Key Points
-
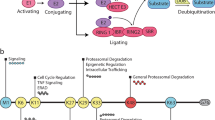
Ubiquitylation is emerging as a general regulatory mechanism in nerve cell development, on a par with phosphorylation or cyclic nucleotide signalling with regard to complexity and functional consequences.
-
The currently available evidence is still restricted to a limited set of example pathways, but these examples provide an exciting view of how ubiquitylation-dependent regulatory processes in neurons intercalate with other, more extensively studied regulatory principles to control all phases of nerve cell development: the generation of neurons from precursor cells, their migration to the appropriate target sites, their extensive arborization and their integration into functional networks through synapse formation. Currently, we see only the tip of the iceberg.
-
Notch-mediated regulation of neurogenesis and gliogenesis is controlled directly and indirectly by multiple ubiquitylation pathways.
-
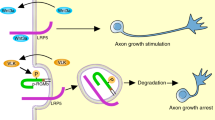
Polyubiquitylation and degradation of disabled homologue 1 (DAB1) — an adaptor protein for reelin receptors, very low-density lipoprotein receptor (VLDLR) and apolipoprotein E receptor type 2 (APOER2) — is crucial for neuronal migration in the cortex by throttling reelin signalling.
-
Signalling pathways controlling neurite development and axon acquisition are controlled by specific monoubiquitylation, diubiquitylation or polyubiquitylation of small GTPases. Typically, these types of ubiquitylation negatively regulate GTPase function or expression.
-
The RING finger E3 ligases RPM1 (also known as highwire) and the SCF complex function in synaptogenesis and synapse elimination by polyubiquitylating target proteins at synaptic or extrasynaptic locations in axons. Spatial restriction of ubiquitylation is essential for synapse elimination.
Abstract
Nerve cell development in the brain is a tightly regulated process. The generation of neurons from precursor cells, their migration to the appropriate target sites, their extensive arborization and their integration into functional networks through synapse formation and refinement are governed by multiple interdependent signalling cascades. The function and turnover of proteins involved in these signalling cascades, in turn, are spatially and temporally controlled by ubiquitylation. Recent advances have provided first insights into the highly complex and intricate molecular pathways that regulate ubiquitylation during all stages of neural development and that operate in parallel with other regulatory processes such as phosphorylation or cyclic nucleotide signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lange, W. Cell number and cell density in the cerebellar cortex of man and some other mammals. Cell Tissue Res. 157, 115–124 (1975).
Shariff, G. A. Cell counts in the primate cerebral cortex. J. Comp. Neurol. 98, 381–400 (1953).
Barnes, A. P. & Polleux, F. Establishment of axon-dendrite polarity in developing neurons. Annu. Rev. Neurosci. 32, 347–381 (2009).
Parrish, J. Z., Emoto, K., Kim, M. D. & Jan, Y. N. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu. Rev. Neurosci. 30, 399–423 (2007).
Spruston, N. Pyramidal neurons: dendritic structure and synaptic integration. Nature Rev. Neurosci. 9, 206–221 (2008).
O'Donnell, M., Chance, R. K. & Bashaw, G. J. Axon growth and guidance: receptor regulation and signal transduction. Annu. Rev. Neurosci. 32, 383–412 (2009).
Sanes, J. R. & Yamagata, M. Many paths to synaptic specificity. Annu. Rev. Cell Dev. Biol. 25, 161–195 (2009).
Marin, O., Valiente, M., Ge, X. & Tsai, L. H. Guiding neuronal cell migrations. Cold Spring Harb. Perspect. Biol. 2, a001834 (2010).
Li, W. et al. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle's dynamics and signaling. PLoS ONE 3, e1487 (2008).
Xu, P. et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137, 133–145 (2009).
Kim, H. C. & Huibregtse, J. M. Polyubiquitination by HECT E3s and the determinants of chain type specificity. Mol. Cell. Biol. 29, 3307–3318 (2009).
