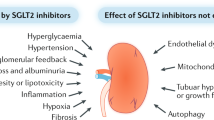
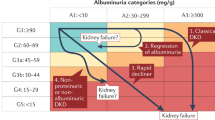
Key Points
-
Diabetes mellitus remains the major cause of end-stage renal disease in the Western world
-
Increasingly, individuals with diabetes mellitus have reduced glomerular filtration rate in the absence of albuminuria; this heterogenous group includes some individuals with classic morphological changes of diabetic nephropathy on renal biopsy
-
Clinical research activity is increasing to identify new biomarkers to predict and monitor diabetic nephropathy
-
Studies focusing on drugs that inhibit the renin–angiotensin system suggest that early administration of such agents in normalbuminuric individuals is not particularly effective at preventing the development of diabetic nephropathy
-
Whether the newer glucose lowering agents, such as glucagon-like peptide 1 analogues and dipeptidyl peptidase-4 inhibitors, will confer renoprotection, independent of their hypoglycaemic effect remains to be determined
Abstract
Nephropathy remains a major cause of morbidity and a key determinant of mortality in patients with type 1 or type 2 diabetes mellitus. Research is ongoing to identify biomarkers that in addition to albuminuria and glomerular filtration rate assist in the prediction and monitoring of renal disease in diabetes mellitus. Current strategies to treat this condition focus on intensification of glycaemic control and excellent control of blood pressure using regimens based on blockade of the renin–angiotensin system. Other approaches to control blood pressure and afford renoprotection are under active clinical investigation, including renal denervation and endothelin receptor antagonism. With increasing understanding of the underlying pathophysiological processes implicated in diabetic nephropathy, new specific renoprotective treatment strategies are anticipated to become available over the next few years.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ritz, E. & Orth, S. R. Nephropathy in patients with type 2 diabetes mellitus. N. Engl. J. Med. 341, 1127–1133 (1999).
Adler, A. I. et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 321, 412–419 (2000).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321, 405–412 (2000).
Reutens, A. T. & Atkins, R. C. Epidemiology of diabetic nephropathy. Contrib. Nephrol. 170, 1–7 (2011).
KDOQI. KDOQI Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am. J. Kidney Dis. 49, S12–S154 (2007).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013).
American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 35 (Suppl 1), S11–S63 (2012).
National Kidney, Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 39, S1–S266 (2002).
Sampson, M. J. & Drury, P. L. Accurate estimation of glomerular filtration rate in diabetic nephropathy from age, body weight, and serum creatinine. Diabetes Care 15, 609–612 (1992).
Krolewski, A. S. et al. Serum concentration of cystatin C and risk of end-stage renal disease in diabetes. Diabetes Care 35, 2311–2316 (2012).
Perkins, B. A. et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J. Am. Soc. Nephrol. 18, 1353–1361 (2007).
Andersen, A. R., Christiansen, J. S., Andersen, J. K., Kreiner, S. & Deckert, T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia 25, 496–501 (1983).
Krolewski, A. S., Laffel, L. M., Krolewski, M., Quinn, M. & Warram, J. H. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N. Engl. J. Med. 332, 1251–1255 (1995).
Cooper, M. E. Is diabetic nephropathy disappearing from clinical practice? Pediatr. Diabetes 7, 237–238 (2006).
Groop, P. H. et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58, 1651–1658 (2009).
Orchard, T. J., Secrest, A. M., Miller, R. G. & Costacou, T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 53, 2312–2319 (2010).
Adler, A. I. et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 63, 225–232 (2003).
Mogensen, C. E. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N. Engl. J. Med. 310, 356–360 (1984).
Lane, P. H., Steffes, M. W. & Mauer, S. M. Glomerular structure in IDDM women with low glomerular filtration rate and normal urinary albumin excretion. Diabetes 41, 581–586 (1992).
Macisaac, R. J. & Jerums, G. Diabetic kidney disease with and without albuminuria. Curr. Opin. Nephrol. Hypertens. 20, 246–257 (2011).
