Abstract
Allergic asthma is a chronic inflammatory disorder characterized by eosinophilia and T helper type 2 (Th2) cell activation. However, little information is available on the mechanisms leading to this pathology. We previously showed that alveolar macrophages (AM) from rats with experimental asthma lose their ability to prevent asthma symptoms. To understand the implication of AM in lung immunity, we investigated the influence of AM sensitization status on lung dendritic cell (DC) activation induced by allergen challenge in vivo. Rat sensitized to ovalbumin developed airway inflammation (eosinophils and Th2 cells) and demonstrated myeloid DC (mDC) activation following allergen exposure. The replacement of AM of sensitized animals by AM from naive animals did not affect allergen-triggered eosinophilia but completely abolished lung mDC allergen capture and migration to the lymph nodes, as well as Th2 cell polarization. Moreover, immunosuppressive functions of naive AM occurred in conjunction with low engulfment of allergens but without variation of major histocompatibility complex II and CD23 expression. Interestingly, sensitized AM that were withdrawn from the inflammatory environment regained their immunosuppressive functions when transferred to sensitized rats. Thus, these are the first in vivo evidences showing that dysregulation of AM functions is sufficient to induce DC-triggered allergic response.
Similar content being viewed by others
Introduction
Lung homeostasis is achieved by complex interactions between resident and infiltrating immune cells, leading to either immune ignorance or tolerance toward innocuous antigens. Dysregulation of these processes induces inadequate immune response leading to pathologies, such as asthma. Chronic T helper type 2 (Th2) inflammatory response, airway hyper-responsiveness (AHR), and remodelling are the hallmarks of allergic asthma.1, 2 Although the roles of dendritic cells (DCs), mast cells, eosinophils, and lymphocytes in asthma pathogenesis are extensively documented, the implication of alveolar macrophages (AM) is still misunderstood.3
This lack of information is surprising given that AM are the first immune cells in contact with inhaled particles.4 Strikingly, our laboratory5, 6, 7 and others8, 9, 10, 11 showed that AM are essential to maintain lung homeostasis and dampen airway inflammation. For instance, AM depletion causes inflammation in naive mice,12 increases inflammation in animals with experimental asthma,8, 9, 10, 13 and causes asthma symptoms in a strain of rat resistant to asthma.7 Although AM functions are by default protective, multiple studies showed that AM from chronic asthmatic patients have shifted towards a pro-inflammatory phenotype,14 suggesting that AM homeostatic functions are altered in asthma. Interestingly, replacing AM of asthmatic animals with AM from naive animals, but not from sensitized rats, inhibits AHR and reduces inflammatory cytokines in bronchoalveolar lavage (BAL), independently of eosinophilia.5, 6 Moreover, the protective capacities of AM can be restored. Indeed, AM from rats with experimental asthma can be reprogrammed to a naive phenotype, when withdrawn from the asthmatic microenvironment.15 Hence, AM in asthma lose their capacity to prevent immunopathological responses. Yet, the mechanisms used by AM to maintain lung homeostasis remain poorly understood.
DCs are likely targets for AM to maintain lung homeostasis. Indeed, DCs are sentinels of the airways and critical activators of immune responses. They are found throughout the lung epithelium and have a central role in allergic airway inflammation. At least two DC subsets are found in the lung, myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), but their respective role in immunity is poorly delineated. Although pDCs are scarcer than mDCs in lung mucosa,16 both subsets are recruited to the lung and BAL of asthmatic subjects after allergen exposure.17, 18 In asthma pathogenesis, DC activation has a central role as they capture and present antigens locally and in draining lymph nodes (dLN).19, 20 In addition, DCs produce soluble mediators that polarize and induce chemotaxis of Th2 cells.21 Thus, inhibition of DCs represents a potential mechanism by which AM could prevent the development of allergic disease.
Circumstantial as well as in vitro evidences suggest that AM may be central to positively and negatively modulate DC activation.22, 23 Indeed, AM are in close proximity with DCs22 and, in asthma, AM produce soluble mediators involved in DC activation.24 Conversely, AM reduce the number of DCs into the airway lumen of naive animals25 and inhibit DC-induced proliferation of T cells in vitro.22 In addition, a recent study showed that local environment, for which AM are significant contributors, is essential to control allergen uptake by DCs.26 Given that DCs are central in allergic asthma pathogenesis19, 20 and that asthma can be prevented by grafting naive AM (nAM) into sensitized rats,5 our general hypothesis is that the ability of AM to prevent DC activation is impaired in the context of allergic airway inflammation.