Hicke, L. Protein regulation by monoubiquitin. Nature Rev. Mol. Cell Biol. 2, 195–201 (2001).
Xu, L., Lubkov, V., Taylor, L. J. & Bar-Sagi, D. Feedback regulation of Ras signaling by Rabex-5-mediated ubiquitination. Curr. Biol. 20, 1372–1377 (2010).
Wilkinson, K. D. et al. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science 246, 670–673 (1989).
Kent, C. & Clarke, P. J. The immunolocalisation of the neuroendocrine specific protein PGP9.5 during neurogenesis in the rat. Brain Res. Dev. Brain Res. 58, 147–150 (1991).
Lowe, J. et al. A filamentous inclusion body within anterior horn neurones in motor neurone disease defined by immunocytochemical localisation of ubiquitin. Neurosci. Lett. 94, 203–210 (1988).
Lennox, G., Lowe, J., Morrell, K., Landon, M. & Mayer, R. J. Ubiquitin is a component of neurofibrillary tangles in a variety of neurodegenerative diseases. Neurosci. Lett. 94, 211–217 (1988).
Tai, H. C. & Schuman, E. M. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nature Rev. Neurosci. 9, 826–838 (2008).
Qian, X. et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28, 69–80 (2000).
Gerhart, J. 1998 Warkany lecture: signaling pathways in development. Teratology 60, 226–239 (1999).
Yun, S. J. et al. Transcriptional regulatory networks associated with self-renewal and differentiation of neural stem cells. J. Cell. Physiol. 225, 337–347 (2010).
Inestrosa, N. C. & Arenas, E. Emerging roles of Wnts in the adult nervous system. Nature Rev. Neurosci. 11, 77–86 (2010).
Kikuchi, A. Regulation of β-catenin signaling in the Wnt pathway. Biochem. Biophys. Res. Commun. 268, 243–248 (2000).
Yoon, K. & Gaiano, N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nature Neurosci. 8, 709–715 (2005).
Schier, A. F. et al. Mutations affecting the development of the embryonic zebrafish brain. Development 123, 165–178 (1996).
Jiang, Y. J. et al. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development 123, 205–216 (1996).
Itoh, M. et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 4, 67–82 (2003). This study shows that the E3 ubiquitin ligase mind bomb ubiquitylates Delta and thereby regulates Notch signalling.
Yoon, K. J. et al. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron 58, 519–531 (2008). Using conditional MIB1 knockout mice, the authors of this study provide important evidence for the regulation of Delta by MIB1 during neurogenesis.
Koo, B. K. et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development 132, 3459–3470 (2005).
Wang, W. & Struhl, G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development 132, 2883–2894 (2005).
Yamamoto, M. et al. Mib–Jag1–Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development 137, 2527–2537 (2010).
Konno, D. et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nature Cell Biol. 10, 93–101 (2008).
Ossipova, O., Ezan, J. & Sokol, S. Y. PAR-1 phosphorylates Mind bomb to promote vertebrate neurogenesis. Dev. Cell 17, 222–233 (2009).
Knoepfler, P. S., Cheng, P. F. & Eisenman, R. N. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 16, 2699–2712 (2002).
Zhao, X. et al. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nature Cell Biol. 10, 643–653 (2008).
Zhao, X. et al. The N.-Myc-DLL3 cascade is suppressed by the ubiquitin ligase Huwe1 to inhibit proliferation and promote neurogenesis in the developing brain. Dev. Cell 17, 210–221 (2009).
Marthiens, V. & ffrench-Constant, C. Adherens junction domains are split by asymmetric division of embryonic neural stem cells. EMBO Rep. 10, 515–520 (2009).
Marquardt, T. et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43–55 (2001).
Hebert, J. M. & Fishell, G. The genetics of early telencephalon patterning: some assembly required. Nature Rev. Neurosci. 9, 678–685 (2008).