Ekinci, E. I. et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care http://dx.doi.org/10.2337/dc12-2572.
Mottl, A. K. et al. Normoalbuminuric diabetic kidney disease in the U. S. population. J. Diabetes Complications 27, 123–127 (2013).
Calcutt, N. A., Cooper, M. E., Kern, T. S. & Schmidt, A. M. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat. Rev. Drug Discov. 8, 417–429 (2009).
Wong, M. G. et al. Circulating bone morphogenetic protein-7 and transforming growth factor-β1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int. 83, 278–284 (2013).
Papale, M. et al. Urine proteome analysis may allow noninvasive differential diagnosis of diabetic nephropathy. Diabetes Care 33, 2409–2415 (2010).
Hansen, T. K. et al. Association between mannose-binding lectin, high-sensitivity C-reactive protein and the progression of diabetic nephropathy in type 1 diabetes. Diabetologia 53, 1517–1524 (2010).
Hovind, P. et al. Mannose-binding lectin as a predictor of microalbuminuria in type 1 diabetes: an inception cohort study. Diabetes 54, 1523–1527 (2005).
Gohda, T. et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J. Am. Soc. Nephrol. 23, 516–524 (2012).
Niewczas, M. A. et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 23, 507–515 (2012).
Makinen, V. P. et al. Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane Study). Metabolomics 8, 369–375 (2012).
Currie, D., McKnight, A. J., Patterson, C. C., Sadlier, D. M. & Maxwell, A. P. Investigation of ACE, ACE2 and AGTR1 genes for association with nephropathy in Type 1 diabetes mellitus. Diabet. Med. 27, 1188–1194 (2010).
Hadjadj, S. et al. Prognostic value of the insertion/deletion polymorphism of the ACE gene in type 2 diabetic subjects: results from the Non-insulin-dependent Diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events, and Ramipril (DIABHYCAR), Diabete de type 2, Nephropathie et Genetique (DIAB2NEPHROGENE), and Survie, Diabete de type 2 et Genetique (SURDIAGENE) studies. Diabetes Care 31, 1847–1852 (2008).
Ng, D. P., Tai, B. C., Koh, D., Tan, K. W. & Chia, K. S. Angiotensin-I converting enzyme insertion/deletion polymorphism and its association with diabetic nephropathy: a meta-analysis of studies reported between 1994 and 2004 and comprising 14,727 subjects. Diabetologia 48, 1008–1016 (2005).
Pezzolesi, M. G. et al. Confirmation of genetic associations at ELMO1 in the GoKinD collection supports its role as a susceptibility gene in diabetic nephropathy. Diabetes 58, 2698–2702 (2009).
Shimazaki, A. et al. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes 54, 1171–1178 (2005).
Boger, C. A. & Sedor, J. R. GWAS of diabetic nephropathy: is the GENIE out of the bottle? PLoS Genet. 8, e1002989 (2012).
Sandholm, N. et al. New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 8, e1002921 (2012).
Kantharidis, P., Wang, B., Carew, R. M. & Lan, H. Y. Diabetes complications: the microRNA perspective. Diabetes 60, 1832–1837 (2011).
Argyropoulos, C. et al. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS ONE 8, e0054662 (2013).
Forbes, J. M. & Cooper, M. E. Mechanisms of diabetic complications. Physiol. Rev. 93, 137–188 (2013).
Tervaert, T. W. et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 21, 556–563 (2010).
Gaede, P., Lund-Andersen, H., Parving, H. H. & Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358, 580–591 (2008).
Zoungas, S. et al. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care 32, 2068–2074 (2009).
Borch-Johnsen, K., Andersen, P. K. & Deckert, T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 28, 590–596 (1985).
Gaede, P. et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393 (2003).
Gaede, P., Vedel, P., Parving, H. H. & Pedersen, O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 353, 617–622 (1999).
Araki, S. et al. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes 54, 2983–2987 (2005).
Fioretto, P., Steffes, M. W., Sutherland, D. E., Goetz, F. C. & Mauer, M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N. Engl. J. Med. 339, 69–75 (1998).
