Key Points
-
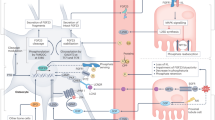
Abnormalities in phosphate homeostasis are common in renal transplant recipients, ranging from hypophosphataemia within 3 months after transplantation to hyperphosphataemia, hyperparathyroidism, and high FGF-23 levels in the late post-transplantation stage
-
Persistent high levels of FGF-23 and parathyroid hormone during the first months post-transplantation, and the influence of immunosuppressive drugs, ischaemia–reperfusion injury, and metabolic acidosis, can result in post-transplantation hypophosphataemia
-
Long-term exposure to high levels of phosphate and FGF-23 might have distinct yet associated adverse effects on the cardiovascular system, kidney, and bone in renal transplant recipients
-
Although data are limited, renal transplant recipients might benefit from dietary and pharmacologic interventions to improve phosphate metabolism; future studies should address whether these measures can improve cardiorenal prognosis
Abstract
Dysregulated phosphate metabolism is a common consequence of chronic kidney disease, and is characterized by a high circulating level of fibroblast growth factor (FGF)-23, hyperparathyroidism, and hyperphosphataemia. Kidney transplantation can elicit specific alterations to phosphate metabolism that evolve over time, ranging from severe hypophosphataemia (<0.5 mmol/l) to hyperphosphataemia (>1.50 mmol/l) and high FGF-23 levels. The majority of renal transplant recipients develop hypophosphataemia during the first 3 months after transplantation as a consequence of relatively slow adaptation of FGF-23 and parathyroid hormone levels to restored renal function, and the influence of immunosuppressive drugs. By 3–12 months after transplantation, phosphate homeostasis is at least partially restored in the majority of recipients, which is paralleled by a substantially reduced risk of cardiovascular-associated morbidity and mortality compared with the pre-transplantation setting. Many renal transplant recipients, however, exhibit persistent abnormalities in phosphate homeostasis, which is often due to multifactorial causes, and may contribute to adverse outcomes on the cardiovascular system, kidney, and bone. Dietary and pharmacologic interventions might improve phosphate homeostasis in renal transplant recipients, but additional insight into the pathophysiology of transplantation-associated abnormalities in phosphate homeostasis is needed to further optimize disease management and improve prognosis for renal transplant recipients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Isakova, T. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 79, 1370–1378 (2011).
Silver, J. & Naveh-Many, T. FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat. Rev. Nephrol. 9, 641–649 (2013).
Scialla, J. J. & Wolf, M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat. Rev. Nephrol. 10, 268–278 (2014).
Wolf, M. et al. A prospective cohort study of mineral metabolism after kidney transplantation. Transplantation http://dx.doi.org/10.1097/TP.0000000000000823.
Lockitch, G., Halstead, A. C., Albersheim, S., MacCallum, C. & Quigley, G. Age- and sex-specific paediatric reference intervals for biochemistry analytes as measured with the Ektachem-700 analyzer. Clin. Chem. 34, 1622–1625 (1988).
Hilfiker, H. et al. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc. Natl Acad. Sci. USA 95, 14564–14569 (1998).
Danisi, G., Bonjour, J. P. & Straub, R. W. Regulation of Na-dependent phosphate influx across the mucosal border of duodenum by 1, 25-dihydroxycholecalciferol. Pflugers Arch. 388, 227–232 (1980).
Hattenhauer, O., Traebert, M., Murer, H. & Biber, J. Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am. J. Physiol. 277, G756–G762 (1999).
Alizadeh Naderi, A. S. & Reilly, R. F. Hereditary disorders of renal phosphate wasting. Nat. Rev. Nephrol. 6, 657–665 (2010).
Virkki, L. V., Biber, J., Murer, H. & Forster, I. C. Phosphate transporters: a tale of two solute carrier families. Am. J. Physiol. Renal Physiol. 293, F643–654 (2007).
Segawa, H., Aranami, F., Kaneko, I., Tomoe, Y. & Miyamoto, K. The roles of Na/Pi-II transporters in phosphate metabolism. Bone 45 (Suppl. 1), S2–S7 (2009).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004).