St-Onge, L., Sosa-Pineda, B., Chowdhury, K., Mansouri, A. & Gruss, P. Pax6 is required for differentiation of glucagon-producing α-cells in mouse pancreas. Nature 387, 406–409 (1997).
Englund, C. et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 25, 247–251 (2005).
Scardigli, R., Baumer, N., Gruss, P., Guillemot, F. & Le Roux, I. Direct and concentration-dependent regulation of the proneural gene Neurogenin2 by Pax6. Development 130, 3269–3281 (2003).
Tuoc, T. C. & Stoykova, A. Trim11 modulates the function of neurogenic transcription factor Pax6 through ubiquitin-proteosome system. Genes Dev. 22, 1972–1986 (2008).
Berger, J. et al. Conditional activation of Pax6 in the developing cortex of transgenic mice causes progenitor apoptosis. Development 134, 1311–1322 (2007).
Sobieszczuk, D. F., Poliakov, A., Xu, Q. & Wilkinson, D. G. A feedback loop mediated by degradation of an inhibitor is required to initiate neuronal differentiation. Genes Dev. 24, 206–218 (2010).
Kageyama, R., Ohtsuka, T., Shimojo, H. & Imayoshi, I. Dynamic regulation of Notch signaling in neural progenitor cells. Curr. Opin. Cell Biol. 21, 733–740 (2009).
Shimojo, H., Ohtsuka, T. & Kageyama, R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 58, 52–64 (2008).
Hirata, H. et al. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 298, 840–843 (2002).
Hirabayashi, Y. et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63, 600–613 (2009). This study describes the role of the PcG components PRC1 and PRC2 in fate decision of neural progenitor cells, and shows that the function of the ubiquitin ligase RING1B in the PRC2 complex is essential for this process.
Fan, G. et al. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 132, 3345–3356 (2005).
Wu, H. et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science 329, 444–448 (2010).
Hatada, I. et al. Astrocyte-specific genes are generally demethylated in neural precursor cells prior to astrocytic differentiation. PLoS ONE 3, e3189 (2008).
Namihira, M., Nakashima, K. & Taga, T. Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS Lett. 572, 184–188 (2004).
Takizawa, T. et al. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell 1, 749–758 (2001).
Cao, R. et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 (2002).
Wang, H. et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878 (2004).
Cao, R., Tsukada, Y. & Zhang, Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 20, 845–854 (2005).
Mohd-Sarip, A., Venturini, F., Chalkley, G. E. & Verrijzer, C. P. Pleiohomeotic can link polycomb to DNA and mediate transcriptional repression. Mol. Cell. Biol. 22, 7473–7483 (2002).
Mohd-Sarip, A., Cleard, F., Mishra, R. K., Karch, F. & Verrijzer, C. P. Synergistic recognition of an epigenetic DNA element by Pleiohomeotic and a Polycomb core complex. Genes Dev. 19, 1755–1760 (2005).
Mohd-Sarip, A. et al. Architecture of a polycomb nucleoprotein complex. Mol. Cell 24, 91–100 (2006).
Chong, J. A. et al. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 80, 949–957 (1995).
Schoenherr, C. J. & Anderson, D. J. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 267, 1360–1363 (1995).
Lee, M. G., Wynder, C., Cooch, N. & Shiekhattar, R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature 437, 432–435 (2005).
Roopra, A., Qazi, R., Schoenike, B., Daley, T. J. & Morrison, J. F. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol. Cell 14, 727–738 (2004).
Lunyak, V. V. et al. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science 298, 1747–1752 (2002).
Ballas, N., Grunseich, C., Lu, D. D., Speh, J. C. & Mandel, G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121, 645–657 (2005).
Westbrook, T. F. et al. SCFβ-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature 452, 370–374 (2008). In this paper, the authors show that the ubiquitylation and degradation of REST by SCF–bTRCP is required for the differentiation of neural progenitor cells to neurons.
Kohyama, J. et al. BMP-induced REST regulates the establishment and maintenance of astrocytic identity. J. Cell Biol. 189, 159–170 (2010).
