Abstract
Immune tolerance to allergens in early-life decreases the risk for asthma in later life. Here we show establishment of stable airway tolerance to the allergen, house dust mite (HDM), by exposing newborn mice repeatedly to a low dose of the allergen. Lung dendritic cells (DCs) from tolerized mice induced a low Th2 response in vitro mirroring impact of tolerance in vivo. In line with our previous finding of increased mitochondrial H2O2 production from lung DCs of mice tolerized to ovalbumin, depletion of mitochondrial H2O2 in MCAT mice abrogated HDM-induced airway tolerance (Tol) with elevated Th2 effector response, airway eosinophilia, and increased airway hyperreactivity. WT-Tol mice displayed a decrease in total, cDC1 and cDC2 subsets in the lung as compared to that in naive mice. In contrast, the lungs of MCAT-Tol mice showed 3-fold higher numbers of cDCs including those of the subsets as compared to that in WT mice. Our study demonstrates an important role of mitochondrial H2O2 in constraining lung DC numbers towards establishment of early-life airway tolerance to allergens.
Similar content being viewed by others
Introduction
In 1951, a study showed that pre-exposure to a high dose of an antigen induced severe asthma, whereas a low dose caused hyposensitization.1 Follow-up studies confirmed that repeated exposure to low doses of an allergen prevents adverse reactions upon subsequent exposure to the same allergen.2,3 First described in 1911,4 allergen immunotherapy (AIT) involving desensitization with low doses of allergens rather than complete avoidance has been effectively practiced to treat a broad spectrum of allergic diseases including food allergy,2,5,6,7 atopic dermatitis,8,9 and allergic rhinitis.10,11 However, clinical trials of AIT in asthma are rare3,12,13 due to safety concerns and lack of adequate experimental evidence.14 This necessitates the development of animal models of airway tolerance to common aeroallergens for future translation.
Many studies suggest a critical time window in early life for achieving tolerance to prevent development of allergic disease,15 as is also evident in immunotherapy to establish tolerance to peanuts.2 This knowledge provides an opportunity to establish early-life tolerance to common aeroallergens associated with the asthmatic diathesis. We previously demonstrated PPARγ-mediated production of the reactive oxygen species, H2O2, from mitochondria in lung CD11c+ cells in the context of inhaled tolerance to the model allergen ovalbumin (OVA).16 A pharmacological approach that indiscriminately scavenged all reactive oxygen species from mitochondria prevented tolerance establishment and instead induced eosinophil-dominated airway inflammation in response to allergen challenge in the mice.16 In this study we show that repeated exposure of young mice to a low dose of the common household allergen, house dust mite (HDM), which has been associated with asthma onset,17 was effective in achieving tolerance to HDM. Additionally, targeted overexpression of catalase in transgenic mice (MCAT) to specifically deplete H2O2 in mitochondria abolished establishment of inhaled tolerance to HDM. Functionally, we describe an impact of tolerance on constraining numbers and gene expression in lung dendritic cells (DCs) that is affected in MCAT mice.
Results
Early-life exposure to low-dose HDM prevents allergic airway inflammations in later life
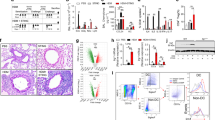
Since early-life exposure to allergens can cause immune tolerance,2,15 we established a mouse model of airway tolerance to HDM by repeatedly exposing young mice to a low dose of HDM. This model consists of two phases (Fig. 1a). First, an early sensitization phase involves intranasal administration of a relatively low dose of HDM to 2-week-old mouse pups, the age being equivalent to <6-month-old humans.18,19 This is followed by a challenge phase involving repeated administration of a higher dose of HDM when the pups reach 6 weeks of age, which is equivalent to the age of puberty onset in humans. Histologic assessment of lung sections following staining for mucus showed that compared to untreated naive animals, mice immunized to mount airway inflammation without any early-life exposure to HDM and pre-exposed instead only to PBS followed by multiple exposures to HDM at 6 weeks of age show increased inflammation around bronchovascular bundles and increased staining for mucus (Fig. 1b, c; labeled No HDM group). In contrast, repeated pre-exposure to a low dose of 0.5 μg HDM (labeled Low HDM) at 2 weeks of age prevented the development of airway inflammation and mucus in the airways when the mice were later challenged at 6 weeks, as in the No HDM group (Fig. 1b, c). However, this protective effect was not observed when a 10× higher dose of 5 μg (labeled High HDM) was used for sensitization at 2 weeks of age (Fig. 1b, c). Mice in the No HDM group displayed a higher total cell number in the bronchoalveolar lavage (BAL) fluid that included increased numbers of eosinophils (predominant cell type), neutrophils and lymphocytes (Fig. 1d) indicative of robust airway inflammation in response to repeated exposure to inhaled HDM at 6 weeks of age. In line with the profiles of lung histologies (Fig. 1b, c), Low HDM mice showed lower BAL fluid cellularity compared to those in the No HDM group (Fig. 1d). In marked contrast, the High HDM group failed to show tolerance to HDM, instead showing a response of increased airway inflammation upon HDM challenge (Fig. 1d), demonstrating a protective role of low-dose allergen exposure.
