrol. Dial. Transplant. 22, 1743–1749 (2007).
Jenssen, T. & Hartmann, A. Prevention and management of transplant-associated diabetes. Expert. Opin. Pharmacother. 12, 2641–2655 (2011).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine 15, 539–553 (1998).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 34, S62–S69 (2011).
Eide, I. A. et al. Limitations of hemoglobin A1c for the diagnosis of posttransplant diabetes mellitus. Transplantation 99, 629–635 (2014).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35, 1364–1379 (2012).
Cole, E. H., Johnston, O., Rose, C. L. & Gill, J. S. Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin. J. Am. Soc. Nephrol. 3, 814–821 (2008).
Hjelmesaeth, J. et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 69, 588–595 (2006).
Wolfe, R. A. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 341, 1725–1730 (1999).
Miller, L. W. Cardiovascular toxicities of immunosuppressive agents. Am. J. Transplant. 2, 807–818 (2002).
[No authors listed] Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis of diagnostic criteria in Europe. Lancet 354, 617–621 (1999).
Valderhaug, T. G. et al. Fasting plasma glucose and glycosylated hemoglobin in the screening for diabetes mellitus after renal transplantation. Transplantation 88, 429–434 (2009).
Valderhaug, T. G. et al. Early posttransplantation hyperglycemia in kidney transplant recipients is associated with overall long-term graft losses. Transplantation 94, 714–720 (2012).
Dinneen, S., Gerich, J. & Rizza, R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 327, 707–713 (1992).
Grodsky, G. M. A new phase of insulin secretion. How will it contribute to our understanding of beta-cell function? Diabetes 38, 673–678 (1989).
Pimenta, W. et al. Pancreatic β-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA 273, 1855–1861 (1995).
Weyer, C., Bogardus, C., Mott, D. M. & Pratley, R. E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 104, 787–794 (1999).
van Haeften, T. W. et al. Relative contributions of beta-cell function and tissue insulin sensitivity to fasting and postglucose-load glycemia. Metabolism 49, 1318–1325 (2000).
Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Strom Halden, T. A., Asberg, A., Vik, K., Hartmann, A. & Jenssen, T. Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol. Dial. Transplant. 29, 926–933 (2014).
Ferrannini, E. et al. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J. Clin. Endocrinol. Metab. 90, 493–500 (2005).
Gerich, J. E. et al. Contribution of impaired muscle glucose clearance to reduced postabsorptive systemic glucose clearance in NIDDM. Diabetes 39, 211–216 (1990).
Kahn, S. E. et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42, 1663–1672 (1993).
Gerich, J. E. Physiology of glucose homeostasis. Diabetes Obes. Metab. 2, 345–350 (2000).
Muller, W. A., Faloona, G. R., Aguilar-Parada, E. & Unger, R. H. Abnormal α-cell function in diabetes. Response to carbohydrate and protein ingestion. N. Engl. J. Med. 283, 109–115 (1970).
Consoli, A., Nurjhan, N., Capani, F. & Gerich, J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes 38, 550–557 (1989).
Yki-Jarvinen, H. Acute and chronic effects of hyperglycaemia on glucose metabolism: implications for the development of new therapies. Diabet. Med. 14 (Suppl. 3), S32–S37 (1997).
Segaert, M. F., De, S. C., Vandewiele, I. & Verbanck, J. Drug-interaction-induced rhabdomyolysis. Nephrol. Dial. Transplant. 11, 1846–1847 (1996).
Maggio, T. G. & Bartels, D. W. Increased cyclosporine blood concentrations due to verapamil administration. Drug Intell. Clin. Pharm. 22, 705–707 (1988).
Drugs.com [Internet]. Mycophenolate mofetil drug interactions [online], (2015).
Arora, S., Tangirala, B., Osadchuk, L. & Sureshkumar, K. K. Belatacept: a new biological agent for maintenance immunosuppression in kidney transplantation. Expert Opin. Biol. Ther. 12, 965–979 (2012).
Su, V. C., Harrison, J., Rogers, C. & Ensom, M. H. Belatacept: a new biologic and its role in kidney transplantation. Ann. Pharmacother. 46, 57–67 (2012).
Gerich, J. E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet. Med. 27, 136–142 (2010).
Meyer, C., Dostou, J. M. & Gerich, J. E. Role of the human kidney in glucose counterregulation. Diabetes 48, 943–948 (1999).
Bruderer, S. G. et al. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes. Metab. (2014).
Koch, M., Gradaus, F., Schoebel, F. C., Leschke, M. & Grabensee, B. Relevance of conventional cardiovascular risk factors for the prediction of coronary artery disease in diabetic patients on renal replacement therapy. Nephrol. Dial. Transplant. 12, 1187–1191 (1997).