Our goal was thus to determine whether restoration of AM functions would interfere with DC activation and DC-associated mechanisms of pulmonary T-cell response in the early events following allergen exposure. To reach this goal, we used an extensively validated model of asthma,26 i.e., ovalbumin (OVA)-induced allergic airway inflammation in Brown Norway rats. This model reflects many features of human asthma, including lung eosinophilia, immunoglobulin E response, airway inflammation, and AHR.27, 28, 29 In addition, rat DC subsets are similar to those observed in human.30 To look into AM functions in lung allergic airway inflammation, we took advantage of an experimental system enabling in vivo replacement of AM of sensitized animals with AM from either naive or sensitized rats. The present study shows, for the first time, that AM dysregulation in asthma is sufficient to induce DC-mediated antigenic allergic response. Most importantly, we determined that AM withdrawn from the asthmatic environment could regain those homeostatic functions, supporting that they could be targeted to interfere with lung inflammation. As a whole, our study supports that AM are dysregulated in asthma pathogenesis, which enables mDC activation. Thus, the restoration of AM regulatory functions could be a promising strategy to interfere with allergic airway inflammation.
Results
Frequency of eosinophils is not affected by the sensitization status of AM
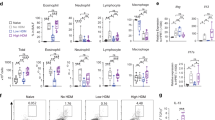
The influence of AM sensitization status on the inflammatory response induced by allergen challenge was first determined. We collected BAL cells 2 h following allergen challenge in four different groups of animals: naive animals (Control), sensitized animals (Asthmatic), and sensitized animals depleted of AM and reconstituted with AM derived from either sensitized (Asthma+sAM) or naive animals (Asthma+nAM). Naive rats displayed a homeostatic response, whereas asthmatic animals showed airway inflammation and AHR in response to aerosolized OVA.27 The total number of cells and the differential cell counts in the BAL were then characterized (Figure 1). Total cell count was similar in all the groups (Figure 1a), which is consistent with the early time point after challenge used in this study.27, 31 Although there was no difference in total cell count, the percentage of eosinophils was significantly increased in the asthmatic group (13.3±3.9%) compared with the control group (0.4±0.3%) (Figure 1b). nAM and sAM transfer did not alter eosinophil recruitment compared with the asthmatic group (Figure 1b). In addition, more neutrophils were observed in the transfer groups (Figure 1b), but the difference was not significant and was independent of AM sensitization status, as previously documented.32 Therefore, AM did not affect early eosinophil influx.
Sensitization status of alveolar macrophages (AM) does not modulate early eosinophil recruitment in the airways. Asthmatic rats were sensitized intraperitoneally with ovalbumin (OVA)/Alum. AM were depleted and replaced with AM from sensitized rats (Asthma+sAM) or naive rats (Asthma+nAM). Bronchoalveolar lavage (BAL) were performed 2 h after OVA challenge. (a) Total cell counts and (b) cellularity were measured after Trypan blue and DiffQuick stainings, respectively. Total cell numbers were similar in all the groups and eosinophilia was present in the asthmatic, Asthma+sAM, and Asthma+nAM groups. Asterisk indicates significant differences (P<0.05) compared with the naive group. Mean±s.e.m.; n=4–7 per group.
Naive AM control mDC allergen uptake but not their recruitment
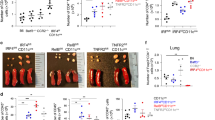
Before addressing the modulation of DC functions, we enumerated lung DC subsets, given that both mDCs and pDCs are recruited in the airways of asthmatic patients.17 major histocompatibility complex (MHC) IIhigh/CD11b+ mDCs represented around 90% (ranging from 87% to 94%) of the total MHC IIhigh/autoflorescence– DCs in naive rats, whereas MHC II+/CD11b–/CD4+ pDCs accounted for the other 10% (ranging from 6% to 13%) (Figure 2). When compared with the control group, allergen challenge induced more than a twofold increase of mDC number in the asthmatic group, reaching 1.6±0.4 × 106 cells per g of lung (Figure 2a), whereas pDC number was not affected in any condition, with approximately 0.14±0.02 × 106 cells per g of lung (Figure 2c). nAM and sAM transfer did not alter the recruitment of DCs in the lungs, when compared with the asthmatic group (Figure 2). Thus, the sensitization status of AM did not affect early DC recruitment in this model of asthma.
Dendritic cell (DC) recruitment in asthma is enhanced but not modulated by alveolar macrophages (AM). Single-cell suspensions were prepared from lungs 2 h after ovalbumin challenge. DCs were identified by flow cytometry using major histocompatibility complex (MHC) II and CD11b expression in either whole-cell suspensions for the myeloid DCs (mDCs) or after gating on CD4+ cells for the plasmacytoid DCs (pDCs). (a, c) mDC and pDC fractions; (b, d) number per g of lung. mDC numbers were increased in the asthmatic, Asthma+sAM, and Asthma+nAM (AM were depleted and replaced with AM from sensitized rats (Asthma+sAM) or naive rats (Asthma+nAM)) groups compared with the naive, whereas pDC population was not affected in any condition. Asterisk indicates significant differences (P<0.05) compared with the naive group. Mean±s.e.m.; n=4–7 per group.