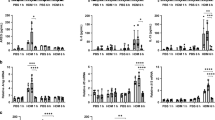
a Schematic for low-dose HDM-induced early-life airway tolerance model. Other groups for comparison included pre-exposure to either PBS (No HDM) or a higher dose of HDM. b, c Analysis of lung tissue isolated from WT mice sensitized to a low dose of HDM (0.5 µg, referred to here as Low HDM) or a high dose of HDM (5.0 µg, referred to here as High HDM) or without any HDM pre-exposure (referred to as No HDM) at 2 weeks of age, followed by challenges with 25 µg of HDM at 6 weeks of age. Animals without any exposure to vehicle or HDM were considered as naïve controls. Histological assessment of lung sections after PAS staining showing cellular infiltration around blood vessels and airways (black arrow) and mucus staining in the small airways (red arrow). Scale bar:100 µm. Data shown in (b) are representative results and in (c) are median ± IQR summarized from two independent experiments with 6 mice per group. d Analysis of differential cell counts showing frequency and absolute number of inflammatory cells in the BAL fluid. Data shown are median ± IQR combined from three independent experiments with 7–13 mice per group in pooled data. e Expression of Ifng, Il-13 and Il-17a mRNA in lung tissue was assessed by RT-qPCR methods. Data shown are median ± IQR of pooled data from two independent experiments with 4–6 mice per group. f Representative flow plots of the percentage of Th2 cells (GATA-3+IL-13+ and GATA-3+IL-5+) (gated on live CD4+TCRβ+FoxP3−cells) and FoxP3−IL-10+ and FoxP3+IL-10+ populations (gated on live CD4+TCRβ+) in the lungs. g quantification of the percentages of cell types shown in (e) in each mouse. Data shown are median ± IQR combined from three independent experiments with 6–10 mice per group (pooled). Kruskal–Wallis with Dunn’s post hoc test (c, d, e, g). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; nsP > 0.05.
HDM is a complex allergen that induces a mixed T-helper cell response.20 Indeed, animals without HDM pre-exposure (No HDM group) displayed increased expression of Ifng, Il-13, and Il-17a mRNA in the lung tissue compared to that detected in the lungs of naïve mice (Fig. 1e). Mice with early-life exposure to either low or high dose of HDM showed comparable Ifng mRNA levels. However, in the Low but not the High HDM group, lower levels of Il-13 and Il-17a mRNA were detected compared to that in the No HDM group (Fig. 1e). Analysis of cytokine expression in lung cells by intracellular cytokine staining (ICS) methods showed a significant reduction in IL-13- and IL-5-producing CD4+ T cells in the Low HDM group as compared to that in the No HDM or High HDM group (Fig. 1f, g), consistent with reduced staining for mucus in the airways (Fig. 1b, c) and lower eosinophil numbers in the BAL fluid of mice in this group (Fig. 1d). We also found a significant reduction in the frequency of CD4+Foxp3− T cells expressing IL-10 in the Low HDM group as compared to that in the No HDM group (Fig. 1f, g). This was in line with increased IL-10 expression previously observed in the context of an effector Th2 response.21 However, the frequency of IL-10+CD4+FoxP3+ T cells (resembling regulatory T cells-Tregs) was also lower in the Low HDM tolerance group (Fig. 1f, g) suggesting that this mode of tolerance establishment in early life did not result in a higher regulatory T-cell response in the lungs of the mice.
Taken together, we were able to establish a mouse model of airway tolerance, which was induced by exposure to a low dose of the common allergen HDM in early life. This model presented an opportunity to study tolerance-inducing mechanisms that effectively blocked allergic responses to a common allergen.