Frier, B. M., Schernthaner, G. & Heller, S. R. Hypoglycemia and cardiovascular risks. Diabetes Care 34 (Suppl. 2), S132–S137 (2011).
Robinson, R. T. et al. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycaemia. Diabetes 52, 1469–1474 (2003).
Koivikko, M. L. et al. Effects of controlled hypoglycaemia on cardiac repolarisation in patients with type 1 diabetes. Diabetologia 51, 426–435 (2008).
Dichtwald, S., Weinbroum, A. A., Sorkine, P., Ekstein, M. P. & Dahan, E. Metformin-associated lactic acidosis following acute kidney injury. Efficacious treatment with continuous renal replacement therapy. Diabet. Med. 29, 245–250 (2012).
Wang, C. H., Weisel, R. D., Liu, P. P., Fedak, P. W. & Verma, S. Glitazones and heart failure: critical appraisal for the clinician. Circulation 107, 1350–1354 (2003).
Sharif, A., Moore, R. & Baboolal, K. Influence of lifestyle modification in renal transplant recipients with postprandial hyperglycemia. Transplantation 85, 353–358 (2008).
Zelle, D. M. et al. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 6, 898–905 (2011).
Kim, Y. et al. Patients with persistent new-onset diabetes after transplantation have greater weight gain after kidney transplantation. J. Korean Med. Sci. 28, 1431–1434 (2013).
Tokodai, K. et al. Posttransplant increase of body mass index is associated with new-onset diabetes mellitus after kidney transplantation. Tohoku J. Exp. Med. 229, 227–232 (2013).
Jardine, A. G., Gaston, R. S., Fellstrom, B. C. & Holdaas, H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet 378, 1419–1427 (2011).
Schacke, H., Docke, W. D. & Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 96, 23–43 (2002).
Woodle, E. S. et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann. Surg. 248, 564–577 (2008).
Rostaing, L. et al. Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation 79, 807–814 (2005).
Midtvedt, K. et al. Insulin resistance after renal transplantation: the effect of steroid dose reduction and withdrawal. J. Am. Soc. Nephrol. 15, 3233–3239 (2004).
Yates, C. J., Fourlanos, S., Colman, P. G. & Cohney, S. J. Divided dosing reduces prednisolone-induced hyperglycaemia and glycaemic variability: a randomized trial after kidney transplantation. Nephrol. Dial. Transplant. 29, 698–705 (2014).
Ekberg, H. et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N. Engl. J. Med. 357, 2562–2575 (2007).
Webster, A. C., Woodroffe, R. C., Taylor, R. S., Chapman, J. R. & Craig, J. C. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ 331, 810 (2005).
Johnston, O., Rose, C. L., Webster, A. C. & Gill, J. S. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J. Am. Soc. Nephrol. 19, 1411–1418 (2008).
Flechner, S. M. et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am. J. Transplant. 11, 1633–1644 (2011).
Suszynski, T. M. et al. Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation. Am. J. Transplant. 13, 961–970 (2013).
Vanrenterghem, Y. et al. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies). Transplantation 91, 976–983 (2011).
Cusi, K., Consoli, A. & DeFronzo, R. A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab 81, 4059–4067 (1996).
Kahn, C. R., Chen, L. & Cohen, S. E. Unraveling the mechanism of action of thiazolidinediones. J. Clin. Invest 106, 1305–1307 (2000).
Hasan, F. M., Alsahli, M. & Gerich, J. E. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res. Clin. Pract. 104, 297–322 (2014).
Inzucchi, S. E. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 287, 360–372 (2002).
Thule, P. M. & Umpierrez, G. Sulfonylureas: a new look at old therapy. Curr. Diab. Rep. 14, 473 (2014).
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998).
Roumie, C. L. et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann. Intern. Med. 157, 601–610 (2012).
Mocanu, M. M. et al. Glimepiride, a novel sulfonylurea, does not abolish myocardial protection afforded by either ischemic preconditioning or diazoxide. Circulation 103, 3111–3116 (2001).
Turk, T. et al. Repaglinide in the management of new-onset diabetes mellitus after renal transplantation. Am. J. Transplant. 6, 842–846 (2006).
Voytovich, M. H. et al. Nateglinide improves postprandial hyperglycemia and insulin secretion in renal transplant recipients. Clin. Transplant. 21, 246–251 (2007).
Califf, R. M. et al. Prevention of diabetes and cardiovascular disease in patients with impaired glucose tolerance: rationale and design of the Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) Trial. Am. Heart J. 156, 623–632 (2008).