Fioretto, P. et al. Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet 342, 1193–1196 (1993).
Wang, P. H., Lau, J. & Chalmers, T. C. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet 341, 1306–1309 (1993).
The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 47, 1703–1720 (1995).
Writing Team for the Diabetes Control Complications Trial/Epidemiology of Diabetes Interventions Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 287, 2563–2569 (2002).
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 837–853 (1998).
Shichiri, M., Kishikawa, H., Ohkubo, Y. & Wake, N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 23 (Suppl. 2), B21–B29 (2000).
Ohkubo, Y. et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res. Clin. Pract. 28, 103–117 (1995).
CONTROL Group. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52, 2288–2298 (2009).
Perkovic, V. et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 83, 517–523 (2013).
Yang, J. et al. Role of PPARγ in renoprotection in type 2 diabetes: molecular mechanisms and therapeutic potential. Clin. Sci. (Lond.) 116, 17–26 (2009).
Thomas, M. C., Jandeleit-Dahm, K. A. & Tikellis, C. The renoprotective actions of peroxisome proliferator-activated receptors agonists in diabetes. PPAR Res. 2012, 456529 (2012).
Graham, D. J. et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 304, 411–418 (2010).
Kermode-Scott, B. Meta-analysis confirms raised risk of bladder cancer from pioglitazone. BMJ 345, e4541 (2012).
Hocher, B., Reichetzeder, C. & Alter, M. L. Renal and cardiac effects of DPP4 inhibitors--from preclinical development to clinical research. Kidney Blood Press. Res. 36, 65–84 (2012).
Alter, M. L. et al. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press. Res. 36, 119–130 (2012).
Groop, P.-H. et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care http://dx.doi.org/10.2337/dc13-0323.
Monami, M., Ahren, B., Dicembrini, I. & Mannucci, E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 15, 112–120 (2013).
Chao, E. C. & Henry, R. R. SGLT2 inhibition–a novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 9, 551–559 (2010).
Ferrannini, E., Veltkamp, S. A., Smulders, R. A. & Kadokura, T. Renal glucose handling: Impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care 36, 1260–1265 (2013).
Vallon, V. & Thomson, S. C. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu. Rev. Physiol. 74, 351–375 (2012).
Bailey, C. J., Gross, J. L., Pieters, A., Bastien, A. & List, J. F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375, 2223–2233 (2010).
ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann. Intern. Med. 134, 370–379 (2001).
Parving, H. H. et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N. Engl. J. Med. 345, 870–878 (2001).
Andersen, S., Brochner-Mortensen, J. & Parving, H. H. Kidney function during and after withdrawal of long-term irbesartan treatment in patients with type 2 diabetes and microalbuminuria. Diabetes Care 26, 3296–3302 (2003).
Viberti, G., Wheeldon, N. M. & MicroAlbuminuria Reduction With VALsartan Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 106, 672–678 (2002).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Mogensen, C. E. et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 321, 1440–1444 (2000).
Yusuf, S. et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 358, 1547–1559 (2008).
Mann, J. F. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 (2008).
Weber, M. A. et al. Cardiovascular events during differing hypertension therapies in patients with diabetes. J. Am. Coll. Cardiol. 56, 77–85 (2010).
Jamerson, K. et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N. Engl. J. Med. 359, 2417–2428 (2008).
Dahlöf, B. et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 366, 895–906 (2005).
Ostergren, J. et al. The Anglo-Scandinavian Cardiac Outcomes Trial: blood pressure-lowering limb: effects in patients with type II diabetes. J. Hypertens. 26, 2103–2111 (2008).
Parving, H. H., Persson, F., Lewis, J. B., Lewis, E. J. & Hollenberg, N. K. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N. Engl. J. Med. 358, 2433–2446 (2008).
Parving, H. H. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367, 2204–2213 (2012).
Imai, E. et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 54, 2978–2986 (2011).