Hamburgh, M. Analysis of the postnatal developmental effects of “Reeler, ” a neurological mutation in mice. A study in developmental genetics. Dev. Biol. 19, 165–185 (1963).
Senzaki, K., Ogawa, M. & Yagi, T. Proteins of the CNR family are multiple receptors for Reelin. Cell 99, 635–647 (1999).
Hiesberger, T. et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24, 481–489 (1999).
D'Arcangelo, G. et al. Reelin is a ligand for lipoprotein receptors. Neuron 24, 471–479 (1999).
Trommsdorff, M. et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 (1999).
Keshvara, L., Benhayon, D., Magdaleno, S. & Curran, T. Identification of reelin-induced sites of tyrosyl phosphorylation on disabled 1. J. Biol. Chem. 276, 16008–16014 (2001).
Sheldon, M. et al. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 389, 730–733 (1997).
Ware, M. L. et al. Aberrant splicing of a mouse disabled homolog, mdab1, in the scrambler mouse. Neuron 19, 239–249 (1997).
Arnaud, L., Ballif, B. A. & Cooper, J. A. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol. Cell. Biol. 23, 9293–9302 (2003).
Feng, L., Allen, N. S., Simo, S. & Cooper, J. A. Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 21, 2717–2730 (2007).
Simo, S., Jossin, Y. & Cooper, J. A. Cullin 5 regulates cortical layering by modulating the speed and duration of Dab1-dependent neuronal migration. J. Neurosci. 30, 5668–5676 (2010).
Tissir, F. & Goffinet, A. M. Reelin and brain development. Nature Rev. Neurosci. 4, 496–505 (2003).
Campbell, D. S. & Holt, C. E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32, 1013–1026 (2001).
Kim, T. H. et al. Netrin induces down-regulation of its receptor, Deleted in Colorectal Cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron. J. Neurochem. 95, 1–8 (2005).
Hu, G. et al. Mammalian homologs of seven in absentia regulate DCC via the ubiquitin-proteasome pathway. Genes Dev. 11, 2701–2714 (1997).
Yuasa-Kawada, J., Kinoshita-Kawada, M., Wu, G., Rao, Y. & Wu, J. Y. Midline crossing and Slit responsiveness of commissural axons require USP33. Nature Neurosci. 12, 1087–1089 (2009). This study identifies the deubiquitylating enzyme USP33 as a binding partner of ROBO1, and shows that USP33 is crucial for midline crossing of axons.
Piper, M. et al. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 49, 215–228 (2006).
Tojima, T., Hines, J. H., Henley, J. R. & Kamiguchi, H. Second messengers and membrane trafficking direct and organize growth cone steering. Nature Rev. Neurosci. 12, 191–203 (2011).
Takai, Y., Sasaki, T. & Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 81, 153–208 (2001).
Arimura, N. & Kaibuchi, K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nature Rev. Neurosci. 8, 194–205 (2007).
da Silva, J. S. & Dotti, C. G. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nature Rev. Neurosci. 3, 694–704 (2002).
Garvalov, B. K. et al. Cdc42 regulates cofilin during the establishment of neuronal polarity. J. Neurosci. 27, 13117–13129 (2007).
Schwamborn, J. C. & Puschel, A. W. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nature Neurosci. 7, 923–929 (2004).
Fu, Z. et al. Differential roles of Rap1 and Rap2 small GTPases in neurite retraction and synapse elimination in hippocampal spiny neurons. J. Neurochem. 100, 118–131 (2007).
Bryan, B. et al. Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 579, 1015–1019 (2005).
Wang, H. R. et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302, 1775–1779 (2003).
Schwamborn, J. C., Muller, M., Becker, A. H. & Puschel, A. W. Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J. 26, 1410–1422 (2007). This paper provides the first evidence that the expression level of a small GTPase is regulated by polyubiquitylation and plays a key part in neuronal development.