Holman, R. R. et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N. Engl. J. Med. 362, 1463–1476 (2010).
McMurray, J. J. et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N. Engl. J. Med. 362, 1477–1490 (2010).
Kolata, G. B. The phenformin ban: is the drug an imminent hazard? Science 203, 1094–1096 (1979).
Shaw, J. S., Wilmot, R. L. & Kilpatrick, E. S. Establishing pragmatic estimated GFR thresholds to guide metformin prescribing. Diabet. Med. 24, 1160–1163 (2007).
Shivaswamy, V., Bennett, R. G., Clure, C. C., Larsen, J. L. & Hamel, F. G. Metformin improves immunosuppressant induced hyperglycemia and exocrine apoptosis in rats. Transplantation 95, 280–284 (2013).
McCarty, M. F. Metformin may antagonize Lin28 and/or Lin28B activity, thereby boosting let-7 levels and antagonizing cancer progression. Med. Hypotheses 78, 262–269 (2012).
Leone, A., Di, G. E., Bruzzese, F., Avallone, A. & Budillon, A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat. Res. 159, 355–376 (2014).
[No authors listed] Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 854–865 (1998).
Boussageon, R. et al. Are concomitant treatments confounding factors in randomized controlled trials on intensive blood-glucose control in type 2 diabetes? a systematic review. BMC Med. Res. Methodol. 13, 107 (2013).
Hemmingsen, B. et al. Comparison of metformin and insulin versus insulin alone for type 2 diabetes: systematic review of randomised clinical trials with meta-analyses and trial sequential analyses. BMJ 344, e1771 (2012).
Kurian, B., Joshi, R. & Helmuth, A. Effectiveness and long-term safety of thiazolidinediones and metformin in renal transplant recipients. Endocr. Pract. 14, 979–984 (2008).
Hecking, M. et al. Novel views on new-onset diabetes after transplantation: development, prevention and treatment. Nephrol. Dial. Transplant. 28, 550–566 (2013).
Sharif, A. Should metformin be our antiglycemic agent of choice post-transplantation? Am. J. Transplant. 11, 1376–1381 (2011).
Bajaj, M. et al. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes 52, 1364–1370 (2003).
Watkins, P. B. Idiosyncratic liver injury: challenges and approaches. Toxicol. Pathol. 33, 1–5 (2005).
Nissen, S. E. & Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 356, 2457–2471 (2007).
Luther, P. & Baldwin, D., Jr Pioglitazone in the management of diabetes mellitus after transplantation. Am. J. Transplant. 4, 2135–2138 (2004).
Dormandy, J. A. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–1289 (2005).
Tan, M. H. et al. Comparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care 28, 544–550 (2005).
Howard, R. J. et al. The changing causes of graft loss and death after kidney transplantation. Transplantation 73, 1923–1928 (2002).
Ojo, A. O. et al. Chronic renal failure after transplantation of a nonrenal organ. N. Engl. J. Med. 349, 931–940 (2003).
Holst, J. J., Orskov, C., Nielsen, O. V. & Schwartz, T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 211, 169–174 (1987).
Pederson, R. A., Schubert, H. E. & Brown, J. C. The insulinotropic action of gastric inhibitory polypeptide. Can. J. Physiol. Pharmacol. 53, 217–223 (1975).
Plamboeck, A. et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1117–G1127 (2013).
Toft-Nielsen, M. B. et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 86, 3717–3723 (2001).
Nauck, M. A. et al. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 91, 301–307 (1993).
Idorn, T. et al. Postprandial responses of incretin and pancreatic hormones in non-diabetic patients with end-stage renal disease. Nephrol. Dial. Transplant. 29, 119–127 (2014).
Orskov, C., Wettergren, A. & Holst, J. J. Biological effects and metabolic rates of glucagonlike peptide-1 7–36 amide and glucagonlike peptide-1 7–37 in healthy subjects are indistinguishable. Diabetes 42, 658–661 (1993).
Haidinger, M. et al. Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantation--a randomized, double-blind, placebo-controlled trial. Am. J. Transplant. 14, 115–123 (2014).
Lane, J. T. et al. Sitagliptin therapy in kidney transplant recipients with new-onset diabetes after transplantation. Transplantation 92, e56–e57 (2011).
Gueler, I. et al. Effects of vildagliptin (Galvus(R)) therapy in patients with type 2 diabetes mellitus after heart transplantation. Drug Des. Devel. Ther. 7, 297–303 (2013).
