Key Points
-
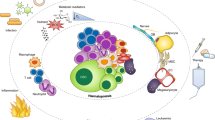
Haematopoietic stem cells (HSCs) are maintained in specialized microenvironments known as 'niches', which are composed of complex cellular and acellular components. Recent advances have contributed significantly to our understanding of what the niche is and how it changes during development, ageing and disease.
-
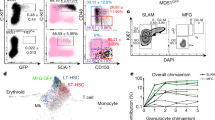
In particular, powerful new imaging techniques permit the direct observation of HSCs in their niches. These techniques provide novel insights concerning the emergence of HSCs from the dorsal aorta in fetal haematopoiesis, as well as the associations between HSCs and niche components in adult haematopoiesis.
-
Previous conceptions of niches as static, anatomically-defined bone marrow microenvironments consisting predominantly of one cell type are being challenged. Increasingly, it is becoming clear that the niche is an anatomically fluid and very dynamic environment, wherein multiple physical and cellular inputs are integrated by HSCs.
-
Physical inputs that are known to be important in haematopoiesis include oxygen tension, calcium concentration, shear force and mechanical support. Identified bone marrow niche cells include osteoblasts, vascular endothelial cells, mesenchymal stem cells, CXCL12-abundant reticular cells, neural cells and adipocytes.
-
Recent work demonstrates that dysfunctional haematopoiesis, such as that seen in patients with myelodysplasia and leukaemia, can result from defects in the microenvironment alone, without genetic perturbation of HSCs. This indicates that strategies for correcting haematopoietic diseases that target only the HSC may be insufficient or incomplete.
-
Many current clinical trials are exploiting our expanding knowledge of niche biology to improve haematopoietic stem cell transplantation. At present, such studies are largely restricted to targeting relevant niche pathways to effect HSC expansion ex vivo before transplantation; however, future clinical trials will be likely to allow more-directed manipulation of haematopoiesis in vivo.
Abstract
Haematopoietic stem cells (HSCs) are multipotent, self-renewing progenitors that generate all mature blood cells. HSC function is tightly controlled to maintain haematopoietic homeostasis, and this regulation relies on specialized cells and factors that constitute the haematopoietic 'niche', or microenvironment. Recent discoveries, aided in part by technological advances in in vivo imaging, have engendered a new appreciation for the dynamic nature of the niche, identifying novel cellular and acellular niche components and uncovering fluctuations in the relative importance of these components over time. These new insights significantly improve our understanding of haematopoiesis and raise fundamental questions about what truly constitutes a stem cell niche.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
07 December 2011
The authors wish to correct two typographical errors in the above article. On page 654, there was an error in the highlighted reference comment. The text "References 74-76 and 103 use groundbreaking imaging technology to visualize HSCs in the niche in mice. The authors of references 74, 75 and 103 image murine bones ex vivo, and the authors of references 75 and 76 image cells in the bone in vivo." should have read "References 74-76 and 103 use groundbreaking imaging technology to visualize HSCs in the niche in mice. The authors of references 74 and 103 image bones ex vivo, and the authors of references 75 and 76 image cells in the bone in vivo." Also, on page 650, 'TPO' should have been 'THPO' in figure 5 and 'TPO, thyroid peroxidase' should have read 'THPO, thrombopoietin' in the legend. The Online version has been corrected, and the authors regret any confusion caused to the readers.
References
Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978). This seminal paper was first to apply the concept of the niche to stem cell biology, postulating that loss of HSC association with the niche would result in differentiation.
Eliasson, P. & Jonsson, J. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J. Cell Physiol. 222, 17–22 (2009).
Eliasson, P. et al. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term- reconstituting hematopoietic stem cells during in vitro culture. Exp. Hematol. 38, 301–310 e2 (2010).
Kulkeaw, K., Ishitani, T., Kanemaru, T., Fucharoen, S. & Sugiyama, D. Cold exposure down-regulates zebrafish hematopoiesis. Biochem. Biophys. Res. Commun. 394, 859–864 (2010).
Adamo, L. et al. Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 (2009). Demonstrates that shear stress increases Runx1 expression and colony-forming potential in embryonic stem cells differentiated in vitro into HSCs, and in haematopoietic precursors in the AGM region of mouse embryos.
Guzmán, A. et al. Formation of micronucleated erythrocytes in mouse bone-marrow under conditions of hypothermia is not associated with stimulation of erythropoiesis. Mutat. Res. 656, 8–13 (2008).