Tomoe, Y. et al. Phosphaturic action of fibroblast growth factor 23 in Npt2 null mice. Am. J. Physiol. Renal Physiol. 298, F1341–F1350 (2010).
Weinman, E. J., Steplock, D., Shenolikar, S. & Biswas, R. Fibroblast growth factor-23-mediated inhibition of renal phosphate transport in mice requires sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) and synergizes with parathyroid hormone. J. Biol. Chem. 286, 37216–37221 (2011).
Lotscher, M. et al. Rapid downregulation of rat renal Na/P(i) cotransporter in response to parathyroid hormone involves microtubule rearrangement. J. Clin. Invest. 104, 483–494 (1999).
Pfister, M. F. et al. Parathyroid hormone-dependent degradation of type II Na+/Pi cotransporters. J. Biol. Chem. 272, 20125–20130 (1997).
Shimada, T. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004).
Wolf, M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 82, 737–747 (2012).
Goldman, R. & Bassett, S. H. Phosphorus excretion in renal failure. J. Clin. Invest. 33, 1623–1628 (1954).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Hu, M. C., Shiizaki, K., Kuro-o, M. & Moe, O. W. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 75, 503–533 (2013).
Green, J. et al. Evidence for a PTH-independent humoral mechanism in post-transplant hypophosphataemia and phosphaturia. Kidney Int. 60, 1182–1196 (2001).
Evenepoel, P. et al. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin. J. Am. Soc. Nephrol. 3, 1829–1836 (2008).
Trombetti, A. et al. Early post-transplantation hypophosphataemia is associated with elevated FGF-23 levels. Eur. J. Endocrinol. 164, 839–847 (2011).
Han, S. Y., Hwang, E. A., Park, S. B., Kim, H. C. & Kim, H. T. Elevated fibroblast growth factor 23 levels as a cause of early post-renal transplantation hypophosphataemia. Transplant. Proc. 44, 657–660 (2012).
Evenepoel, P., Naesens, M., Claes, K., Kuypers, D. & Vanrenterghem, Y. Tertiary 'hyperphosphatoninism' accentuates hypophosphataemia and suppresses calcitriol levels in renal transplant recipients. Am. J. Transplant. 7, 1193–1200 (2007).
Boudville, N. C. & Hodsman, A. B. Renal function and 25-hydroxyvitamin D concentrations predict parathyroid hormone levels in renal transplant patients. Nephrol. Dial. Transplant. 21, 2621–2624 (2006).
Trombetti, A. et al. Early post-transplantation hypophosphataemia is associated with elevated FGF-23 levels. Eur. J. Endocrinol. 164, 839–847 (2011).
Kawarazaki, H. et al. The relative role of fibroblast growth factor 23 and parathyroid hormone in predicting future hypophosphataemia and hypercalcemia after living donor kidney transplantation: a 1-year prospective observational study. Nephrol. Dial. Transplant. 26, 2691–2695 (2011).
Wesseling-Perry, K. et al. FGF23 and mineral metabolism in the early post-renal transplantation period. Pediatr. Nephrol. 28, 2207–2215 (2013).
Higgins, R. M., Richardson, A. J., Endre, Z. H., Frostick, S. P. & Morris, P. J. Hypophosphataemia after renal transplantation: relationship to immunosuppressive drug therapy and effects on muscle detected by 31P nuclear magnetic resonance spectroscopy. Nephrol. Dial. Transplant. 5, 62–68 (1990).
Loffing, J. et al. Renal Na/H exchanger NHE-3 and Na-PO4 cotransporter NaPi-2 protein expression in glucocorticoid excess and deficient states. J. Am. Soc. Nephrol. 9, 1560–1567 (1998).
Levi, M. et al. Dexamethasone modulates rat renal brush border membrane phosphate transporter mRNA and protein abundance and glycosphingolipid composition. J. Clin. Invest. 96, 207–216 (1995).
Borowitz, S. M. & Granrud, G. S. Glucocorticoids inhibit intestinal phosphate absorption in developing rabbits. J. Nutr. 122, 1273–1279 (1992).
Kahan, B. D. Cyclosporine. N. Engl. J. Med. 321, 1725–1738 (1989).
