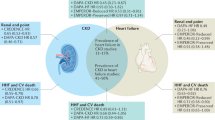
Abstract
Diabetic kidney disease (DKD), defined as co-existing diabetes and chronic kidney disease in the absence of other clear causes of kidney injury, occurs in approximately 20–40% of patients with diabetes mellitus. As the global prevalence of diabetes has increased, DKD has become highly prevalent and a leading cause of kidney failure, accelerated cardiovascular disease, premature mortality and global health care expenditure. Multiple pathophysiological mechanisms contribute to DKD, and single lifestyle or pharmacological interventions have shown limited efficacy at preserving kidney function. For nearly two decades, renin–angiotensin system inhibitors were the only available kidney-protective drugs. However, several new drug classes, including sodium glucose cotransporter-2 inhibitors, a non-steroidal mineralocorticoid antagonist and a selective endothelin receptor antagonist, have now been demonstrated to improve kidney outcomes in people with type 2 diabetes mellitus. In addition, emerging preclinical and clinical evidence of the kidney-protective effects of glucagon-like-peptide-1 receptor agonists has led to the prospective testing of these agents for DKD. Research and clinical efforts are geared towards using therapies with potentially complementary efficacy in combination to safely halt kidney disease progression. As more kidney-protective drugs become available, the outlook for people living with DKD should improve in the next few decades.
Key points
-
Diabetic kidney disease (DKD) is associated with substantial morbidity and cardiovascular mortality, and for nearly two decades, the only kidney-protective treatment for DKD was renin–angiotensin system blockade.
-
Positive kidney outcome data have now been reported for SGLT2 inhibitors, the mineralocorticoid antagonist finerenone and endothelin receptor antagonists.
-
GLP1 receptor agonists have been shown to have beneficial effects on surrogate kidney end points, and a kidney outcomes trial is underway.
-
The advent of novel kidney-protective drugs provides new therapeutic options for people living with diabetes and kidney disease but increases the complexity of treatment decisions for caregivers.
-
Combining drugs may enhance their kidney protective efficacy and could potentially reduce the risk of adverse effects that are associated with specific drug classes.
-
An important goal for the future pharmacological management of DKD is to individualize treatment with optimal drug combinations based on the underlying pathophysiology and guided by tissue or serum biomarkers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
de Boer, I. H. et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305, 2532–2539 (2011).
de Boer, I. H., Group DER. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 37, 24–30 (2014).
Afkarian, M. et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316, 602–610 (2016).
Koye, D. N., Magliano, D. J., Nelson, R. G. & Pavkov, M. E. The global epidemiology of diabetes and kidney disease. Adv. Chronic Kidney Dis. 25, 121–132 (2018).
Agrawal, L. et al. Intensive glycemic control improves long-term renal outcomes in type 2 diabetes in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 42, e181–e182 (2019).
Agrawal, L. et al. Observation on renal outcomes in the Veterans Affairs Diabetes Trial. Diabetes Care 34, 2090–2094 (2011).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Fried, L. F. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 369, 1892–1903 (2013).
Parving, H. H. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367, 2204–2213 (2012).
Mann, J. F. et al. Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 21, 527–535 (2010).
de Zeeuw, D. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 (2013).
Packham, D. K. et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 23, 123–130 (2012).
The E-KCG. et al. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 388, 117–127 (2023).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Bakris, G. L. et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 383, 2219–2229 (2020).
Heerspink, H. J. L. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 393, 1937–1947 (2019).
International Diabetes Federation. IDF Diabetes Atlas, 10th edn. https://www.diabetesatlas.org (2021).
Menke, A., Casagrande, S., Geiss, L. & Cowie, C. C. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 314, 1021–1029 (2015).
Jager, K. J. et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 96, 1048–1050 (2019).
Toppe, C. et al. Decreasing cumulative incidence of end-stage renal disease in young patients with type 1 diabetes in Sweden: a 38-year prospective nationwide study. Diabetes Care 42, 27–31 (2019).
Gregg, E. W., Hora, I. & Benoit, S. R. Resurgence in diabetes-related complications. JAMA 321, 1867–1868 (2019).