We then studied the capacity of DCs to capture allergen in vivo with intranasal delivery of fluorescent OVA. mDCs were the main DC population capturing allergen. Indeed, in naive rats, 13±6 × 103 mDCs per g of lung were OVA+, compared with 0.23±0.09 × 103 for OVA+ pDCs (Figure 3). Furthermore, mDC allergen capture was enhanced by 3.9-fold in the asthmatic group (51±18 × 103 OVA+ mDCs per g of lung) compared with naive rats. Asthmatic and Asthma+sAM groups had similar number and frequency of OVA+ mDCs, whereas transfer of nAM completely abolished the increase of OVA uptake by mDCs, bringing it back to the baseline level (Figure 3a). By contrast, the frequency of OVA+ pDCs remained at the control level, averaging 0.25±0.08 × 103 OVA+ pDCs per g of lung tissue in all the conditions. These data suggest that mDC is the major DC subset sampling lung antigen in this asthma model and that the dysregulation of AM functions can profoundly affect antigen uptake by mDCs.
Dendritic cell (DC) allergen capture is decreased by alveolar macrophages (AM) from naive rats. Single-cell suspensions were prepared from lungs 2 h after instillation of ovalbumin (OVA)-AlexaFluor647 challenge. Allergen capture was assessed by measuring the frequency of AlexaFluor647+ cells on DC subsets, using flow cytometry. (a, c) Myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) OVA+ frequency; (b, d) number per g of lung. Compared with naive rats, allergen uptake was increased in the asthmatic and Asthma+sAM groups but not in the Asthma+nAM group (AM were depleted and replaced with AM from sensitized rats (Asthma+sAM) or naive rats (Asthma+nAM)). Allergen uptake was also significantly lower in the Asthma+nAM group compared with the Asthma+sAM group. pDC allergen uptake was low in all the groups tested. *P<0.05. Mean±s.e.m.; n=4–7 per group.
Transfer of nAM to rats with experimental asthma reduces accumulation of antigen-bearing DCs in dLN
Activated DCs migrate from lungs to dLN to activate T cells. To confirm that reduced antigen uptake in the lungs correlates with a lower accumulation of antigen-loaded DCs in the dLN, we characterized OVA+ DC subsets in dLN. In contrast to the lungs, dLN mDC and pDC subsets were almost equally represented in naive rats, and their number remained the same in all the groups (Figure 4). However, allergen-loaded DCs that migrated from the lungs to dLN were mainly mDCs. Indeed, <5% of OVA+ DCs were OVA+ pDCs in all the conditions tested, and this frequency was not modulated between any group (data not shown). In asthmatic rats, OVA+ mDC number increased 3.4-fold compared with naive rats, reaching 8.2±2.3 × 104 cells per dLN (Figure 5). Although the transfer of sAM did not reduce the number of OVA-bearing DCs when compared with asthmatic rats, nAM transfer reduced the number of OVA+ mDCs in the dLN to that of the level of naive rats (Figure 5). Thus, nAM transfer inhibited the accumulation of OVA+ DCs in dLN of asthmatic rats, in agreement with reduced OVA uptake in the lungs.
Dendritic cell (DC) accumulation in draining lymph nodes (dLN) is not modulated. Single-cell suspensions were prepared from dLN 2 h after ovalbumin challenge. Myeloid DCs (mDCs) and plasmacytoid DC (pDCs) were identified by flow cytometry using major histocompatibility complex II and CD11b expression. Proportion of mDCs and pDCs in dLN was similar and their numbers were not modulated in any group. *P<0.05. Mean±s.e.m.; n=4–7 per group. Asthma+sAM and Asthma+nAM, alveolar macrophages (AM) were depleted and replaced with AM from sensitized rats or naive rats.
Ovalbumin+ (OVA+) myeloid dendritic cells (mDCs) accumulation in draining lymph nodes (dLN) is inhibited by alveolar macrophages (AM) from naive rats. Single-cell suspensions were prepared from dLN 2 h after instillation of OVA-AlexaFluor647. Allergen capture was assessed by measuring the frequency of AlexaFluor647+ cells on mDC subsets, using flow cytometry. (a) OVA+ mDCs/dLN frequency; (b) number of OVA+ mDCs/dLN. Allergen uptake was increased in the asthmatic group compared with naive rats. Allergen uptake was similar between the asthmatic and Asthma+sAM groups, but was strongly reduced in the Asthma+nAM group (AM were depleted and replaced with AM from sensitized rats (Asthma+sAM) or naive rats (Asthma+nAM)). *P<0.05. Mean±s.e.m.; n=4–7 per group. MHC, major histocompatibility complex.
AM from sensitized rats fail to dampen Th2 cell polarization in the lungs
A critical step in allergic airway inflammation is the establishment of a Th2 response. The control of local T-cell activation is not only mainly orchestrated by DCs,31 but also influenced by other immune cells, including AM.33 Thus, lymphocyte populations were quantified in the lungs. Total lung Th cell (CD3+/CD4+) number was not modulated in the four groups studied, neither was the number of anti-inflammatory Foxp3+/CD25+ regulatory T cells (data not shown). However, the numbers of interleukin (IL)-4+/STAT6+ (signal transducer and activator of transcription factor 6+) Th2 cells increased by 2.4- and 1.8-fold in asthmatic and Asthma+sAM rats, respectively, compared with naive animals (Figure 6), supporting an early local polarization of T cells toward a Th2 phenotype. Strikingly, nAM transfer reduced Th2 cell number to control level. These data suggest that AM inhibit local polarization of Th2 cells in the lung, either directly or via DC modulation.