Moz, Y. et al. Calcineurin Abeta is central to the expression of the renal type II Na/Pi co-transporter gene and to the regulation of renal phosphate transport. J. Am. Soc. Nephrol. 15, 2972–2980 (2004).
Demeule, M. & Beliveau, R. Cyclosporin inhibits phosphate transport and stimulates alkaline phosphatase activity in renal BBMV. Am. J. Physiol. 260, F518–524 (1991).
Palestine, A. G., Austin, H. A. 3rd & Nussenblatt, R. B. Renal tubular function in cyclosporine-treated patients. Am. J. Med. 81, 419–424 (1986).
Kempe, D. S. et al. Rapamycin-induced phosphaturia. Nephrol. Dial. Transplant. 25, 2938–2944 (2010).
Schwarz, C., Bohmig, G. A., Steininger, R., Mayer, G. & Oberbauer, R. Impaired phosphate handling of renal allografts is aggravated under rapamycin-based immunosuppression. Nephrol. Dial. Transplant. 16, 378–382 (2001).
Yao, J. C. et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J. Clin. Oncol. 26, 4311–4318 (2008).
Grignani, G. et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 16, 98–107 (2015).
Tataranni, T. et al. Rapamycin-induced hypophosphataemia and insulin resistance are associated with mTORC2 activation and Klotho expression. Am. J. Transplant. 11, 1656–1664 (2011).
Nowik, M. et al. Renal phosphaturia during metabolic acidosis revisited: molecular mechanisms for decreased renal phosphate reabsorption. Pflugers Arch. 457, 539–549 (2008).
Kwon, T. H., Frokiaer, J., Han, J. S., Knepper, M. A. & Nielsen, S. Decreased abundance of major Na(+) transporters in kidneys of rats with ischemia-induced acute renal failure. Am. J. Physiol. Renal Physiol. 278, F925–939 (2000).
Tanaka, Y. et al. Role of 1, 25-dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc. Natl. Acad. Sci. USA 71, 1040–1044 (1974).
Farrington, K. et al. Dissociation of absorptions of calcium and phosphate after successful cadaveric renal transplantation. Br. Med. J. 1, 712–714 (1979).
Moorhead, J. F. et al. Hypophosphataemic osteomalacia after cadaveric renal transplantation. Lancet 1, 694–697 (1974).
Rosenbaum, R. W., Hruska, K. A., Korkor, A., Anderson, C. & Slatopolsky, E. Decreased phosphate reabsorption after renal transplantation: Evidence for a mechanism independent of calcium and parathyroid hormone. Kidney Int. 19, 568–578 (1981).
Felsenfeld, A. J., Gutman, R. A., Drezner, M. & Llach, F. Hypophosphataemia in long-term renal transplant recipients: effects on bone histology and 1, 25-dihydroxycholecalciferol. Miner. Electrolyte Metab. 12, 333–341 (1986).
Levi, M. Post-transplant hypophosphataemia. Kidney Int. 59, 2377–2387 (2001).
Wolf, M. Forging forward with 10 burning questions on FGF23 in kidney disease. J. Am. Soc. Nephrol. 21, 1427–1435 (2010).
Wesseling-Perry, K., Tsai, E. W., Ettenger, R. B., Juppner, H. & Salusky, I. B. Mineral abnormalities and long-term graft function in paediatric renal transplant recipients: a role for FGF-23? Nephrol. Dial. Transplant. 26, 3779–3784 (2011).
Tomida, K. et al. Dialysis vintage and parathyroid hormone level, not fibroblast growth factor-23, determines chronic-phase phosphate wasting after renal transplantation. Bone 51, 729–736 (2012).
Bacchetta, J. et al. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in paediatric chronic kidney disease. J. Clin. Endocrinol. Metab. 95, 1741–1748 (2010).
Sanchez Fructuoso, A. I. et al. Serum level of fibroblast growth factor 23 in maintenance renal transplant patients. Nephrol. Dial. Transplant. 27, 4227–4235 (2012).
Olauson, H. & Larsson, T. E. FGF23 and Klotho in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 22, 397–404 (2013).
Li, X., Yang, H. Y. & Giachelli, C. M. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 98, 905–912 (2006).
Jono, S. et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 87, E10–17 (2000).