Narres, M. et al. Incidence and relative risk of renal replacement therapy in people with and without diabetes between 2002 and 2016 in a German region. Diabetologia 63, 648–658 (2020).
Koye, D. N. et al. Trends in incidence of ESKD in people with type 1 and type 2 diabetes in Australia, 2002–2013. Am. J. Kidney Dis. 73, 300–308 (2019).
Wu, H. et al. Trends in kidney failure and kidney replacement therapy in people with diabetes in Hong Kong, 2002–2015: a retrospective cohort study. Lancet Reg. Health West Pac. 11, 100165 (2021).
Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E. & Gregg, E. W. Global trends in diabetes complications: a review of current evidence. Diabetologia 62, 3–16 (2019).
Afkarian, M. et al. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 24, 302–308 (2013).
Fox, C. S. et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 380, 1662–1673 (2012).
Wen, C. P. et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 92, 388–396 (2017).
Fioretto, P. & Mauer, M. Histopathology of diabetic nephropathy. Semin. Nephrol. 27, 195–207 (2007).
Fiorentino, M. et al. Renal biopsy in patients with diabetes: a pooled meta-analysis of 48 studies. Nephrol. Dial. Transpl. 32, 97–110 (2017).
Fioretto, P. et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 39, 1569–1576 (1996).
Jin, Q. et al. Nonalbuminuric diabetic kidney disease and risk of all-cause mortality and cardiovascular and kidney outcomes in type 2 diabetes: findings from the Hong Kong Diabetes Biobank. Am. J. Kidney Dis. 80, 196–206 e1 (2022).
Nosadini, R. et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes 49, 476–484 (2000).
Fioretto, P., Steffes, M. W., Sutherland, D. E., Goetz, F. C. & Mauer, M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N. Engl. J. Med. 339, 69–75 (1998).
Scholtes, R. A. et al. Renal haemodynamic and protective effects of renoactive drugs in type 2 diabetes: Interaction with SGLT2 inhibitors. Nephrology 26, 377–390 (2021).
Scholtes, R. A. et al. Kidney hemodynamic effects of angiotensin receptor blockade, sodium-glucose cotransporter-2 inhibition alone, and their combination: a crossover randomized trial in people with type 2 diabetes. Circulation 146, 1895–1897 (2022).
Romero, C. A., Orias, M. & Weir, M. R. Novel RAAS agonists and antagonists: clinical applications and controversies. Nat. Rev. Endocrinol. 11, 242–252 (2015).
Rayego-Mateos, S. et al. Targeting inflammation to treat diabetic kidney disease: the road to 2030. Kidney Int. 103, 282–296 (2023).
Ma, T. K., Kam, K. K., Yan, B. P. & Lam, Y. Y. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br. J. Pharmacol. 160, 1273–1292 (2010).
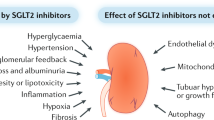
van Bommel, E. J. et al. SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin. J. Am. Soc. Nephrol. 12, 700–710 (2017).
Persson, F. et al. Efficacy and safety of dapagliflozin by baseline glycemic status: a prespecified analysis from the DAPA-CKD trial. Diabetes Care 44, 1894–1897 (2021).
van Bommel, E. J. M. et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 97, 202–212 (2020).
Cherney, D. Z. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014).
Heerspink, H. J., Perkins, B. A., Fitchett, D. H., Husain, M. & Cherney, D. Z. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134, 752–772 (2016).
Nuffield Department of Population Health Renal Studies G, Consortium SiM-AC-RT. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 400, 1788–1801 (2022).
Neuen, B. L. et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation 145, 1460–1470 (2022).
Bolignano, D., Palmer, S. C., Navaneethan, S. D. & Strippoli, G. F. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 10, CD007004 (2014).
Alexandrou, M. E. et al. Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: a systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 37, 2307–2324 (2019).
Kolkhof, P. et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J. Cardiovasc. Pharmacol. 64, 69–78 (2014).
Gerisch, M. et al. Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab. Dispos. 46, 1546–1555 (2018).