T helper type 2 (Th2)-polarized cell accumulation is enhanced locally after allergen challenge and modulated by alveolar macrophages (AM) transfer. Single-cell suspensions were prepared from lungs 2 h after ovalbumin challenge. Th2 cells were identified by flow cytometry using CD4, CD3, STAT6 (signal transducer and activator of transcription factor 6), and interleukin (IL)-4 expression. (a) Th2 cell frequency and (b) number per g of lung were higher in the asthmatic and Asthma+sAM groups compared with naive and Asthma+nAM groups (AM were depleted and replaced with AM from sensitized rats (Asthma+sAM) or naive rats (Asthma+nAM)), respectively. *P<0.05. Mean±s.e.m.; n=4–7 per group.
Reduced antigen uptake by DCs is not caused by AM elimination of antigens
As AM are the first professional phagocytes to encounter allergens, we evaluated whether the reduction of DC allergen capture observed in the Asthma+nAM group could be explained by antigen scavenging by AM in vivo. AM were identified as high autofluorescence and CD172α+ (ED9) cells. The number of AM was not affected in any condition (data not shown), but allergen uptake was modulated. Indeed, AM phagocytosis capacity in vivo was enhanced in the asthmatic group (2.2±0.3 × 105 OVA+ AM) compared with naive rats (0.8±0.2 × 105 OVA+ AM) (Figure 7). Expectedly, sAM or nAM transfer groups had similar level of AM allergen capture compared with asthmatic and naive AM, respectively (Figure 7). Hence, low allergen uptake by AM correlates with reduced DC activation (data not shown), and thus, AM allergen scavenging cannot explain the reduced allergen uptake by DCs observed in the Asthma+nAM group.
Alveolar macrophages (AM) allergen capture is enhanced in asthma but is not modulated by adoptive transfer. Bronchoalveolar lavage (BAL) were performed 2 h after instillation of ovalbumin (OVA)-AlexaFluor647. Allergen capture was assessed by measuring the frequency of AlexaFluor647+ cells in autofluoresent+/CD172α+ AM, using flow cytometry. (a) AM OVA+ frequency; (b) number of AM OVA+. Allergen capture was higher in the asthmatic and Asthma+sAM groups compared with the naive and Asthma+nAM groups (AM were depleted and replaced with AM from sensitized rats (Asthma+sAM) or naive rats (Asthma+nAM)). *P<0.05. Mean±s.e.m.; n=4–7 per group.
AM dysregulation in asthma is not associated with the modulation of MHC II and CD23
AM activation is well known in asthma. Previous studies reported that treatment of AM with granulocyte macrophage colony-stimulating factor (GM-CSF) was associated with an increased MHC II expression and an induction of DC-triggered T-cell activation.34, 35 Given that AM homeostatic functions are altered in our model of asthma, we investigated whether the GM-CSF/MHC II axis is modified. At this early time point after allergen exposure, GM-CSF levels in lung homogenate were similar between naive and asthmatic rats (2.0±1.7 vs. 2.5±0.7 ng of GM-CSF per g of lung, respectively) and the expression of MHC II on AM was not modulated 2 h after allergen challenge in any of the condition (Figure 8a). In addition, similar expression of the low immunoglobulin E receptor CD23, which is involved in allergen uptake, was measured on AM (Figure 8). These data suggest that AM dysregulation in acute airway inflammation is independent of GM-CSF, MHC II, and CD23 expression.
Major histocompatibility complex (MHC) II and CD23 expression are not modulated on alveolar macrophages (AM) early on asthma development. Bronchoalveolar lavage (BAL) were performed 2 h after instillation of ovalbumin. MHC II and CD23 expression was measured on autofluoresent+/CD172α+ AM, using flow cytometry. AM expression of (a) MHC II and (b) CD23 is depicted as the ratio of mean fluorescence intensity over naive rats. No modulation was observed. *P<0.05. Mean±s.e.m.; n=3–5 per group. Asthma+sAM and Asthma+nAM, AM were depleted and replaced with AM from sensitized rats or naive rats.
AM homeostatic functions can be restored ‘ex vivo’
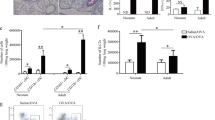
We previously demonstrated that sAM can be reprogrammed by withdrawal from the asthmatic environment and that those AM could reinstate lung homeostasis.15 As a follow-up to these observations, we investigated the modulation of DC functions by replacing AM of sensitized rats with sAM withdrawn from the asthmatic environment for 24 h (Asthma+cAM). Strikingly, mDC allergen capture in Asthma+cAM animals was markedly decreased compared with asthmatic controls (Figure 9a). Furthermore, allergen uptake by AM (Figure 9b) as well as lung Th2 cell number (Figure 9c) returned to baseline values in animals transferred with cAM. Thus, AM withdrawn from the asthmatic environment showed properties similar to that of AM from naive animals, suggesting that AM functions can be targeted to restore the homeostatic balance of the lung.