Jura, N., Scotto-Lavino, E., Sobczyk, A. & Bar-Sagi, D. Differential modification of Ras proteins by ubiquitination. Mol. Cell 21, 679–687 (2006).
Kawabe, H. et al. Regulation of Rap2A by the ubiquitin ligase Nedd4–1 controls neurite development. Neuron 65, 358–372 (2010). This study shows that the HECT-type E3 ligase NEDD4 controls dendrite growth by monoubiquitylation and inhibition of RAP2 and consequent inhibition of the kinase TNIK.
Drinjakovic, J. et al. E3 ligase Nedd4 promotes axon branching by downregulating PTEN. Neuron 65, 341–357 (2010).
Trotman, L. C. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 (2007).
Wang, X. et al. NEDD4–1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128, 129–139 (2007).
Fouladkou, F. et al. The ubiquitin ligase Nedd4–1 is dispensable for the regulation of PTEN stability and localization. Proc. Natl Acad. Sci. USA 105, 8585–8590 (2008).
Rotin, D. & Kumar, S. Physiological functions of the HECT family of ubiquitin ligases. Nature Rev. Mol. Cell Biol. 10, 398–409 (2009).
Cheng, P. L., Lu, H., Shelly, M., Gao, H. & Poo, M. M. Phosphorylation of E3 ligase Smurf1 switches its substrate preference in support of axon development. Neuron 69, 231–243 (2011). This paper shows that phosphorylation of SMURF1 switches its substrate preference from PAR6 to RHOA in axons.
Thornton, B. R. & Toczyski, D. P. Precise destruction: an emerging picture of the APC. Genes Dev. 20, 3069–3078 (2006).
Konishi, Y., Stegmuller, J., Matsuda, T., Bonni, S. & Bonni, A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 303, 1026–1030 (2004). This is the first paper to demonstrate a key function of APC in postmitotic neurons.
Stegmuller, J. et al. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron 50, 389–400 (2006).
Kim, A. H. et al. A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell 136, 322–336 (2009).
Huynh, M. A., Stegmuller, J., Litterman, N. & Bonni, A. Regulation of Cdh1-APC function in axon growth by Cdh1 phosphorylation. J. Neurosci. 29, 4322–4327 (2009).
Zhou, Y., Ching, Y. P., Chun, A. C. & Jin, D. Y. Nuclear localization of the cell cycle regulator CDH1 and its regulation by phosphorylation. J. Biol. Chem. 278, 12530–12536 (2003).
Yang, Y. et al. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science 326, 575–578 (2009).
Margeta, M. A. & Shen, K. Molecular mechanisms of synaptic specificity. Mol. Cell. Neurosci. 43, 261–267 (2010).
Huttenlocher, P. R., de Courten, C., Garey, L. J. & Van der Loos, H. Synaptogenesis in human visual cortex-evidence for synapse elimination during normal development. Neurosci. Lett. 33, 247–252 (1982).
Zecevic, N. & Rakic, P. Synaptogenesis in monkey somatosensory cortex. Cereb. Cortex 1, 510–523 (1991).
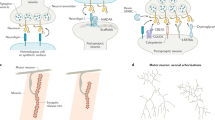
DiAntonio, A. et al. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 412, 449–452 (2001). This paper is of particular importance as it demonstrates that protein ubiquitylation is crucial for synaptogenesis.
Wan, H. I. et al. Highwire regulates synaptic growth in Drosophila. Neuron 26, 313–329 (2000).
Zhen, M., Huang, X., Bamber, B. & Jin, Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron 26, 331–343 (2000).
Schaefer, A. M., Hadwiger, G. D. & Nonet, M. L. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 26, 345–356 (2000).
McCabe, B. D. et al. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron 41, 891–905 (2004).
Nakata, K. et al. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 120, 407–420 (2005). This study extends the characterization of the PHR ligase and identifies the DLK-1–MKK-4–PMK-3 cascade as a downstream effector pathway of PHR.