Agarwal, R. et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur. Heart J. 43, 474–484 (2022).
Agarwal, R. et al. Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J. Am. Soc. Nephrol. 33, 225–237 (2022).
Ito, S. et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin. J. Am. Soc. Nephrol. 15, 1715–1727 (2020).
Barton, M. & Yanagisawa, M. Endothelin: 30 years from discovery to therapy. Hypertension 74, 1232–1265 (2019).
Chung, E. Y. M., Badve, S. V., Heerspink, H. J. L. & Wong, M. G. Endothelin receptor antagonists in kidney protection for diabetic kidney disease and beyond? Nephrology 28, 97–108 (2023).
Weber, M. A. et al. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet 374, 1423–1431 (2009).
Muskiet, M. H. A. et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat. Rev. Nephrol. 13, 605–628 (2017).
Drucker, D. J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756 (2018).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Shaman, A. M. et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 145, 575–585 (2022).
Tuttle, K. R. et al. Post hoc analysis of SUSTAIN 6 and PIONEER 6 trials suggests that people with type 2 diabetes at high cardiovascular risk treated with semaglutide experience more stable kidney function compared with placebo. Kidney Int. 103, 772–781 (2023).
Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N. Engl. J. Med. 377, 839–848 (2017).
Mann, J. F. E. et al. Potential kidney protection with liraglutide and semaglutide: exploratory mediation analysis. Diabetes Obes. Metab. 23, 2058–2066 (2021).
Muskiet, M. H. A. et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 6, 859–869 (2018).
Alicic, R. Z., Cox, E. J., Neumiller, J. J. & Tuttle, K. R. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 17, 227–244 (2021).
Pichler, R., Afkarian, M., Dieter, B. P. & Tuttle, K. R. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Renal Physiol. 312, F716–F731 (2017).
Ronn, J., Jensen, E. P., Wewer Albrechtsen, N. J., Holst, J. J. & Sorensen, C. M. Glucagon-like peptide-1 acutely affects renal blood flow and urinary flow rate in spontaneously hypertensive rats despite significantly reduced renal expression of GLP-1 receptors. Physiol. Rep. 5, e13503 (2017).
Gutzwiller, J. P. et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 89, 3055–3061 (2004).
Muskiet, M. H. et al. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes. Metab. 18, 178–185 (2016).
Tonneijck, L. et al. Renal tubular effects of prolonged therapy with the GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes mellitus. Am. J. Physiol. Renal Physiol. 316, F231–F240 (2019).
Rossing, P. et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol. Dial. Transpl. 38, 2041–2051 (2023).
A research study to find out how semaglutide works in the kidneys compared to placebo, in people with type 2 diabetes and chronic kidney disease (the REMODEL trial) (REMODEL). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04865770 (2024).
Kalantar-Zadeh, K. & Fouque, D. Nutritional management of chronic kidney disease. N. Engl. J. Med. 377, 1765–1776 (2017).
Suckling R. J., He F. J., Macgregor G. A. Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst. Rev. 2010:CD006763.
de Boer, I. H. et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence-based advances in monitoring and treatment. Kidney Int. 98, 839–848 (2020).
Kwakernaak, A. J. et al. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trial. Lancet Diabetes Endocrinol. 2, 385–395 (2014).
Dietary sodium intake effects on ertugliflozin-induced changes in GFR, renal oxygenation and systemic hemodynamics: the DESIGN study (DESIGN). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05727579 (2023).
Yan, B., Su, X., Xu, B., Qiao, X. & Wang, L. Effect of diet protein restriction on progression of chronic kidney disease: a systematic review and meta-analysis. PLoS One 13, e0206134 (2018).
Bosch, J. P. et al. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am. J. Med. 75, 943–950 (1983).
Look ARG. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2, 801–809 (2014).
Shulman, A. et al. Incidence of end-stage renal disease following bariatric surgery in the Swedish Obese Subjects Study. Int. J. Obes. 42, 964–973 (2018).
O’Hare, A. M., Tawney, K., Bacchetti, P. & Johansen, K. L. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am. J. Kidney Dis. 41, 447–454 (2003).