Alveolar macrophages (AM) withdrawal from the asthmatic environment restores their protective functions. Asthmatic rats were sensitized intraperitoneally with ovalbumin (OVA)/Alum and AM were replaced with ex vivo cultured sAM (AM were depleted and replaced with AM from sensitized rats) (Asthma+cAM). The effect of cAM on allergen capture by (a) myeloid dendritic cells (mDCs) and (b) AM, as well as on (c) T helper type 2 (Th2) response were measured 2 h after instillation of OVA-AlexaFluor647. (a) Frequency (left panel) and number (right panel) of OVA+ mDCs per g of lung were lower in the Asthma+cAM group compared with asthmatic rats. (b) OVA+ AM in bronchoalveolar lavage (BAL) were less frequent in Asthma+cAM than in asthmatic rats. (c) cAM transfer reduced considerably the number of Th2 cells in the lungs compared with asthmatic animals. *P<0.05. Mean±s.e.m.; n=3–5 per group. IL, interleukin; STAT, signal transducer and activator of transcription factor 6.
Discussion
AM are often forgotten in lung immunity even though they have enhanced anti-inflammatory capacities compared with resident macrophages elsewhere.11, 36 AM are central to lung homeostasis and their depletion in asthma-resistant rats results in increased AHR and airway inflammation.7 Interestingly, depletion of AM in rats with established asthma does not worsen the disease, suggesting that the homeostatic functions of AM are lost in the course of asthma pathogenesis. In accordance with this idea, the transfer of AM from naive rats into asthmatic rats reinstates homeostasis by reducing AHR and restoring a naive cytokine profile.5 Here, we show for the first time that AM are able to control mDC allergen capture in vivo and that the dysregulation of AM functions in asthma is sufficient to induce DC-triggered allergen inflammatory cascade. Indeed, AM abrogate DC allergen uptake and blunt the accumulation of Th2-polarized cells in the lungs. This is in agreement with the theory that AM insure the lack of response against innocuous antigen by increasing the immune activation threshold. Importantly, we determined that the control of DC functions could be restored by AM withdrawn from the asthmatic environment, supporting that AM could be targeted to short-circuit the early events following allergen exposure. In addition, we found that mDCs, not pDCs, are the prominent lung DC subset and that they are the first DC subtype uptaking and carrying the allergen to dLN. This supports their critical involvement in allergic airway disease.
Our data strongly argue for a central role of AM to maintain lung homeostasis by limiting DC allergen uptake. This in vivo study design allowed the validation of previous findings obtained in vitro that suggested that AM can inhibit T-cell proliferation induced by DCs.22 However, this is in contradiction to the recent report of Bedoret et al.,37 which showed that interstitial macrophages (IM), and not AM, have an important role in lung homeostasis in allergic asthma. This discrepancy could be explained, at least in part, by the experimental design of their study. Instead of challenging animal with nebulized or instilled allergens, they administered allergen-loaded DCs (bone marrow DCs primed with lipopolysaccharide (LPS) and OVA) to induce asthma, which bypasses many immunological processes, including DC allergen capture. Given that naive AM reduced allergen uptake by DCs, this could explain why their study overlooked AM immunosuppressive functions. Furthermore, IM mechanisms of action are likely different from the ones used by AM. Indeed, IM produce IL-10 in a Toll-like receptor 4–dependent fashion,37 whereas our study used an LPS-free model of allergy, and no increase of IL-10 level is detected in the BAL of Asthma+nAM rats compared with Asthma+sAM and asthmatic animals.5 Thus, the protective effects of AM in the current model are likely independent of IL-10.
To further investigate the mechanisms involved in AM regulation of mDCs, we explored a likely mechanism described under generic inflammatory condition (LPS exposition), i.e., the alteration of AM phenotype by GM-CSF.34, 35 Indeed, the increase of GM-CSF in LPS-exposed lungs blunted the protective functions of AM on DC activation and increased AM expression of MHC II.34, 35 The current model addresses early steps of asthma, and no modulation of GM-CSF level was observed, even though increased production is observed in adult patients with a history of asthma.38 Given that neither modulation of GM-CSF in lung tissue nor MHC II expression on AM was observed, it is unlikely that AM dysregulation is caused by GM-CSF at this early stage of the disease. Furthermore, CD23 (pro-inflammatory) involvement may also be discarded to explain AM dysregulation, as it was not modulated in the conditions studied here. Thus, our asthma model enabled the study of early steps involved in asthma exacerbation, including natural DC activation, which allowed the identification of a novel role of AM in lung homeostasis: the early inhibition of allergen uptake by mDCs.