Navaneethan, S. D., Nigwekar, S. U., Sehgal, A. R. & Strippoli, G. F. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 4, 542–551 (2009).
Epstein, M. et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 1, 940–951 (2006).
Mann, J. F. et al. Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 21, 527–535 (2010).
Kohan, D. E. et al. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J. Am. Soc. Nephrol. 22, 763–772 (2011).
Krum, H. et al. Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation 123, 209–215 (2011).
Luippold, G., Beilharz, M. & Muhlbauer, B. Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol. Dial. Transplant. 19, 342–347 (2004).
Mahfoud, F. et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 123, 1940–1946 (2011).
Heusser, K. et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 55, 619–626 (2010).
Morton, J. et al. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care 35, 2201–2206 (2012).
Jenkins, A. J. et al. Serum lipoproteins in the diabetes control and complications trial/epidemiology of diabetes intervention and complications cohort: associations with gender and glycemia. Diabetes Care 26, 810–818 (2003).
Tolonen, N. et al. Lipid abnormalities predict progression of renal disease in patients with type 1 diabetes. Diabetologia 52, 2522–2530 (2009).
Bonnet, F. & Cooper, M. E. Potential influence of lipids in diabetic nephropathy: insights from experimental data and clinical studies. Diabetes Metab. 26, 254–264 (2000).
Koya, D. & Campese, V. M. Statin use in patients with diabetes and kidney disease: the Japanese experience. J. Atheroscler. Thromb. 20, 407–424 (2013).
Colhoun, H. M. et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am. J. Kidney Dis. 54, 810–819 (2009).
Jun, M. et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 60, 2061–2071 (2012).
Davis, T. M. et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 54, 280–290 (2011).
Mychaleckyj, J. C. et al. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care 35, 1008–1014 (2012).
Thomas, M. C. Emerging drugs for managing kidney disease in patients with diabetes. Expert Opin. Emerg. Drugs 18, 55–70 (2013).
Lassila, M. et al. Accelerated nephropathy in diabetic apolipoprotein e-knockout mouse: role of advanced glycation end products. J. Am. Soc. Nephrol. 15, 2125–2138 (2004).
Forbes, J. M. et al. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 17, 1762–1764 (2003).
Soulis-Liparota, T., Cooper, M., Papazoglou, D., Clarke, B. & Jerums, G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes 40, 1328–1334 (1991).
Degenhardt, T. P. et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 61, 939–950 (2002).
Thomas, M. C. et al. Interactions between renin angiotensin system and advanced glycation in the kidney. J. Am. Soc. Nephrol. 16, 2976–2984 (2005).
Forbes, J. M. et al. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes 51, 3274–3282 (2002).
Bolton, W. K. et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am. J. Nephrol. 24, 32–40 (2004).
Williams, M. E. et al. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am. J. Nephrol. 27, 605–614 (2007).
Hovind, P., Rossing, P., Tarnow, L., Johnson, R. J. & Parving, H. H. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 58, 1668–1671 (2009).
Rosolowsky, E. T. et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin. J. Am. Soc. Nephrol. 3, 706–713 (2008).
Sanchez-Lozada, L. G. et al. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrol. Dial. Transplant. 23, 1179–1185 (2008).
Achour, A. et al. One year course of oral sulodexide in the management of diabetic nephropathy. J. Nephrol. 18, 568–574 (2005).
Packham, D. K. et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 23, 123–130 (2012).
Thomas, M. C. & Cooper, M. E. Into the light? Diabetic nephropathy and vitamin D. Lancet 376, 1521–1522 (2010).
de Zeeuw, D. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376, 1543–1551 (2010).
Kim, M. J. et al. Oral cholecalciferol decreases albuminuria and urinary TGF-beta1 in patients with type 2 diabetic nephropathy on established renin-angiotensin-aldosterone system inhibition. Kidney Int. 80, 851–860 (2011).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001).
Koya, D. et al. Characterization of protein kinase C β isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J. Clin. Invest. 100, 115–126 (1997).