Proulx, C., Dupuis, N., St-Amour, I., Boyer, L. & Lemieux, R. Increased megakaryopoiesis in cultures of CD34-enriched cord blood cells maintained at 39°C. Biotechnol. Bioeng. 88, 675–680 (2004).
Xie, T. & Spradling, A. C. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251–260 (1998).
Xie, T. & Spradling, A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000).
Kimble, J. E. & White, J. G. On the control of germ cell development in Caenorhabditis elegans. Dev. Biol. 81, 208–219 (1981).
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Kiel, M. J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Calvi, L. M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Gordon, M. D., Vetto, J., Meshul, C. K. & Schmidt, W. A. FNA of extraskeletal myxoid chondrosarcoma: cytomorphologic, EM, and X-ray microanalysis features. Diagn. Cytopathol. 10, 352–356 (1994).
Wright, D. E., Wagers, A. J., Gulati, A. P., Johnson, F. L. & Weissman, I. L. Physiological migration of hematopoietic stem and progenitor cells. Science 294, 1933–1936 (2001).
Dzierzak, E. & Speck, N. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nature Immunol. 9, 129–136 (2008).
Badillo, A. T. & Flake, A. W. The regulatory role of stromal microenvironments in fetal hematopoietic ontogeny. Stem Cell Rev. 2, 241–246 (2006).
Ottersbach, K., Smith, A., Wood, A. & Gottgens, B. Ontogeny of haematopoiesis: recent advances and open questions. Br. J. Haematol. 148, 343–355 (2010).
Weissman, I., Papaioannou, V. & Gardner, R. in Differentiation of Normal and Neoplastic Hematopoietic Cells (Cold Spring Harbor Laboratory, New York, 1978).
Samokhvalov, I. M., Samokhvalova, N. I. & Nishikawa, S.-I. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 446, 1056–1061 (2007).
Boisset, J.-C. et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120 (2010).
Eilken, H. M., Nishikawa, S.-I. & Schroeder, T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457, 896–900 (2009).
Cumano, A., Dieterlen-Lievre, F. & Godin, I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86, 907–916 (1996).
Kalev-Zylinska, M. L. et al. Runx3 is required for hematopoietic development in zebrafish. Dev. Dyn. 228, 323–336 (2003).
Kalev-Zylinska, M. L. et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development 129, 2015–2030 (2002).
Burns, C. E. et al. Isolation and characterization of runxa and runxb, zebrafish members of the runt family of transcriptional regulators. Exp. Hematol. 30, 1381–1389 (2002).
Kissa, K. & Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 (2010).
Rosselló, C. A. & Torres, M. Gene transfer by electroporation into hemogenic endothelium in the avian embryo. Dev. Dyn. 239, 1748–1754 (2010).
Bigas, A., Robert-Moreno, A. & Espinosa, L. The Notch pathway in the developing hematopoietic system. Int. J. Dev. Biol. 54, 1175–1188 (2010).
Wolber, F. M. et al. Roles of spleen and liver in development of the murine hematopoietic system. Exp. Hematol. 30, 1010–1019 (2002).
Yin, T. & Li, L. The stem cell niches in bone. J. Clin. Invest. 116, 1195–1201 (2006).
Askmyr, M., Sims, N. A., Martin, T. J. & Purton, L. E. What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends Endocrinol. Metab. 20, 303–309 (2009).
Kiel, M. J. & Morrison, S. J. Uncertainty in the niches that maintain haematopoietic stem cells. Nature Rev. Immunol. 8, 290–301 (2008).
Arai, F. et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004).
Nagasawa, T. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 (1996).
Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 (2006).
Barker, J. E. Sl/Sld hematopoietic progenitors are deficient in situ. Exp. Hematol. 22, 174–177 (1994).
Yoshihara, H. et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1, 685–697 (2007).
Qian, H. et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1, 671–684 (2007).
Weber, J. M. & Calvi, L. M. Notch signaling and the bone marrow hematopoietic stem cell niche. Bone 46, 281–285 (2010).
Luis, T. C. & Staal, F. J. WNT proteins: environmental factors regulating HSC fate in the niche. Ann. N. Y. Acad. Sci. 1176, 70–76 (2009).
Qian, H. et al. Distinct roles of integrins α6 and α4 in homing of fetal liver hematopoietic stem and progenitor cells. Blood 110, 2399–2407 (2007).