Wada, T., McKee, M. D., Steitz, S. & Giachelli, C. M. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ. Res. 84, 166–178 (1999).
Scialla, J. J. et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 83, 1159–1168 (2013).
Scialla, J. J. et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 25, 349–360 (2014).
Humalda, J. K. et al. Fibroblast growth factor 23 and the antiproteinuric response to dietary sodium restriction during renin-angiotensin-aldosterone system blockade. Am. J. Kidney Dis. 65, 259–266 (2015).
Andrukhova, O. et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 5, 744–759 (2014).
Sarnak, M. J. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108, 2154–2169 (2003).
Marechal, C. et al. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am. J. Kidney Dis. 59, 258–269 (2012).
DeLoach, S. S., Joffe, M. M., Mai, X., Goral, S. & Rosas, S. E. Aortic calcification predicts cardiovascular events and all-cause mortality in renal transplantation. Nephrol. Dial. Transplant. 24, 1314–1319 (2009).
Demer, L. L. & Tintut, Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler. Thromb. Vasc. Biol. 34, 715–723 (2014).
Cianciolo, G. et al. Importance of vascular calcification in kidney transplant recipients. Am. J. Nephrol. 39, 418–426 (2014).
Cianciolo, G., Scolari, M. P., Angelini, M. L. & Stefoni, S. Progression of vascular calcification in kidney transplantation: the need to assess the calcium burden and to understand why some patients have a calcium score of 0. Am. J. Kidney Dis. 62, 644–645 (2013).
Pasch, A. et al. Nanoparticle-based test measures overall propensity for calcification in serum. J. Am. Soc. Nephrol. 23, 1744–1752 (2012).
Keyzer, C. A. et al. Calcification propensity and survival among renal transplant recipients. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2014070670.
Connolly, G. M., Cunningham, R., McNamee, P. T., Young, I. S. & Maxwell, A. P. Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation 87, 1040–1044 (2009).
Wolf, M. et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J. Am. Soc. Nephrol. 22, 956–966 (2011).
Baia, L. C. et al. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin. J. Am. Soc. Nephrol. 8, 1968–1978 (2013).
Malyszko, J., Koc-Zorawska, E., Matuszkiewicz-Rowinska, J. & Malyszko, J. FGF23 and Klotho in relation to markers of endothelial dysfunction in kidney transplant recipients. Transplant. Proc. 46, 2647–2650 (2014).
Yilmaz, M. I. et al. Longitudinal analysis of vascular function and biomarkers of metabolic bone disorders before and after renal transplantation. Am. J. Nephrol. 37, 126–134 (2013).
Asicioglu, E. et al. Fibroblast growth factor-23 levels are associated with uric acid but not carotid intima media thickness in renal transplant recipients. Transplant. Proc. 46, 180–183 (2014).
Gungor, O. et al. The relationships between serum sTWEAK, FGF-23 levels, and carotid atherosclerosis in renal transplant patients. Ren. Fail. 35, 77–81 (2013).
Mitsnefes, M. M. Cardiovascular disease in children with chronic kidney disease. J. Am. Soc. Nephrol. 23, 578–585 (2012).
Haut, L. L., Alfrey, A. C., Guggenheim, S., Buddington, B. & Schrier, N. Renal toxicity of phosphate in rats. Kidney Int. 17, 722–731 (1980).
Mackay, E. M. & Oliver, J. Renal damage following the ingestion of a diet containing an excess of inorganic phosphate. J. Exp. Med. 61, 319–334 (1935).
Fliser, D. et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J. Am. Soc. Nephrol. 18, 2600–2608 (2007).
Lundberg, S. et al. FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin. J. Am. Soc. Nephrol. 7, 727–734 (2012).
Benavente, D. et al. Serum phosphate measured at 6 and 12 months after successful kidney transplant is independently associated with subsequent graft loss. Exp. Clin. Transplant. 10, 119–124 (2012).
Bonthuis, M. et al. Mineral metabolism in European children living with a renal transplant: a European Society for Paediatric Nephrology/European Renal Association-European Dialysis and Transplant Association Registry Study. Clin. J. Am. Soc. Nephrol. 10, 767–775 (2015).