Wilkinson, T. J., McAdams-DeMarco, M., Bennett, P. N. & Wilund, K., Global Renal Exercise N. Advances in exercise therapy in predialysis chronic kidney disease, hemodialysis, peritoneal dialysis, and kidney transplantation. Curr. Opin. Nephrol. Hypertens. 29, 471–479 (2020).
Zelle, D. M. et al. Physical inactivity: a risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 13, 318 (2017).
Efficacy of a high-intensity physical activity program on renal function in high risk patients with type 2 diabetes (ACTIDIANE). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03184662 (2021).
Liu, J. et al. Multi-scalar data integration links glomerular angiopoietin-tie signaling pathway activation with progression of diabetic kidney disease. Diabetes 71, 2664–2676 (2022).
Stefansson, V. T. N. et al. Molecular programs associated with glomerular hyperfiltration in early diabetic kidney disease. Kidney Int. 102, 1345–1358 (2022).
Nair, V. et al. A molecular morphometric approach to diabetic kidney disease can link structure to function and outcome. Kidney Int. 93, 439–449 (2018).
Sas, K. M. et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1, e86976 (2016).
Wilson, P. C. et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl Acad. Sci. USA 116, 19619–19625 (2019).
Wu, H. et al. Mapping the single-cell transcriptomic response of murine diabetic kidney disease to therapies. Cell Metab. 34, 1064–78 e6 (2022).
Hodgin, J. B. et al. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes 62, 299–308 (2013).
Brosius, F. C. III et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 20, 2503–2512 (2009).
Kolkhof, P. et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am. J. Nephrol. 52, 642–652 (2021).
Vergara, A. et al. Enhanced cardiorenal protective effects of combining SGLT2 inhibition, endothelin receptor antagonism and RAS blockade in type 2 diabetic mice. Int. J. Mol. Sci. 23, 12823 (2022).
Seidu, S., Kunutsor, S. K., Topsever, P. & Khunti, K. Benefits and harms of sodium-glucose co-transporter-2 inhibitors (SGLT2-I) and renin-angiotensin-aldosterone system inhibitors (RAAS-I) versus SGLT2-Is alone in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 5, e00303 (2022).
Lytvyn, Y. et al. Renal and vascular effects of combined SGLT2 and angiotensin-converting enzyme inhibition. Circulation 146, 450–462 (2022).
Phadke, G. et al. Osmotic nephrosis and acute kidney injury associated with SGLT2 inhibitor use: a case report. Am. J. Kidney Dis. 76, 144–147 (2020).
Promoting effective renoprotection in cardiac surgery patients by inhibition of SGLT-2 (MERCURI-2). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05590143 (2023).
Hesp, A. C. et al. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: a promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. 98, 579–589 (2020).
Puglisi, S. et al. Effects of SGLT2 inhibitors and GLP-1 receptor agonists on renin-angiotensin-aldosterone system. Front. Endocrinol. 12, 738848 (2021).
Jabbour, S. A. et al. Efficacy and safety over 2 years of exenatide plus dapagliflozin in the DURATION-8 study: a multicenter, double-blind, phase 3, randomized controlled trial. Diabetes Care 43, 2528–2536 (2020).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Lam, C. S. P. et al. Efpeglenatide and clinical outcomes with and without concomitant sodium-glucose cotransporter-2 inhibition use in type 2 diabetes: exploratory analysis of the AMPLITUDE-O trial. Circulation 145, 565–574 (2022).
van der Aart-van der Beek, A. B. et al. Albuminuria-lowering effect of dapagliflozin, exenatide, and their combination in patients with type 2 diabetes: a randomized cross-over clinical study. Diabetes Obes. Metab. 25, 1758–1768 (2023).
van Ruiten, C. C. et al. Effect of exenatide twice daily and dapagliflozin, alone and in combination, on markers of kidney function in obese patients with type 2 diabetes: a prespecified secondary analysis of a randomized controlled clinical trial. Diabetes Obes. Metab. 23, 1851–1858 (2021).
Gullaksen, S. et al. Separate and combined effects of semaglutide and empagliflozin on kidney oxygenation and perfusion in people with type 2 diabetes: a randomised trial. Diabetologia 66, 813–825 (2023).