Magnon, C. & Frenette, P. S. Hematopoietic stem cell trafficking. StemBook 14 Jul 2008 (doi:10.3824/stembook.1.8.1).
Bertrand, J. Y. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 (2010). References 22, 23, 28 and 45 use real-time imaging to demonstrate that HSCs arise from the aortic endothelium in the AGM. References 22 and 23 show this in mice, whereas references 28 and 45 examine zebrafish.
His, W. Lecithoblast und Angioblast der Wirbelthiere. Histogenetische studien. Abhandl. Math.-Phys. Classe Konigl. Sachs. Ges. Wiss. 26, 171–328 (1900).
Murray, P. D. F. The development in vitro of the blood of the early chick embryo. Proc. R. Soc. Lond. B 111, 497–521 (1932).
Oostendorp, R. A. J. et al. Stromal cell lines from mouse aorta–gonads–mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood 99, 1183–1189 (2002).
Mascarenhas, M. I., Parker, A., Dzierzak, E. & Ottersbach, K. Identification of novel regulators of hematopoietic stem cell development through refinement of stem cell localization and expression profiling. Blood 114, 4645–4653 (2009).
Yamazaki, S. et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 25, 3515–3523 (2006).
Esner, M. et al. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development 133, 737–749 (2006).
Gekas, C., Dieterlen-Lièvre, F., Orkin, S. H. & Mikkola, H. K. A. The placenta is a niche for hematopoietic stem cells. Dev. Cell 8, 365–375 (2005).
Ottersbach, K. & Dzierzak, E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev. Cell 8, 377–387 (2005).
Kumaravelu, P. et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development 129, 4891–4899 (2002).
Ma, Q. et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl Acad. Sci. USA 95, 9448–9453 (1998).
McGrath, K. E., Koniski, A. D., Maltby, K. M., McGann, J. K. & Palis, J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev. Biol. 213, 442–456 (1999).
Christensen, J. L., Wright, D. E., Wagers, A. J. & Weissman, I. L. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2, e75 (2004).
McCulloch, E. A., Siminovitch, L., Till, J. E., Russell, E. S. & Bernstein, S. E. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype Sl/Sld. Blood 26, 399–410 (1965).
Broxmeyer, H. E. et al. The kit receptor and its ligand, steel factor, as regulators of hemopoiesis. Cancer Cells 3, 480–487 (1991).
Martin, M. A. & Bhatia, M. Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev. 14, 493–504 (2005).
Kieusseian, A. et al. Expression of Pitx2 in stromal cells is required for normal hematopoiesis. Blood 107, 492–500 (2006).
Krosl, J. et al. A mutant allele of the Swi/Snf member BAF250a determines the pool size of fetal liver hemopoietic stem cell populations. Blood 116, 1678–1684 (2010).
Chan, C. et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494 (2009).
Broxmeyer, H. E. et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 201, 1307–1318 (2005).
Shiojiri, N. Development and differentiation of bile ducts in the mammalian liver. Microsc. Res. Tech. 39, 328–335 (1997).
Ehninger, A. & Trumpp, A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J. Exp. Med. 208, 421–428 (2011).
Arai, F. et al. Niche regulation of hematopoietic stem cells in the endosteum. Ann. N. Y. Acad. Sci. 1176, 36–46 (2009).
Parmar, K., Mauch, P., Vergilio, J.-A., Sackstein, R. & Down, J. D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA 104, 5431–5436 (2007).
Winkler, I. G. et al. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood 116, 375–385 (2010). Makes the case that HSCs reside in a functionally hypoxic niche because HSCs that do not stain with Hoechst dye (and are therefore poorly-perfused) exhibit superior long-term reconstitution compared with HSCs that do take up Hoechst dye.
Bourke, V. et al. Spatial gradients of blood vessels and hematopoietic stem and progenitor cells within the marrow cavities of the human skeleton. Blood 114, 4077–4080 (2009).
Boitano, A. E. et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348 (2010). Uses a high-throughput chemical screen to identify a small molecule antagonist of the aryl hydrocarbon receptor pathway that potently expands engraftable UCB HSCs ex vivo.
Takubo, K. et al. Regulation of the HIF-1α level is essential for hematopoietic stem cells. Stem Cell 7, 391–402 (2010).
Simsek, T. et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Stem Cell 7, 380–390 (2010).
Takaku, T. et al. Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy. Blood 116, e41–e55 (2010).
Kohler, A. et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood 114, 290–298 (2009).