Our study shows for the first time that, rapidly after allergen exposure, mDCs are the main DC subset capturing allergens in the lung and that this capacity is drastically increased in asthmatic animals. This observation is in agreement with the preferential uptake of house-dust mite by lung mDCs in a mouse model of inflammation.39 However, the capacity of pDCs to capture allergens is somewhat more controversial. A first study showed a strong accumulation of OVA+ pDCs in dLN 36 h after allergen challenge,40 whereas another study showed no lung or dLN pDCs positive for house-dust mite 3 days after exposition.41 The different types of allergens could explain this discrepancy, but in our hand pDCs did not capture allergens. Given that allergen capture is a rapid process16, 42 and that allergen can be transferred between DC populations,43 this discrepancy is more likely to be the result of timing. Thus, our data support that, early after allergen exposure, resident mDCs, but not pDCs, quickly sample allergens from the airways and then migrate to the dLN.
In a previous work,5 we reported the dissociation between eosinophilia and AHR, as observed in several experimental models, as well as in humans.44, 45, 46 Interestingly, the present study showed that Th2 cell accumulation, rather than eosinophilia, could be linked with AHR. These observations are in agreement with Venkayya et al.47 who showed that Th2 cell–conditioned media can induce AHR via IL-4 and/or IL-13. The present study thus unravels that AM could control allergic airway disease–associated AHR by interfering with the Th2 cellular response. Secondarily, our study strengthens the observation that AM can shift between different phenotypes.15, 48, 49 Indeed, we showed that the plasticity of AM allows the restoration of the homeostatic functions of sAM, when they are withdrawn for the inflammatory environment. AM plasticity is an interesting point for future therapeutic options but complicates the identification of key mechanisms using in vitro studies.
Overall, these findings are the first to show in vivo that AM are dysregulated in asthma, which is sufficient to unleash mDC allergen sampling and Th2 cell accumulation in the lungs. Thus, AM immunomodulatory functions are essential to short-circuit the pathogenesis of allergic airway diseases. We showed that AM protective functions are mediated, at least in part, by effects on mDCs (not pDCs), rather than by their ability to scavenge allergens. In addition, asthmatic AM lose their capacity to inactivate DCs, but AM plasticity enables them to regain their homeostatic functions, making AM an interesting target for novel therapies. Current studies in our laboratory are performed to evaluate how AM functions could be controlled to reduce DC activation and asthma pathogenesis in patients.
Methods
Animals, treatments, and allergen exposure. Eight- to-twelve-week-old pathogen-free Brown Norway rats from Harlan Laboratory (Indianapolis, IN) were used. They were maintained at the animal facility of the IUCPQ on a 12-h light/dark cycle in filter top cages to ensure virus/pathogen-free conditions. Food and water were given ad libitum. The protocol was approved by Laval University Animal Care Committee. OVA (Sigma Chemical, St Louis, MO) sensitization was performed once by intraperitoneal injection of 1 mg OVA/10 mg aluminum hydroxide (Sigma Chemical), and rats were challenged 21 days later by intranasal instillation of 75 μg of OVA-Alexa647 (Invitrogen, Grand Island, NY) in 100 μl of saline. All the groups were challenged with OVA, and tissues were harvested 2 h after exposure.
AM elimination and grafting. AM depletion was achieved by Clodronate liposome (Clodrosome; Encapsula, Nashville, TN) administration as previously described.6 Briefly, 17 days after sensitization, 100 μl of 5 mg ml−1 Clodrosome was instilled in each lobe (right and left). At the maximal depletion time, 3 days later (day 20), these animals received AM intratracheally, which were obtained from sensitized (Asthma+sAM) or naive (Asthma+nAM) rats. Also, in some experiments, AM-depleted rats received sAM kept ex vivo in serum-free complete RPMI medium (Gibco BRL, Burlington, Canada) in non-adherent tubes for 24 h (Asthma+cAM).15
Cell isolation and preparation. To identify cell types and to measure AM allergen capture, BAL were performed as previously described,6 with 50 mL of phosphate-buffered saline–EDTA. Cell types were identified using cytospins stained with Diff-Quik (Gibco BRL). Lung tissue and dLN were prepared as previously reported.26 Briefly, tissues were digested with Collagenase IV and DNase I (Worthington Biochemical, Lakewood, NJ) to obtain single-cell suspension. Identification of cell populations was performed using monoclonal antibodies (Biolegend, San Diego, CA; BD Pharmingen, San Diego, CA; R&D, Minneapolis, MN) directed against cell-surface antigens and intracellular markers. mDCs are CD11b+/MHC IIhigh, pDCs are CD11b–/MHC II+/CD4+, and AM are CD172α+(ED9)/autofluorescencehigh. T-cell populations were identified using CD4, CD3, IL-4, FoxP3, STAT6, and CD25 expression. Allergen capture was measured by AlexaFluor647 fluorescence, and AM activation was assessed with MHC II and CD23 expression. Data were acquired on a BD FACS Aria II (Becton Dickinson, Franklin Lakes, NJ) and analyzed using Flowjo software (Tree star, Ashland, OR).