Egbuna, O. I., Taylor, J. G., Bushinsky, D. A. & Zand, M. S. Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure. Clin. Transplant. 21, 558–566 (2007).
Baia, L. C. et al. Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin. J. Am. Soc. Nephrol. 8, 1968–1978 (2013).
McGregor, R. et al. Vitamin D in renal transplantation - from biological mechanisms to clinical benefits. Am. J. Transplant. 14, 1259–1270 (2014).
Bienaime, F. et al. Vitamin D status and outcomes after renal transplantation. J. Am. Soc. Nephrol. 24, 831–841 (2013).
Obi, Y. et al. Vitamin D deficiency predicts decline in kidney allograft function: a prospective cohort study. J. Clin. Endocrinol. Metab. 99, 527–535 (2014).
Keyzer, C. A. et al. Associations of 25(OH) and 1, 25(OH)2 vitamin D with long-term outcomes in stable renal transplant recipients. J. Clin. Endocrinol. Metab. 100, 81–89 (2015).
Weisinger, J. R., Carlini, R. G., Rojas, E. & Bellorin-Font, E. Bone disease after renal transplantation. Clin. J. Am. Soc. Nephrol. 1, 1300–1313 (2006).
Sanchez, C. P. et al. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int. 53, 1358–1364 (1998).
Mainra, R. & Elder, G. J. Individualized therapy to prevent bone mineral density loss after kidney and kidney–pancreas transplantation. Clin. J. Am. Soc. Nephrol. 5, 117–124 (2010).
Rojas, E. et al. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. 63, 1915–1923 (2003).
Ghanekar, H., Welch, B. J., Moe, O. W. & Sakhaee, K. Post-renal transplantation hypophosphataemia: a review and novel insights. Curr. Opin. Nephrol. Hypertens. 15, 97–104 (2006).
Kanaan, N. et al. Fibroblast growth factor-23 and parathyroid hormone are associated with post-transplant bone mineral density loss. Clin. J. Am. Soc. Nephrol. 5, 1887–1892 (2010).
Monier-Faugere, M. C., Mawad, H., Qi, Q., Friedler, R. M. & Malluche, H. H. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J. Am. Soc. Nephrol. 11, 1093–1099 (2000).
Seeherunvong, W. & Wolf, M. Tertiary excess of fibroblast growth factor 23 and hypophosphataemia following kidney transplantation. Pediatr. Transplant. 15, 37–46 (2011).
Sitara, D. et al. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 8, e1000154 (2008).
Wang, H. et al. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J. Bone Miner. Res. 23, 939–948 (2008).
Grotz, W. H. et al. Bone loss after kidney transplantation: a longitudinal study in 115 graft recipients. Nephrol. Dial. Transplant. 10, 2096–2100 (1995).
Bubenicek, P., Sotornik, I., Vitko, S. & Teplan, V. Early bone mineral density loss after renal transplantation and pre-transplant PTH: a prospective study. Kidney Blood Press. Res. 31, 196–202 (2008).
Casez, J. P., Lippuner, K., Horber, F. F., Montandon, A. & Jaeger, P. Changes in bone mineral density over 18 months following kidney transplantation: the respective roles of prednisone and parathyroid hormone. Nephrol. Dial. Transplant. 17, 1318–1326 (2002).
Evenepoel, P. et al. A randomized study evaluating cinacalcet to treat hypercalcemia in renal transplant recipients with persistent hyperparathyroidism. Am. J. Transplant. 14, 2545–2555 (2014).
Neves, C. L. et al. Persistence of bone and mineral disorders 2 years after successful kidney transplantation. Transplantation 96, 290–296 (2013).
Bover, J. & Cozzolino, M. Mineral and bone disorders in chronic kidney disease and end-stage renal disease patients: new insights into vitamin D receptor activation. Kidney Int. Suppl. 1, 122–129 (2011).
Chen, T. L. & Feldman, D. Glucocorticoid receptors and actions in subpopulations of cultured rat bone cells. Mechanism of dexamethasone potentiation of parathyroid hormone-stimulated cyclic AMP production. J. Clin. Invest. 63, 750–758 (1979).
Velasquez-Forero, F., Mondragon, A., Herrero, B. & Pena, J. C. Adynamic bone lesion in renal transplant recipients with normal renal function. Nephrol. Dial. Transplant. 11, 58–64 (1996).