Kelly, D. J. et al. Protein kinase C β inhibition attenuates the progression of experimental diabetic nephropathy in the presence of continued hypertension. Diabetes 52, 512–518 (2003).
Tuttle, K. R. et al. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 28, 2686–2690 (2005).
Menne, J. et al. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-α-deficient diabetic mice. Diabetes 53, 2101–2109 (2004).
Thallas-Bonke, V. & Cooper, M. E. Tandem inhibition of PKC in diαβetic nephropathy: it takes two to tango? Diabetes 62, 1010–1011 (2013).
Menne, J. et al. Dual inhibition of classical protein kinase C-α and protein kinase C-β isoforms protects against experimental murine diabetic nephropathy. Diabetes 62, 1167–1174 (2013).
Sharma, K. et al. Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 22, 1144–1151 (2011).
Adler, S. G. et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin. J. Am. Soc. Nephrol. 5, 1420–1428 (2010).
Gilbert, R. E. et al. A purpose-synthesised anti-fibrotic agent attenuates experimental kidney diseases in the rat. PLoS ONE 7, e47160 (2012).
Thomas, M. C. & Cooper, M. E. Diabetes: bardoxolone improves kidney function in type 2 diabetes. Nat. Rev. Nephrol. 7, 552–553 (2011).
Motohashi, H. & Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 10, 549–557 (2004).
Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011).
de Zeeuw, D. et al. Rationale and trial design of bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes: the occurrence of renal events (BEACON). Am. J. Nephrol. 37, 212–222 (2013).
Tayek, J. A. & Kalantar-Zadeh, K. The extinguished BEACON of bardoxolone: not a Monday morning quarterback story. Am. J. Nephrol. 37, 208–211 (2013).
Zoja, C. et al. Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am. J. Physiol. Renal Physiol. 304, F808–F819 (2013).
Mauer, M. et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 361, 40–51 (2009).
Bilous, R. et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann. Intern. Med. 151, 11–20, W3–W4 (2009).
Haller, H. et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N. Engl. J. Med. 364, 907–917 (2011).
Remuzzi, G., Macia, M. & Ruggenenti, P. Prevention and treatment of diabetic renal disease in type 2 diabetes: the BENEDICT study. J. Am. Soc. Nephrol. 17 (4 Suppl. 2), S90–S97 (2006).
de Galan, B. E. et al. Lowering blood pressure reduces renal events in type 2 diabetes. J. Am. Soc. Nephrol. 20, 883–892 (2009).
Author information
Authors and Affiliations
Contributions
D. Fineberg researched data for the article, contributed to discussion of the content, wrote the article and reviewed and edited the manuscript before submission. K. A. M. Jandeleit-Dahm researched data for the article, contributed to discussion of the content and reviewed and edited the manuscript before submission. M. E. Cooper contributed to discussion of the content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. E. Cooper declares associations with the following companies: AstraZeneca (advisory board), Boehringer Ingelheim (speakers fees), Lilly (speakers fees), MSD (advisory board), Novo Nordisk (advisory board), Servier (speakers fees), Takeda (advisory board). The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Fineberg, D., Jandeleit-Dahm, K. & Cooper, M. Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol 9, 713–723 (2013). https://doi.org/10.1038/nrendo.2013.184
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.184
This article is cited by
-
Dapagliflozin improves podocytes injury in diabetic nephropathy via regulating cholesterol balance through KLF5 targeting the ABCA1 signalling pathway
Diabetology & Metabolic Syndrome (2024)
-
The cardio-renal-metabolic connection: a review of the evidence
Cardiovascular Diabetology (2023)
-
Biomarkers and signaling pathways of diabetic nephropathy and peripheral neuropathy: possible therapeutic intervention of rutin and quercetin
Diabetology International (2023)
-
Hsa_circ_0003928 regulates the progression of diabetic nephropathy through miR-136-5p/PAQR3 axis
Journal of Endocrinological Investigation (2023)
-
The landscape of immune cell infiltration in the glomerulus of diabetic nephropathy: evidence based on bioinformatics
BMC Nephrology (2022)