Lung digestion and enzyme-linked immunosorbent assay. To measure cytokine level in lung tissue, approximately 10 mg of lung tissue was placed in ice-cold phosphate-buffered saline supplemented with protease and phosphastase inhibitor cocktail (Roche, Mannheim, Germany) and homogenized with a Polytron homogenizer (Omni International, Marietta, GA). Enzyme-linked immunosorbent assays were performed with DuoSet kit (R&D Systems) to measure GM-CSF level, and results are expressed per gram of tissue.
Statistics. Prism (GraphPad Software, Inc., La Jolla, CA) was used for all statistical analysis, which includes one-way and two-way analysis of variance with Bonferroni post hoc test. Data are presented as mean±s.e.m., and P values <0.05 were considered significant.
References
Elias, J.A., Lee, C.G., Zheng, T., Ma, B., Homer, R.J. & Zhu, Z. New insights into the pathogenesis of asthma. J. Clin. Invest. 111, 291–297 (2003).
Holgate, S.T. Pathogenesis of asthma. Clin. Exp. Allergy 38, 872–897 (2008).
Peters-Golden, M. The alveolar macrophage: the forgotten cell in asthma. Am. J. Respir. Cell Mol. Biol. 31, 3–7 (2004).
Lambrecht, B.N. Alveolar macrophage in the driver's seat. Immunity 24, 366–368 (2006).
Careau, E., Proulx, L.I., Pouliot, P., Spahr, A., Turmel, V. & Bissonnette, E.Y. Antigen sensitization modulates alveolar macrophage functions in an asthma model. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L871–L879 (2006).
Careau, E., Turmel, V., Lauzon-Joset, J.F. & Bissonnette, E.Y. Alveolar macrophages reduce airway hyperresponsiveness and modulate cytokine levels. Exp. Lung Res. 36, 255–261 (2010).
Careau, E. & Bissonnette, E.Y. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 31, 22–27 (2004).
Thepen, T., McMenamin, C., Oliver, J., Kraal, G. & Holt, P.G. Regulation of immune response to inhaled antigen by alveolar macrophages: differential effects of in vivo alveolar macrophage elimination on the induction of tolerance vs. immunity. Eur.J. Immunol. 21, 2845–2850 (1991).
Bang, B.R. et al. Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Exp. Mol. Med. 43, 275–280 (2011).
Tang, C. et al. Th type 1-stimulating activity of lung macrophages inhibits Th2-mediated allergic airway inflammation by an IFN-gamma-dependent mechanism. J. Immunol. 166, 1471–1481 (2001).
Naessens, T. et al. Innate imprinting of murine resident alveolar macrophages by allergic bronchial inflammation causes a switch from hypoinflammatory to hyperinflammatory reactivity. Am. J. Pathol. 181, 174–184 (2012).
Thepen, T., Van Rooijen, N. & Kraal, G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J. Exp. Med. 170, 499–509 (1989).
Thepen, T., Kraal, G. & Holt, P.G. The role of alveolar macrophages in regulation of lung inflammation. Ann. N Y Acad. Sci. 725, 200–206 (1994).
Byers, D.E. & Holtzman, M.J. Alternatively activated macrophages and airway disease. Chest 140, 768–774 (2011).
Pouliot, P., Spahr, A., Careau, E., Turmel, V. & Bissonnette, E.Y. Alveolar macrophages from allergic lungs are not committed to a pro-allergic response and can reduce airway hyperresponsiveness following ex vivo culture. Clin. Exp. Allergy 38, 529–538 (2008).
Fear, V.S. et al. Restricted aeroallergen access to airway mucosal dendritic cells in vivo limits allergen-specific CD4+ T cell proliferation during the induction of inhalation tolerance. J. Immunol. 187, 4561–4570 (2011).
Bratke, K. et al. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax 62, 168–175 (2007).
Jahnsen, F.L., Moloney, E.D., Hogan, T., Upham, J.W., Burke, C.M. & Holt, P.G. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax 56, 823–826 (2001).
Holt, P.G. Dendritic cells as sentinel cells in asthma. Clin. Exp. Allergy Rev. 1, 77–79 (2001).
van Rijt, L.S. et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201, 981–991 (2005).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3, 673–680 (2002).
Holt, P.G. et al. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Exp. Med. 177, 397–407 (1993).
Kim, J.Y. et al. Alveolar macrophages play a key role in cockroach-induced allergic inflammation via TNF-alpha pathway. PloS one 7, e47971 (2012).
Moreira, A.P. & Hogaboam, C.M. Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution. J. Interferon Cytokine Res. 31, 485–491 (2011).
Jakubzick, C., Tacke, F., Llodra, J., van Rooijen, N. & Randolph, G.J. Modulation of dendritic cell trafficking to and from the airways. J. Immunol. 176, 3578–3584 (2006).
Strickland, D.H. et al. Defective aeroallergen surveillance by airway mucosal dendritic cells as a determinant of risk for persistent airways hyper-responsiveness in experimental asthma. Mucosal Immunol. 5, 332–341 (2012).