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 9, S1–155 (2009).
Sakhaee, K. Post-renal transplantation hypophosphataemia. Pediatr. Nephrol. 25, 213–220 (2010).
Desmeules, S., Bergeron, M. J. & Isenring, P. Acute phosphate nephropathy and renal failure. N. Engl. J. Med. 349, 1006–1007 (2003).
Singh, N. & Qadir, M. Do no harm: calcium and phosphate supplementation in kidney transplant recipients. Transplantation 96, e81–e82 (2013).
Michaut, P., Prie, D., Amiel, C. & Friedlander, G. Dipyridamole for renal phosphate leak? N. Engl. J. Med. 331, 58–59 (1994).
Prie, D., Blanchet, F. B., Essig, M., Jourdain, J. P. & Friedlander, G. Dipyridamole decreases renal phosphate leak and augments serum phosphorus in patients with low renal phosphate threshold. J. Am. Soc. Nephrol. 9, 1264–1269 (1998).
Balal, M., Paydas, S., Seyrek, N., Sertdemir, Y. & Karayaylali, I. Dipyridamole for renal phosphate leak in successfully renal transplanted hypophosphatemic patients. Clin. Nephrol. 63, 87–91 (2005).
Friedlander, G., Couette, S., Coureau, C. & Amiel, C. Mechanisms whereby extracellular adenosine 3′,5′-monophosphate inhibits phosphate transport in cultured opossum kidney cells and in rat kidney. Physiological implication. J. Clin. Invest. 90, 848–858 (1992).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 113, S1–S130 (2009).
Kalantar-Zadeh, K. et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 5, 519–530 (2010).
Ritz, E. et al. Phosphate additives in food—a health risk. Dtsch. Arztebl. Int. 109, 49–55 (2012).
Moore, L. W. et al. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am. J. Clin. Nutr. 102, 444–453 (2015).
Powles, J. et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 3, e003733-2013-003733 (2013).
Burnett, S. M. et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J. Bone Miner. Res. 21, 1187–1196 (2006).
Adema, A. Y., de Borst, M. H., Ter Wee, P. M., Vervloet, M. G. & NIGRAM Consortium. Dietary and Pharmacological Modification of Fibroblast Growth Factor-23 in Chronic Kidney Disease. J. Ren. Nutr. 24, 143–150 (2014).
Sullivan, C. et al. Effect of food additives on hyperphosphataemia among patients with end-stage renal disease: a randomized controlled trial. JAMA 301, 629–635 (2009).
Sandberg, A. S., Andersson, H., Kivisto, B. & Sandstrom, B. Extrusion cooking of a high-fibre cereal product. 1. Effects on digestibility and absorption of protein, fat, starch, dietary fibre and phytate in the small intestine. Br. J. Nutr. 55, 245–254 (1986).
Moe, S. M. et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 257–264 (2011).
Noori, N. et al. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 5, 683–692 (2010).
Sherman, R. A. & Mehta, O. Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis. Clin. J. Am. Soc. Nephrol. 4, 1370–1373 (2009).
Rho, M. R. et al. Evaluation of nutrient intake in early post kidney transplant recipients. Clin. Nutr. Res. 2, 1–11 (2013).
Oliveira, R. B. et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin. J. Am. Soc. Nephrol. 5, 286–291 (2010).
Pieper, A. K. et al. The effect of sevelamer on the pharmacokinetics of cyclosporin A and mycophenolate mofetil after renal transplantation. Nephrol. Dial. Transplant. 19, 2630–2633 (2004).
Ketteler, M. & Biggar, P. H. Use of phosphate binders in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 22, 413–420 (2013).
Isakova, T. et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol. Dial. Transplant. 26, 584–591 (2011).
Akaberi, S. et al. Impact of parathyroid hormone on bone density in long-term renal transplant patients with good graft function. Transplantation 82, 749–752 (2006).
Bleskestad, I. H. et al. Parathyroid hormone and clinical outcome in kidney transplant patients with optimal transplant function. Clin. Transplant. 28, 479–486 (2014).
Courbebaisse, M. et al. Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 75, 646–651 (2009).
