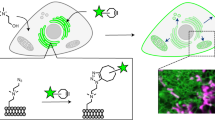
Abstract
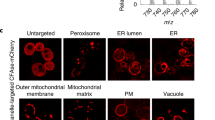
Dissecting the subcellular distribution of a fatty-acylated protein is key to the understanding of the molecular mechanisms regulating protein movement and function in a cell. This protocol describes how to perform single-cell imaging of palmitoylation in a fatty-acylated protein of interest with high sensitivity using click chemistry, proximity ligation and fluorescence microscopy. The initial steps in this protocol involve optimization of conditions for (i) metabolic incorporation of an alkynyl analog of palmitic acid into cellular proteins coupled with click chemistry and (ii) detecting a specific protein of interest with primary antibodies using automated fluorescence microscopy, followed by (iii) imaging palmitoylation of the target fatty-acylated protein of interest, such as Wnt, Sonic Hedgehog or H-Ras. Furthermore, we outline strategies for imaging specific fatty-acylated proteins with subcellular organelles and/or total proteome palmitoylation, and we discuss special considerations that need to be given depending on the experimental design. The use of clickable fatty acids with proximity ligation may have promising applications to the investigation of fatty acylation cell biology. The entire protocol takes ∼3 weeks to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hannoush, R.N. & Sun, J. The chemical toolbox for monitoring protein fatty acylation and prenylation. Nat. Chem. Biol. 6, 498–506 (2010).
Salaun, C., Greaves, J. & Chamberlain, L.H. The intracellular dynamic of protein palmitoylation. J. Cell Biol. 191, 1229–1238 (2010).
Eisenberg, S. et al. The role of palmitoylation in regulating Ras localization and function. Biochem. Soc. Trans. 41, 79–83 (2013).
Linder, M.E. & Deschenes, R.J. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8, 74–84 (2007).
Pepinsky, R.B. et al. Identification of a palmitic acid-modified form of human Sonic Hedgehog. J. Biol. Chem. 273, 14037–14045 (1998).
Porter, J.A., Young, K.E. & Beachy, P.A. Cholesterol modification of Hedgehog signaling proteins in animal development. Science 274, 255–259 (1996).
Takada, R. et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell 11, 791–801 (2006).
Gao, X. & Hannoush, R.N. Single-cell imaging of Wnt palmitoylation by the acyltransferase porcupine. Nat. Chem. Biol. 10, 61–68 (2014).
Peseckis, S.M., Deichaite, I. & Resh, M.D. Iodinated fatty acids as probes for myristate processing and function. Incorporation into pp60v-src. J. Biol. Chem. 268, 5107–5114 (1993).
Schlesinger, M.J., Magee, A.I. & Schmidt, M.F. Fatty acid acylation of proteins in cultured cells. J. Biol. Chem. 255, 10021–10024 (1980).
Gao, X. & Hannoush, R.N. Method for cellular imaging of palmitoylated proteins with clickable probes and proximity ligation applied to Hedgehog, tubulin and Ras. J. Am. Chem. Soc. 136, 4544–4550 (2014).
Hannoush, R.N. & Arenas-Ramirez, N. Imaging the lipidome: ù-alkynyl fatty acids for detection and cellular visualization of lipid-modified proteins. ACS Chem. Biol. 4, 581–587 (2009).
Fredriksson, S. et al. Protein detection using proximity-dependent DNA ligation assays. Nat. Biotechnol. 20, 473–477 (2002).
Gullberg, M. et al. Cytokine detection by antibody-based proximity ligation. Proc. Natl. Acad. Sci. USA 101, 8420–8424 (2004).
Soderberg, O. et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 (2006).
Gao, X., Arenas-Ramirez, N., Scales, S.J. & Hannoush, R.N. Membrane targeting of palmitoylated Wnt and Hedgehog revealed by chemical probes. FEBS Lett. 585, 2501–2506 (2011).
Charron, G. et al. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J. Am. Chem. Soc. 131, 4967–4975 (2009).
Martin, B.R. & Cravatt, B.F. Large-scale profiling of protein palmitoylation in mammalian cells. Nat. Methods 6, 135–138 (2009).
Yap, M.C. et al. Rapid and selective detection of fatty acylated proteins using omega-alkynyl-fatty acids and click chemistry. J. Lipid Res. 51, 1566–1580 (2010).
Hannoush, R.N. Development of chemical probes for biochemical detection and cellular imaging of myristoylated and palmitoylated proteins. Curr. Protoc. Chem. Biol. 3, 15–26 (2011).
Hannoush, R.N. Profiling cellular myristoylation and palmitoylation using omega-alkynyl fatty acids. Methods Mol. Biol. 800, 85–94 (2012).
Wang, Q. et al. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 125, 3192–3193 (2003).
Vocadlo, D.J., Hang, H.C., Kim, E.J., Hanover, J.A. & Bertozzi, C.R. A chemical approach for identifying O-GlcNAc–modified proteins in cells. Proc. Natl. Acad. Sci. USA 100, 9116–9121 (2003).