Wright, A. K. et al. Primary prevention of cardiovascular and heart failure events with SGLT2 inhibitors, GLP-1 receptor agonists, and their combination in type 2 diabetes. Diabetes Care 45, 909–918 (2022).
Pitt, B. et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med. 385, 2252–2263 (2021).
Rossing, P. et al. Finerenone in patients with chronic kidney disease and type 2 diabetes by sodium-glucose cotransporter 2 inhibitor treatment: the FIDELITY analysis. Diabetes Care 45, 2991–2998 (2022).
Rossing, P. et al. Finerenone in patients across the spectrum of chronic kidney disease and type 2 diabetes by glucagon-like peptide-1 receptor agonist use. Diabetes Obes. Metab. 25, 407–416 (2023).
Provenzano, M. et al. Albuminuria-lowering effect of dapagliflozin, eplerenone, and their combination in patients with chronic kidney disease: a randomized crossover clinical trial. J. Am. Soc. Nephrol. 33, 1569–1580 (2022).
A study to learn how well the treatment combination of finerenone and empagliflozin works and how safe it is compared to each treatment alone in adult participants with long-term kidney disease (chronic kidney disease) and type 2 diabetes (CONFIDENCE). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05254002 (2024).
Heerspink, H. J. L., Kohan DE & de Zeeuw, D. New insights from SONAR indicate adding sodium glucose co-transporter 2 inhibitors to an endothelin receptor antagonist mitigates fluid retention and enhances albuminuria reduction. Kidney Int. 99, 346–349 (2021).
Zibotentan and dapagliflozin for the treatment of CKD (ZENITH-CKD Trial) (ZENITH-CKD). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04724837 (2023).
Zibotentan and dapagliflozin in patients with type 2 diabetes and elevated albuminuria (ZODIAC). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05570305 (2023).
Rosenstock, J. et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 41, 2560–2569 (2018).
Phillip, M. et al. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: pooled 52-week outcomes from the DEPICT-1 and -2 studies. Diabetes Obes. Metab. 23, 549–560 (2021).
Sands, A. T. et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care 38, 1181–1188 (2015).
Garg, S. K. et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N. Engl. J. Med. 377, 2337–2348 (2017).
Buse, J. B. et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 41, 1970–1980 (2018).
van Raalte, D. H. et al. The impact of sotagliflozin on renal function, albuminuria, blood pressure, and hematocrit in adults with type 1 diabetes. Diabetes Care 42, 1921–1929 (2019).
Cherney, D. Z. I. et al. Kidney effects of empagliflozin in people with type 1 diabetes. Clin. J. Am. Soc. Nephrol. 16, 1715–1719 (2021).
Stougaard, E. B., Rossing, P., Cherney, D., Vistisen, D. & Persson, F. Sodium-glucose cotransporter 2 inhibitors as adjunct therapy for type 1 diabetes and the benefit on cardiovascular and renal disease evaluated by Steno risk engines. J. Diabetes Complications 36, 108257 (2022).
Wheeler, D. C. et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 9, 22–31 (2021).
Semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03574597 (2024).
Semaglutide and albuminuria reduction trial in obese individuals without diabetes (SMART). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04889183 (2023).
A trial to learn how well finerenone works and how safe it is in adult participants with non-diabetic chronic kidney disease (FIND-CKD). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05047263 (2024).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45, 2753–2786 (2022).
Kidney Disease: Improving Global Outcomes Diabetes Work G. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 102, S1–S127 (2022).
Joseph, J. J. et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 145, e722–e759 (2022).
Blonde, L. et al. American Association of Clinical Endocrinology clinical practice guideline: developing a diabetes mellitus comprehensive care plan — 2022 update. Endocr. Pract. 28, 923–1049 (2022).
American Diabetes Association Professional Practice C. 11. Chronic kidney disease and risk management: standards of medical care in diabetes — 2022. Diabetes Care 45, S175–S184 (2022).
de Boer, I. H. et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 45, 3075–3090 (2022).
van der Sande, N. G. et al. Individualized prediction of the effect of angiotensin receptor blockade on renal and cardiovascular outcomes in patients with diabetic nephropathy. Diabetes Obes. Metab. 18, 1120–1127 (2016).