Amer, H. et al. Oral paricalcitol reduces the prevalence of posttransplant hyperparathyroidism: results of an open label randomized trial. Am. J. Transplant. 13, 1576–1585 (2013).
Trillini, M. et al. Paricalcitol for secondary hyperparathyroidism in renal transplantation. J. Am. Soc. Nephrol. 26, 1205–1214 (2014).
Maccubbin, D., Tipping, D., Kuznetsova, O., Hanlon, W. A. & Bostom, A. G. Hypophosphatemic effect of niacin in patients without renal failure: a randomized trial. Clin. J. Am. Soc. Nephrol. 5, 582–589 (2010).
Kruse, A. E., Eisenberger, U., Frey, F. J. & Mohaupt, M. G. The calcimimetic cinacalcet normalizes serum calcium in renal transplant patients with persistent hyperparathyroidism. Nephrol. Dial. Transplant. 20, 1311–1314 (2005).
Serra, A. L., Schwarz, A. A., Wick, F. H., Marti, H. P. & Wuthrich, R. P. Successful treatment of hypercalcemia with cinacalcet in renal transplant recipients with persistent hyperparathyroidism. Nephrol. Dial. Transplant. 20, 1315–1319 (2005).
Srinivas, T. R. et al. Improvement in hypercalcemia with cinacalcet after kidney transplantation. Clin. J. Am. Soc. Nephrol. 1, 323–326 (2006).
Szwarc, I. et al. Cinacalcet chloride is efficient and safe in renal transplant recipients with posttransplant hyperparathyroidism. Transplantation 82, 675–680 (2006).
Leca, N. et al. Early and severe hyperparathyroidism associated with hypercalcemia after renal transplant treated with cinacalcet. Am. J. Transplant. 6, 2391–2395 (2006).
Falck, P. et al. Cinacalcet's effect on the pharmacokinetics of tacrolimus, cyclosporine and mycophenolate in renal transplant recipients. Nephrol. Dial. Transplant. 23, 1048–1053 (2008).
Courbebaisse, M. et al. Effects of cinacalcet in renal transplant patients with hyperparathyroidism. Am. J. Nephrol. 35, 341–348 (2012).
Ix, J. H., Ganjoo, P., Tipping, D., Tershakovec, A. M. & Bostom, A. G. Sustained hypophosphatemic effect of once-daily niacin/laropiprant in dyslipidemic CKD stage 3 patients. Am. J. Kidney Dis. 57, 963–965 (2011).
Labonte, E. D. et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J. Am. Soc. Nephrol. 26, 1138–1149 (2015).
Acknowledgements
G.N. and M.H.d.B. participate in the NIGRAM consortium, which is supported by a grant from the Dutch Kidney Foundation (grant number CP10.11). The NIGRAM consortium consists of the following principal investigators: Pietter Wee, Marc Vervloet (VU University Medical Centre, Amsterdam, Netherlands), René Bindels, Joost Hoenderop (Radboud University Medical Centre Nijmegen, the Netherlands), Gerjan Navis, Jan-Luuk Hillebrands and Martin de Borst (University Medical Centre Groningen, the Netherlands). The research of M.H.d.B. is supported by a grant from the Netherlands Organization for Scientific Research (Veni grant).
Author information
Authors and Affiliations
Consortia
Contributions
All authors researched the data for the article, provided a substantial contribution to discussions of the content, and contributed equally to writing the article and to review and/or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Baia, L., Heilberg, I., Navis, G. et al. Phosphate and FGF-23 homeostasis after kidney transplantation. Nat Rev Nephrol 11, 656–666 (2015). https://doi.org/10.1038/nrneph.2015.153
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2015.153
This article is cited by
-
An unusual cause of elevated serum creatinine after kidney transplantation in an adolescent: Answers
Pediatric Nephrology (2022)
-
Clinical factors associated with severe hypophosphataemia after kidney transplant
BMC Nephrology (2021)
-
The Causes of Hypo- and Hyperphosphatemia in Humans
Calcified Tissue International (2021)
-
CKD-MBD post kidney transplantation
Pediatric Nephrology (2021)
-
New Therapies for Hypophosphatemia-Related to FGF23 Excess
Calcified Tissue International (2021)