Lo Celso, C. et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–97 (2009).
Adams, G. B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2006).
Lam, B. S., Cunningham, C. & Adams, G. B. Pharmacologic modulation of the calcium-sensing receptor enhances hematopoietic stem cell lodgment in the adult bone marrow. Blood 117, 1167–1175 (2011).
Lymperi, S., Ersek, A., Ferraro, F., Dazzi, F. & Horwood, N. J. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood 117, 1540–1549 (2011).
Kollet, O. et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nature Med. 12, 657–664 (2006).
North, T. E. et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16, 661–672 (2002).
Keung, A. J., Healy, K. E., Kumar, S. & Schaffer, D. V. Biophysics and dynamics of natural and engineered stem cell microenvironments. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 49–64 (2010).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Gilbert, P. M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010).
Visnjic, D. et al. Conditional ablation of the osteoblast lineage in Col2.3Δtk transgenic mice. J. Bone Miner. Res. 16, 2222–2231 (2001).
Visnjic, D. et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258–3264 (2004).
Taichman, R. S., Reilly, M. J. & Emerson, S. G. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood 87, 518–524 (1996).
Nilsson, S. K. et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106, 1232–1239 (2005).
Stier, S. et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 201, 1781–1791 (2005).
Haug, J. S. et al. N-cadherin expression level distinguishes reserved versus primed states of hematopoietic stem cells. Cell Stem Cell 2, 367–379 (2008).
Fleming, H. et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2, 274–283 (2008).
Hosokawa, K. et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell Stem Cell 6, 194–198 (2010).
Hosokawa, K. et al. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood 116, 554–563 (2010).
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Lapidot, T., Dar, A. & Kollet, O. How do stem cells find their way home? Blood 106, 1901–1910 (2005).
Raaijmakers, M. H. G. P. et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852–857 (2010). Demonstrates that ablation of miRNA processing machinery in osteoblasts can give rise to myelodysplasia and leukaemia.
Chitteti, B. R. et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood 115, 3239–3248 (2010).
Nakamura, Y. et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood 116, 1422–1432 (2010).
Jung, Y. et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 38, 497–508 (2006).
Ponomaryov, T. et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Invest. 106, 1331–1339 (2000).
Porter, R. L. & Calvi, L. M. Communications between bone cells and hematopoietic stem cells. Arch. Biochem. Biophys. 473, 193–200 (2008).
Lewandowski, D. et al. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood 115, 443–452 (2009).
Xie, Y. et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 457, 97–101 (2009). References 74–76 and 103 use groundbreaking imaging technology to visualize HSCs in the niche in mice. The authors of references 74 and 103 image bones ex vivo , and the authors of references 75 and 76 image cells in the bone in vivo.
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010). Identifies NES+ MSCs as important niche cells, which are capable of maintaining HSC numbers and function.
Tzeng, Y.-S. et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood 117, 429–439 (2011).
Chen, J. et al. Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood 107, 3764–3771 (2006).
Katayama, Y. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006).
Wu, J., Scadden, D. & Kronenberg, H. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J. Bone Miner. Res. 24, 759–764 (2009).
Zhu, J. et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 109, 3706–3712 (2007).
Omatsu, Y. et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 1–13 (2010). Identifies the CAR cell as an adipo-osteogenic precursor cell capable of serving an HSC niche function, maintaining HSC numbers and function.
Spiegel, A. et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nature Immunol. 8, 1123–1131 (2007).
Spiegel, A., Kalinkovich, A., Shivtiel, S., Kollet, O. & Lapidot, T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell 3, 484–492 (2008).
Butler, J. M. et al. Endothelial cells are essential for the self-renewal and repopulation of notch-dependent hematopoietic stem cells. Stem Cell 6, 251–264 (2010).
Kobayashi, H. et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nature Cell Biol. 12, 1046–1056 (2010).
Hooper, A. T. et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Stem Cell 4, 263–274 (2009).
Cao, J. J., Sun, L. & Gao, H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann. N. Y. Acad. Sci. 1192, 292–297 (2010).
Rosen, C. J., Ackert-Bicknell, C., Rodriguez, J. P. & Pino, A. M. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit. Rev. Eukaryot. Gene Expr. 19, 109–124 (2009).
Halade, G. V., Rahman, M. M., Williams, P. J. & Fernandes, G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J. Nutr. Biochem. 21, 1162–1169 (2010).