Li, H., Kulkarni, G. & Wadsworth, W. G. RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity. J. Neurosci. 28, 3595–3603 (2008).
Grill, B. et al. C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1. Neuron 55, 587–601 (2007).
Collins, C. A., Wairkar, Y. P., Johnson, S. L. & DiAntonio, A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron 51, 57–69 (2006).
Yan, D., Wu, Z., Chisholm, A. D. & Jin, Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 138, 1005–1018 (2009).
Liao, E. H., Hung., W., Abrams, B. & Zhen, M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature 430, 345–350 (2004).
Wu, C., Daniels, R. W. & DiAntonio, A. DFsn collaborates with Highwire to down-regulate the Wallenda/DLK kinase and restrain synaptic terminal growth. Neural Dev. 2, 16 (2007).
Burgess, R. W. et al. Evidence for a conserved function in synapse formation reveals Phr1 as a candidate gene for respiratory failure in newborn mice. Mol. Cell. Biol. 24, 1096–1105 (2004).
Tada, H. et al. Fbxo45, a novel ubiquitin ligase, regulates synaptic activity. J. Biol. Chem. 285, 3840–3849 (2010).
Bloom, A. J., Miller, B. R., Sanes, J. R. & DiAntonio, A. The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes Dev. 21, 2593–2606 (2007).
Ding, M., Chao, D., Wang, G. & Shen, K. Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science 317, 947–951 (2007). This study describes the mechanism by which the regulation of a specific SCF complex controls synapse elimination.
Yao, I. et al. SCRAPPER-dependent ubiquitination of active zone protein RIM1 regulates synaptic vesicle release. Cell 130, 943–957 (2007).
Greer, P. L. et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 140, 704–16 (2010).
Wiesner, S. et al. Autoinhibition of the HECT-type ubiquitin ligase Smurf2 through its C2 domain. Cell 130, 651–662 (2007).
Wang, J. et al. Calcium activates Nedd4 E3 ubiquitin ligases by releasing the C2 domain-mediated auto-inhibition. J. Biol. Chem. 285, 12279–12288 (2010).
Plant, P. J., Yeger, H., Staub, O., Howard, P. & Rotin, D. The C2 domain of the ubiquitin protein ligase Nedd4 mediates Ca2+-dependent plasma membrane localization. J. Biol. Chem. 272, 32329–32336 (1997).
Saha, A. & Deshaies, R. J. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol. Cell 32, 21–31 (2008).
Reyes-Turcu, F. E., Ventii, K. H. & Wilkinson, K. D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 78, 363–397 (2009).
Zeng, W. et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 141, 315–330 (2010).
Acknowledgements
We thank J. Stegmüller for helpful discussions and comments on this manuscript. Work in the authors' laboratories was supported by grants from the Max Planck Society (to N.B.), the German Research Foundation (SFB271/B11 to N.B.), the European Commission (EUSynapse, EUROSPIN and SynSys to N.B) and the Fritz Thyssen Foundation (to H.K.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Kawabe, H., Brose, N. The role of ubiquitylation in nerve cell development. Nat Rev Neurosci 12, 251–268 (2011). https://doi.org/10.1038/nrn3009
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3009
This article is cited by
-
ZNRF2 as an oncogene is transcriptionally regulated by CREB1 in breast cancer models
Human Cell (2023)
-
Genome-wide analysis of genes encoding core components of the ubiquitin system during cerebral cortex development
Molecular Brain (2022)
-
The murine ortholog of Kaufman oculocerebrofacial syndrome protein Ube3b regulates synapse number by ubiquitinating Ppp3cc
Molecular Psychiatry (2021)
-
Identification of biological mechanisms underlying a multidimensional ASD phenotype using machine learning
Translational Psychiatry (2020)
-
The role of ubiquitin ligase E3A in polarized contact guidance and rescue strategies in UBE3A-deficient hippocampal neurons
Molecular Autism (2019)