Tye, S. C. et al. Initiation of the SGLT2 inhibitor canagliflozin to prevent kidney and heart failure outcomes guided by HbA1c, albuminuria, and predicted risk of kidney failure. Cardiovasc. Diabetol. 21, 194 (2022).
Chertow, G. M. et al. Effects of dapagliflozin in chronic kidney disease, with and without other cardiovascular medications: DAPA-CKD trial. J. Am. Heart Assoc. 12, e028739 (2023).
Curovic, V. R. et al. Optimization of albuminuria-lowering treatment in diabetes by crossover rotation to four different drug classes: a randomized crossover trial. Diabetes Care 46, 593–601 (2023).
A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03819153 (2024).
Heerspink, H. J. L. et al. Design of FLAIR: a phase 2b study of the 5-lipoxygenase activating protein inhibitor AZD5718 in patients with proteinuric CKD. Kidney Int. Rep. 6, 2803–2810 (2021).
A phase 2b diabetic kidney disease study. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04170543 (2023).
Frimodt-Moller, M., Persson, F. & Rossing, P. Mitigating risk of aldosterone in diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 29, 145–151 (2020).
Stasch, J. P., Schlossmann, J. & Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Curr. Opin. Pharmacol. 21, 95–104 (2015).
Fantus, D., Rogers, N. M., Grahammer, F., Huber, T. B. & Thomson, A. W. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat. Rev. Nephrol. 12, 587–609 (2016).
Heerspink, H. J. L. et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 10, 774–785 (2022).
Hammoud, R. & Drucker, D. J. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat. Rev. Endocrinol. 19, 201–216 (2023).
A study of tirzepatide (LY3298176) in participants with overweight or obesity and chronic kidney disease with or without type 2 diabetes (TREASURE-CKD). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05536804 (2024).
Ruiz-Andres, O. et al. Downregulation of kidney protective factors by inflammation: role of transcription factors and epigenetic mechanisms. Am. J. Physiol. Renal Physiol. 311, F1329–F1340 (2016).
Mora-Fernandez, C. et al. Sodium-glucose co-transporter-2 inhibitors increase Klotho in patients with diabetic kidney disease: a clinical and experimental study. Biomed. Pharmacother. 154, 113677 (2022).
Acknowledgements
D.H.V.R. is supported by a senior fellowship of the Dutch Diabetes Foundation and by a PIONEER + grant of the Dutch Kidney Foundation. P.B. receives salary and research support from the NIDDK (R01 DK129211, R01 DK132399, RO1 HL165433, R21 DK129720 and UC2 DK114886), JDRF (3-SRA-2022-1097-M-B, 3-SRA-2022-1243-M-B, and 3-SRA-2022-1230-M-B), the Boettcher Foundation, the American Heart Association (20IPA35260142), the Ludeman Family Center for Women’s Health Research at the University of Colorado, the Department of Paediatrics, Section of Endocrinology, and the Barbara Davis Center for Diabetes at University of Colorado School of Medicine. I.H.D.B. is supported by NIH grants R01DK125084, R01DK132399, R01DK126373, and grant funding from JDRF. D.G. is supported by Wilhelm and Else Stockmann Foundation, Liv och Hälsa Society, Medical Society of Finland (Finska Läkaresällskapet), Sigrid Juselius Foundation, Helsinki University Hospital, University of Helsinki, Minerva Foundation Institute for Medical Research Clinician Scientist, and Academy of Finland (UAK1021MRI). S.E.R. reports research support from AstraZeneca, Bayer and NIH/NIDDK [U01DK133092 and U01DK116102] to Joslin Diabetes Center. She also benefited from participation in the Joslin Diabetes research enrichment program funded by P30 DK03836. J.A.S. received salary support provided by K08DK124449. K.T. is supported by NIH research grants R01MD014712, U2CDK114886, UL1TR002319, U54DK083912, U01DK100846, OT2HL161847, UM1AI109568, OT2OD032581 and CDC project number 75D301-21-P-12254. S.S.W. is supported by research grants R01DK108803, U01DK130060, U01DK133092, U01AG076789 and R25DK128858.