Casamassima, F. et al. Hematopoietic bone marrow recovery after radiation therapy: MRI evaluation. Blood 73, 1677–1681 (1989).
Bredella, M. A. et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 19, 49–53 (2011).
Naveiras, O. et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263 (2009). Indicates a negative influence of bone marrow adipocytes on HSC function.
Claycombe, K., King, L. E. & Fraker, P. J. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc. Natl Acad. Sci. USA 105, 2017–2021 (2008).
Pietramaggiori, G. et al. Improved cutaneous healing in diabetic mice exposed to healthy peripheral circulation. J. Invest. Dermatol. 129, 2265–2274 (2009).
Belaid-Choucair, Z. et al. Human bone marrow adipocytes block granulopoiesis through neuropilin-1-induced granulocyte colony-stimulating factor inhibition. Stem Cells 26, 1556–1564 (2008).
Lane, S. W. et al. The Apcmin mouse has altered hematopoietic stem cell function and provides a model for MPD/MDS. Blood 115, 3489–3497 (2010). Demonstrates that expression of a hypomorphic allele of APC causes myelodysplastic/myeloproliferative disease (MDS/MPD) in an HSC-extrinsic fashion.
Walkley, C. et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor-γ deficiency. Cell 129, 1097–1110 (2007).
Walkley, C. R., Shea, J. M., Sims, N. A., Purton, L. E. & Orkin, S. H. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081–1095 (2007).
Sudo, K., Ema, H., Morita, Y. & Nakauchi, H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 192, 1273–1280 (2000).
de Haan, G., Nijhof, W. & Van Zant, G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood 89, 1543–1550 (1997).
Dykstra, B. & Haan, G. Hematopoietic stem cell aging and self-renewal. Cell Tissue Res. 331, 91–101 (2008).
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005).
Liang, Y., Van Zant, G. & Szilvassy, S. J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106, 1479–1487 (2005).
Wagner, W., Horn, P., Bork, S. & Ho, A. D. Aging of hematopoietic stem cells is regulated by the stem cell niche. Exp. Gerontol. 43, 974–980 (2008).
Chen, C., Liu, Y., Liu, Y. & Zheng, P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2, ra75 (2009). Shows that increased expression of mTOR in HSCs from old mice is responsible for the phenotypic and functional changes in haematopoiesis seen in ageing and that forced upregulation of mTOR in young HSCs makes them assume an 'aged' phenotype.
Wang, W. et al. Proteomic analysis of interstitial fluid in bone marrow identified that peroxiredoxin 2 regulates H2O2 level of bone marrow during aging. J. Proteome Res. 9, 3812–3819 (2010).
Milyavsky, M. et al. A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis-independent role for p53 in self-renewal. Stem Cell 7, 1–12 (2010).
Mohrin, M. et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell 7, 174–185 (2010).
Stevens, S. K., Moore, S. G. & Kaplan, I. D. Early and late bone-marrow changes after irradiation: MR evaluation. Am. J. Roentgenol. 154, 745–750 (1990).
Dominici, M. et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 114, 2333–2343 (2009).
Lichtman, M. A. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist 15, 1083–1101 (2010).
Busik, J. V. et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J. Exp. Med. 206, 2897–2906 (2009).
Song, J. et al. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood 115, 2592–2600 (2010).
Vunjak-Novakovic, G. & Scadden, David T. Biomimetic platforms for human stem cell research. Cell Stem Cell 8, 252–261 (2011).
McNiece, I., Harrington, J., Turney, J., Kellner, J. & Shpall, E. J. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy 6, 311–317 (2004).
Delaney, C. et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nature Med. 16, 232–236 (2010). Demonstrates that UBC HSCs can be expanded ex vivo using engineered Notch ligands and that expanded HSCs can successfully be used in clinical bone marrow transplantation.
Lutolf, M. P., Gilbert, P. M. & Blau, H. M. Designing materials to direct stem-cell fate. Nature 462, 433–441 (2009).
Goessling, W. et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136–1147 (2009).
North, T. E. & Goessling, W. NOTCHing an arrow at cord blood: translating stem cell knowledge into clinical practice. Stem Cell 6, 186–187 (2010).
Tesio, M. et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood 117, 419–428 (2011).