Zaro, B.W., Yang, Y.Y., Hang, H.C. & Pratt, M.R. Chemical reporters for fluorescent detection and identification of O-GlcNAc–modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc. Natl. Acad. Sci. USA 108, 8146–8151 (2011).
Clark, P.M. et al. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc–modified proteins. J. Am. Chem. Soc. 130, 11576–11577 (2008).
Golks, A., Tran, T.T., Goetschy, J.F. & Guerini, D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 26, 4368–4379 (2007).
Lamarre-Vincent, N. & Hsieh-Wilson, L.C. Dynamic glycosylation of the transcription factor CREB: a potential role in gene regulation. J. Am. Chem. Soc. 125, 6612–6613 (2003).
Ozcan, S., Andrali, S.S. & Cantrell, J.E. Modulation of transcription factor function by O-GlcNAc modification. Biochim. Biophys. Acta 1799, 353–364 (2010).
Yang, W.H. et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat. Cell Biol. 8, 1074–1083 (2006).
Heal, W.P. et al. Bioorthogonal chemical tagging of protein cholesterylation in living cells. Chem. Commun. (Camb) 47, 4081–4083 (2011).
Bothwell, I.R. et al. Se-adenosyl-L-selenomethionine cofactor analogue as a reporter of protein methylation. J. Am. Chem. Soc. 134, 14905–14912 (2012).
Willnow, S., Martin, M., Luscher, B. & Weinhold, E. A selenium-based click AdoMet analogue for versatile substrate labeling with wild-type protein methyltransferases. Chembiochem 13, 1167–1173 (2012).
Yang, Y.Y., Ascano, J.M. & Hang, H.C. Bioorthogonal chemical reporters for monitoring protein acetylation. J. Am. Chem. Soc. 132, 3640–3641 (2010).
Grammel, M., Luong, P., Orth, K. & Hang, H.C. A chemical reporter for protein AMPylation. J. Am. Chem. Soc. 133, 17103–17105 (2011).
Paulsen, C.E. et al. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 8, 57–64 (2012).
Schermelleh, L. et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, 1332–1336 (2008).
Requejo-Isidro, J. Fluorescence nanoscopy. Methods and applications. J. Chem. Biol. 6, 97–120 (2013).
Koos, B. et al. Analysis of protein interactions in situ by proximity ligation assays. Curr. Top. Microbiol. Immunol. 377, 11–26 (2013).
Leuchowius, K.J., Weibrecht, I. & Soderberg, O. In situ proximity ligation assay for microscopy and flow cytometry. Curr. Protoc. Cytom. 56, 9.36.1–9.36.15 (2011).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Petrova, E., Rios-Esteves, J., Ouerfelli, O., Glickman, J.F. & Resh, M.D. Inhibitors of Hedgehog acyltransferase block Sonic Hedgehog signaling. Nat. Chem. Biol. 9, 247–249 (2013).
Proffitt, K.D. et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 73, 502–507 (2013).
Liu, J. et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 110, 20224–20229 (2013).
Zheng, B. et al. 2-Bromopalmitate analogues as activity-based probes to explore palmitoyl acyltransferases. J. Am. Chem. Soc. 135, 7082–7085 (2013).
Jennings, B.C. et al. 2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro. J. Lipid Res. 50, 233–242 (2009).
Ducker, C.E. et al. Discovery and characterization of inhibitors of human palmitoyl acyltransferases. Mol. Cancer Ther. 5, 1647–1659 (2006).
Lawrence, D.S., Zilfou, J.T. & Smith, C.D. Structure-activity studies of cerulenin analogues as protein palmitoylation inhibitors. J. Med. Chem. 42, 4932–4941 (1999).
Draper, J.M. & Smith, C.D. Palmitoyl acyltransferase assays and inhibitors (Review). Mol. Membr. Biol. 26, 5–13 (2009).
Patterson, S.I. & Skene, J.H. Inhibition of dynamic protein palmitoylation in intact cells with tunicamycin. Methods Enzymol. 250, 284–300 (1995).
Acknowledgements
We acknowledge the use of microscopes at the Center for Advanced Light Microscopy (CALM) at Genentech.
Author information
Authors and Affiliations
Contributions
X.G. performed the experiments. X.G. and R.N.H. designed the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gao, X., Hannoush, R. Single-cell in situ imaging of palmitoylation in fatty-acylated proteins. Nat Protoc 9, 2607–2623 (2014). https://doi.org/10.1038/nprot.2014.179
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.179
This article is cited by
-
A small molecule modulating monounsaturated fatty acids and Wnt signaling confers maintenance to induced pluripotent stem cells against endodermal differentiation
Stem Cell Research & Therapy (2021)
-
Improved efficiency of in situ protein analysis by proximity ligation using UnFold probes
Scientific Reports (2018)
-
An activity-dependent proximity ligation platform for spatially resolved quantification of active enzymes in single cells
Nature Communications (2017)
-
Nonradioactive quantification of autophagic protein degradation with L-azidohomoalanine labeling
Nature Protocols (2017)
-
Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome
Nature Communications (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.