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.H.V.R. has consulting relationships with Bayer, Boehringer Ingelheim, Eli Lilly, Merck and Sanofi, and receives research operating funding from AstraZeneca, Boehringer Ingelheim-Eli Lilly Diabetes Alliance and MSD. Honoraria are transferred to employer Amsterdam UMC. P.B. reports serving or having served as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, LG Chemistry, Sanofi, Novo Nordisk and Horizon Pharma. P.B. also serves or has served on the advisory boards and/or steering committees of AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk and XORTX. P.B. receives grant support from Eli Lilly, Novo Nordisk, AstraZeneca, Merck, and Horizon Pharma. D.Z.I.C. reports serving or having served as a consultant for Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, AbbVie, Janssen, Bayer, Prometic, BMS, Maze, Gilead, CSL-Behring, Otsuka, Novartis, Youngene, Lexicon, Inversago, GSK and Novo-Nordisk. He has received operational funding from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca, CSL-Behring and Novo-Nordisk. I.H.D.B. has received consulting fees from AstraZeneca, Bayer, Boehringer-Ingelheim, Cyclerion Therapeutics, George Clinical, Gilead, Goldfinch Bio, Ironwood, Medscape and Otsuka. P.F. has received fees as consultant and speaker for AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly company and Novo Nordisk. D.G. has received lecture or Advisory Board Honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Delta Medical Communications, EASD eLearning, Finnish Association for Vascular Surgery, Finnish Nephrology Association, Kidney and Liver Foundation in Finland. F.P. has served as a consultant, on advisory boards or as educator for AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Sanofi, Mundipharma, MSD, Novartis and Amgen, and has received research grants to institution from Novo Nordisk, Boehringer Ingelheim, Amgen and AstraZeneca. S.E.R. has participated as a member of scientific advisory boards for AstraZeneca, Bayer, and Travere. P.R. has received research support and personal fees from AstraZeneca, Bayer, and Novo Nordisk and personal fees from Abbott, Astellas, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead and Sanofi. All fees are given to Steno Diabetes Center Copenhagen. K.T. reports consulting relationships with Eli Lilly, Boehringer Ingelheim, AstraZeneca, Pfizer, Bayer and Novo Nordisk, and receives research grants from Travere paid to employer, Providence Inland Northwest Health. S.S.W. is consultant for Wolters Kluwer, Bain, BioMarin, Goldfinch, GSK, Ikena, Strataca, Google, CANbridge, NovoNordisk, PepGen, Sironax and NovoNordisk, and expert witness for Davita, Pfizer, Dechert and Aurinia Pharmaceuticals. S.S.W. receives research funding from Vertex, Pfizer, JNJ and Natera. H.J.L.H. is consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli-Lilly, Gilead, Janssen, Merck, Novo Nordisk, ProKidney, Travere Therapeutics and Vifor Fresenius. He has received research support from AstraZeneca, Boehringer Ingelheim, Janssen and Novo Nordisk.
Peer review
Peer-review information
Nature Reviews Nephrology thanks Mark Cooper, Beatriz Fernandez-Fernandez and Pierre-Jean Saulnier for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Diabetes case-finding
-
A systematic approach to identifying patients with a disease. Active screening for hyperglycaemia has increased the detection of diabetes in the general population.
- Mediation analyses
-
Statistical models that seek to explain a mechanism that underlies a relationship between two variables or conditions by including a mediation variable.
- Steno Type 1 Risk Engine
-
A risk calculator that estimates the 10-year risk of fatal or non-fatal cardiovascular disease in patients with type 1 diabetes mellitus. The calculator incorporates clinical and biochemical risk parameters.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Raalte, D.H., Bjornstad, P., Cherney, D.Z.I. et al. Combination therapy for kidney disease in people with diabetes mellitus. Nat Rev Nephrol (2024). https://doi.org/10.1038/s41581-024-00827-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41581-024-00827-z