Acknowledgements
We thank L. Silberstein and T. Reya for provision of unpublished data and C. Dall'Osso, A. Hartigan and T. Nageswara Rao for helpful comments on the manuscript. This work was funded, in part, by grants from the US National Institutes of Health (NIH) (HL088582 and HL100402 to A.J.W.) and the Keck Foundation. L.D.W. is supported by an NIH T32 Training Grant (T32CA136432). Content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding agencies.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Aorta–gonad–mesonephros
-
(AGM). A region of embryonic mesoderm that develops from the para-aortic splanchnopleura during embryogenesis and gives rise to definitive haematopoietic stem cells.
- Myelodysplasia
-
General term for haematologic defects involving deficiencies in the myeloid blood lineages, which in fact manifest dysfunction in multiple blood lineages. Myelodysplasias are sometimes referred to as 'preleukaemias' because they can transform over time.
- Extramedullary
-
'Outside the marrow.' Used to describe blood formation occurring in anatomical locations that are distinct from the bone marrow. In adults, extramedullary haematopoiesis often signifies haematopoietic stress.
- Haemogenic endothelium
-
A layer of committed endothelial cells that have the capacity to differentiate into blood cells, losing endothelial characteristics and adopting haematopoietic features.
- Haemangioblast
-
An undifferentiated precursor cell formed during development that has the potential to give rise (at the single cell level) to blood cells and endothelial cells, but not to other cell lineages.
- Long-term HSCs
-
(Long-term haematopoietic stem cells; LT-HSCs). Cells that are capable of nearly indefinite self-renewal and differentiation into the mature cells of blood lineages (as assayed by transplantation). These are the bona fide stem cells of haematopoiesis. They are operationally distinct from short-term HSCs, which are capable of only limited self-renewal (but competent to differentiate into the mature cells of all blood lineages) and therefore cannot reconstitute haematopoiesis for the life of an organism.
- Cancellous bone
-
(Also known as trabecular bone). The spongy osseous tissue located at the ends of long bones and within vertebral bodies. It contains haematopoietic (red) marrow but, unlike the medullary cavity of the long bones, also contains trabeculae (tiny osseous beams that traverse the cancellous bone, creating an anatomical and functional structure through which blood and bone marrow elements transit).
- Homing
-
The process whereby haematopoietic stem cells (HSCs) find their way from the circulation to the haematopoietic 'niche'. It is distinct from lodgement, which is the ability of HSCs to enter into the niche and stay there, as well as from engraftment, which is the ability of HSCs to respond to appropriate maintenance and differentiation signals when lodged.
- Endosteal structures
-
Elements of the endosteum, which is a connective-tissue membrane that lines the inner surface of the medullary (marrow) cavity of the bone. Many researchers also refer to the endosteum as the inner surfaces of the bone (that is, the location of osteoblasts; see the glossary entry for osteoid cells).
- Osteoid cells
-
Cells of the bone lineage: osteocytes, osteoblasts and their precursors. Osteoclasts are not osteoid cells, as they are haematopoietic in origin. Anatomically, osteoid is the unmineralized immature bone matrix laid down by osteoblasts and containing osteocytes.
- CXCL12-abundant reticular cells
-
(CAR cells). Cells that were originally identified on the basis of their high expression of CXC chemokine ligand 12 (CXCL12). CAR cells can be perivascular or endosteal in location.
- Vascular sinusoid
-
A fenestrated capillary-sized blood vessel. Sinusoids are quite porous, permitting the free passage of many blood components.
- Parabiosis
-
A surgical procedure whereby the circulatory systems of two animals are joined such that there is free flow of blood between them.
- Non-homologous end joining
-
A double-strand DNA break repair mechanism that does not make use of a homologous DNA template and is therefore more error-prone than homologous recombination.
Rights and permissions
About this article
Cite this article
Wang, L., Wagers, A. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol 12, 643–655 (2011). https://doi.org/10.1038/nrm3184
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3184
This article is cited by
-
MiR-9-1 controls osteoblastic regulation of lymphopoiesis
Leukemia (2023)
-
Extracellular vesicle-mediated remodeling of the bone marrow microenvironment in myeloid malignancies
International Journal of Hematology (2023)
-
Advanced Electrospun Nanofibrous Stem Cell Niche for Bone Regenerative Engineering
Regenerative Engineering and Translational Medicine (2023)
-
Thioredoxin-1 regulates self-renewal and differentiation of murine hematopoietic stem cells through p53 tumor suppressor
Experimental Hematology & Oncology (2022)
-
Bone marrow imaging reveals the migration dynamics of neonatal hematopoietic stem cells
Communications Biology (2022)