Careau, E., Sirois, J. & Bissonnette, E.Y. Characterization of lung hyperresponsiveness, inflammation, and alveolar macrophage mediator production in allergy resistant and susceptible rats. Am. J. Respir. Cell Mol. Biol. 26, 579–586 (2002).
Tschernig, T., Neumann, D., Pich, A., Dorsch, M. & Pabst, R. Experimental bronchial asthma - the strength of the species rat. Curr. Drug Targets 9, 466–469 (2008).
Martin, J.G. & Tamaoka, M. Rat models of asthma and chronic obstructive lung disease. Pulm. Pharmacol. Ther. 19, 377–385 (2006).
Sung, S.S. & Bolton, W.K. Editorial: Are men rats? Dendritic cells in autoimmune glomerulonephritis. J. Leuk. Biol. 88, 831–835 (2010).
Out, T.A., Wang, S.Z., Rudolph, K. & Bice, D.E. Local T-cell activation after segmental allergen challenge in the lungs of allergic dogs. Immunology 105, 499–508 (2002).
Berg, J.T., Lee, S.T., Thepen, T., Lee, C.Y. & Tsan, M.F. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J. Appl. Physiol. 74, 2812–2819 (1993).
Strickland, D.H., Thepen, T., Kees, U.R., Kraal, G. & Holt, P.G. Regulation of T-cell function in lung tissue by pulmonary alveolar macrophages. Immunology 80, 266–272 (1993).
Bilyk, N. & Holt, P.G. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 177, 1773–1777 (1993).
Stampfli, M.R. et al. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J. Clin. Invest. 102, 1704–1714 (1998).
Snelgrove, R.J. et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 9, 1074–1083 (2008).
Bedoret, D. et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Cin. Invest. 119, 3723–3738 (2009).
Woolley, K.L., Adelroth, E., Woolley, M.J., Ellis, R., Jordana, M. & O'Byrne, P.M. Granulocyte-macrophage colony-stimulating factor, eosinophils and eosinophil cationic protein in subjects with and without mild, stable, atopic asthma. Eur. Respir. J. 7, 1576–1584 (1994).
Lewkowich, I.P., Lajoie, S., Clark, J.R., Herman, N.S., Sproles, A.A. & Wills-Karp, M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PloS one 3, e3879 (2008).
de Heer, H.J. et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 200, 89–98 (2004).
Plantinga, M. et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38, 322–335 (2013).
Huh, J.C. et al. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J. Exp. Med. 198, 19–30 (2003).
Knight, S.C., Iqball, S., Roberts, M.S., Macatonia, S. & Bedford, P.A. Transfer of antigen between dendritic cells in the stimulation of primary T cell proliferation. Eur. j. immunol. 28, 1636–1644 (1998).
Bradley, B.L. et al. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J. Allergy Clin. Immunol. 88, 661–674 (1991).
Wardlaw, A.J., Dunnette, S., Gleich, G.J., Collins, J.V. & Kay, A.B. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am. Rev. Respir. Dis. 137, 62–69 (1988).
Birrell, M.A., Battram, C.H., Woodman, P., McCluskie, K. & Belvisi, M.G. Dissociation by steroids of eosinophilic inflammation from airway hyperresponsiveness in murine airways. Respir. Res. 4, 3 (2003).
Venkayya, R., Lam, M., Willkom, M., Grunig, G., Corry, D.B. & Erle, D.J. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am. J. Respir. Cell Mol. Biol. 26, 202–208 (2002).
Korf, J.E. et al. Macrophage reprogramming by mycolic acid promotes a tolerogenic response in experimental asthma. Am. J. Respir. Crit. Care Med. 174, 152–160 (2006).
Johnston, L.K., Rims, C.R., Gill, S.E., McGuire, J.K. & Manicone, A.M. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am. J. Respir. Cell Mol. Biol. 47, 417–426 (2012).
Acknowledgements
This work was supported by Canadian Institutes of Health Research (MOP-84346) and Fondation JD Bégin. J.F.L.J. was supported by Fonds de Recherche du Québec - Santé. We thank Emilie Bernatchez and Marc Veillette for technical help. We also thank Dr Ynuk Bossé and Dr Yvon Cormier for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lauzon-Joset, JF., Marsolais, D., Langlois, A. et al. Dysregulation of alveolar macrophages unleashes dendritic cell–mediated mechanisms of allergic airway inflammation. Mucosal Immunol 7, 155–164 (2014). https://doi.org/10.1038/mi.2013.34
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2013.34
This article is cited by
-
The role of extracellular vesicles when innate meets adaptive
Seminars in Immunopathology (2018)
-
A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes
Nature Immunology (2017)
-
The contributions of lung macrophage and monocyte heterogeneity to influenza pathogenesis
Immunology & Cell Biology (2017)
-
Curcumin Nanoparticles Attenuate Production of Pro-inflammatory Markers in Lipopolysaccharide-Induced Macrophages
Pharmaceutical Research (2016)