Sanyal, D., Gupta, S. & Das, P. A retrospective study evaluating efficacy and safety of linagliptin in treatment of NODAT (in renal transplant recipients) in a real world setting. Indian J. Endocrinol. Metab. 17 (Suppl. 1), S203–S205 (2013).
Wang, X. M., Yang, Y. J. & Wu, Y. J. The emerging role of dipeptidyl peptidase-4 inhibitors in cardiovascular protection: current position and perspectives. Cardiovasc. Drugs Ther. 27, 297–307 (2013).
Scirica, B. M. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013).
White, W. B. et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335 (2013).
Nielsen, L. L., Young, A. A. & Parkes, D. G. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul. Pept. 117, 77–88 (2004).
Kim, D. et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 30, 1487–1493 (2007).
Kaakeh, Y., Kanjee, S., Boone, K. & Sutton, J. Liraglutide-induced acute kidney injury. Pharmacotherapy 32, e7–e11 (2012).
Weise, W. J., Sivanandy, M. S., Block, C. A. & Comi, R. J. Exenatide-associated ischemic renal failure. Diabetes Care 32, e22–e23 (2009).
Rahmoune, H. et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54, 3427–3434 (2005).
Mogensen, C. E. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand. J. Clin. Lab. Invest. 28, 101–109 (1971).
Nauck, M. A. et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes. Metab. 16, 1111–1120 (2014).
Yale, J. F. et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes. Metab. 16, 1016–1027 (2014).
Gilbert, R. E. Sodium-glucose linked transporter-2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int. 86, 693–700 (2013).
Kohan, D. E., Fioretto, P., Tang, W. & List, J. F. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 85, 962–971 (2014).
Hornum, M., Lindahl, J. P., von Zur-Muhlen, B., Jenssen, T. & Feldt-Rasmussen, B. Diagnosis, management and treatment of glucometabolic disorders emerging after kidney transplantation: a position statement from the Nordic Transplantation Societies. Transpl. Int. 26, 1049–1060 (2013).
Hecking, M. et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J. Am. Soc. Nephrol. 23, 739–749 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Weng, J. et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 371, 1753–1760 (2008).
Gerstein, H. C. et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 367, 319–328 (2012).
Schmitz, O., Brock, B. & Rungby, J. Amylin agonists: a novel approach in the treatment of diabetes. Diabetes 53 (Suppl. 3), S233–S238 (2004).
Ryan, G., Briscoe, T. A. & Jobe, L. Review of pramlintide as adjunctive therapy in treatment of type 1 and type 2 diabetes. Drug Des. Devel. Ther. 2, 203–214 (2009).
Matschinsky, F. M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 8, 399–416 (2009).
Bagger, J. I., Knop, F. K., Holst, J. J. & Vilsboll, T. Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes. Metab. 13, 965–971 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Hansen, M., Sonne, D. P. & Knop, F. K. Bile acid sequestrants: glucose-lowering mechanisms and efficacy in type 2 diabetes. Curr. Diab. Rep. 14, 482 (2014).
Bronden, A. et al. Sevelamer in a diabetologist's perspective: a phosphate-binding resin with glucose-lowering potential. Diabetes Obes. Metab. 17, 116–120 (2014).
Yates, C. J., Fourlanos, S., Hjelmesaeth, J., Colman, P. G. & Cohney, S. J. New-onset diabetes after kidney transplantation-changes and challenges. Am. J. Transplant. 12, 820–828 (2012).
Author information
Authors and Affiliations
Contributions
T.J. wrote the article. Both authors researched the data for the article, provided substantial contributions to discussions of its content, and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.J. has received lecture honoraria from AstraZeneca, Novartis, Sanofi, and Merck Sharp & Dohme. A.H. declares no competing interests.
Rights and permissions
About this article
Cite this article
Jenssen, T., Hartmann, A. Emerging treatments for post-transplantation diabetes mellitus. Nat Rev Nephrol 11, 465–477 (2015). https://doi.org/10.1038/nrneph.2015.59
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.59
This article is cited by
-
Glomerular proteomic profiling reveals early differences between preexisting and de novo type 2 diabetes in human renal allografts
BMC Nephrology (2023)
-
Single-nucleotide polymorphisms as important risk factors of diabetes among Middle East population
Human Genomics (2022)
-
Remnant lipoprotein cholesterol is associated with incident new onset diabetes after transplantation (NODAT) in renal transplant recipients: results of the TransplantLines Biobank and cohort Studies
Cardiovascular Diabetology (2022)
-
Alginate@TiO2 hybrid microcapsules as a reservoir of beta INS-1E cells with controlled insulin delivery
Journal of Materials Science (2020)
-
Post-Transplantation Diabetes Mellitus
Diabetes Therapy (2020)