Differential impact of dendritic cells on T cells depending on dose of HDM exposure
DCs play a critical role in antigen processing and presentation to naive T cells inducing T-cell activation, proliferation and differentiation. To assess the impact of low dose HDM exposure on DC function, we performed an in vitro DC-T-cell short-term co-culture assay using DCs from mice exposed to either high or low-dose HDM and naïve T cells from OVA-specific OT-II TCR transgenic mice. DCs isolated and purified from the lungs of the pups exposed to High HDM dose (5.0 µg) that primed for inflammation or Low HDM dose (0.5 µg) that primed for tolerance were co-cultured for 5 days with naïve CD4+ T cells from OT-II mice, and the OVA peptide, OVA323–339, OT-II mice being TCR transgenic (Tg) with specificity for this epitope of OVA. Since an HDM TCR Tg mouse was not available to us, we tested the function of the DCs isolated from our model by using T cells from OT-II mice as a surrogate. In lieu of the cognate TCR Tg system, this approach has been used in multiple studies to assess the functional capacity of DCs exposed to an allergen or pathogen in vivo.22,23 The ability of the DCs for priming and polarizing naive CD4+ T cells was determined by assessing the profile of the co-cultured CD4+ T cells by flow cytometry. Of note, these experiments involved only a priming step over a 5-day period and did not involve any antigen-restimulation to boost the initial response. Consistent with the in vivo data, lung DCs from mice in the Low HDM group resulted in fewer polarized Th2 cells (GATA-3+IL-13+) compared to their counterparts isolated from the High HDM group (Fig. 2a, b). Also, in accordance with the in vivo data, lung DCs from the tolerized group induced a lower frequency of FoxP3-IL-10+ CD4+ T cells as compared to those from the latter group (Fig. 2e, f). We did not, however, detect any difference in the frequency of Foxp3+IL-10+ cells (Tregs) in the two groups (Fig. 2e, f). In additional experiments performed to assess the level of secreted cytokines in the culture supernatants, we also detected significantly lower levels of Th2 cytokines in the supernatants originating from co-culture with DCs from exposure to Low dose HDM as compared to those corresponding to the High HDM dose exposure (Fig. 2g). Interestingly, we detected comparable IFN-γ levels in the supernatants of the two groups (Fig. 2g) akin to observations in vivo (Fig. 1e) suggesting a specific inability of DCs from the Low dose HDM-exposed group to stimulate a Th2 response rather than a general impairment in T-cell-stimulatory function, as also evident in data shown in Fig. 1 after repeated challenge with HDM in vivo. However, one limitation of the experimental setup for assessment of DC function ex vivo was that correspondingly we were unable to assess the functional capacity of the DCs using T cells from transgenic mice with TCR specificity for one of the HDM antigens, Der p 1.24
24 h after the last HDM sensitization regimen, DCs purified from lungs of WT mice sensitized to high-dose HDM (5.0 µg) or low-dose HDM (0.5 µg) were co-cultured with naive CD4+ T cells from OT-II mice in vitro for 5 days in the presence of OVA peptide. DCs in each group were pooled from 3 to 5 mice. a, c, e Representative flow plots of the percentage of Th2 cells (GATA-3+IL-13+) (gated on live CD4+ CD25+ cells), total IL-10+ population (gated on live CD4+ cells), FoxP3− IL-10+, and FoxP3+ IL-10+ populations (gated on live CD4+ cells) in the lungs. b, d, f Quantifications of the percentages of cell types shown in (a, c, e) in each mouse. g ELISA measurements of the indicated cytokines present in culture supernatants after 5 days of co-culture. Data shown are median ± IQR combined from three independent experiments that includes technical replicates in each group. Each data point represents cytokine level in a single well. Two-tailed Mann–Whitney U test (b, d, f, g). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; nsP > 0.05.
Depletion of mitochondrial H2O2 abolishes active suppression of allergic airway inflammation
We next investigated the molecular mechanism by which airway tolerance is established after early-life exposure to a low dose of HDM. In our previous study, we showed production of hydrogen peroxide (H2O2) by lung DCs in our model of immune tolerance using inhaled OVA.16 However, we did not establish that it is H2O2 per se that has a specific role in immune tolerance among the different reactive oxygen species induced in the context of an immune response that also include superoxide and hydroxyl radicals. Superoxide radicals undergo dismutation to produce H2O2. To address this question, we used transgenic mice with mitochondrion-targeted overexpression of the human catalase gene (MCAT mice).25 Scavenging of H2O2 by the catalase enzyme leads to depletion of mitochondrial H2O2 in the expressing cells. MCAT mice have been widely used in studies of mitochondrial function in different tissues.26,27 To test that the catalase gene is also expressed and functional in other tissues that can mount an immune response, we characterized spleens and lungs of MCAT mice for catalase activity. Total spleen cells from MCAT mice were found to have significantly higher catalase activity than those from their WT littermate controls (see Supplementary Fig. 1a, left panel online). In the lung tissue, both CD11c+ (APCs) and CD11c− (mostly non-APCs other than B cells) cells were found to have higher catalase activity (see Supplementary Fig. 1a, middle and right panels online) and lower H2O2 was produced from cells in the MCAT mice compared to that from cells in WT controls (see Supplementary Fig. 1b online) thereby establishing this transgenic mouse as a reliable animal tool to directly assess the role of mitochondrial H2O2 in induction of tolerance by low-dose HDM.
To test whether mitochondrial H2O2 is associated with suppression of airway inflammation in response to inhaled low-dose HDM, we compared the H2O2-depleted MCAT mice with the H2O2-intact WT littermate controls under both inflammation and tolerance conditions. As shown in Fig. 1, inflammation-inducing conditions that did not involve pre-exposure of the mice to low dose of HDM at 2 weeks of age (mice were pre-exposed to the vehicle PBS), induced airway inflammation and mucus production in WT mice (WT-Infl group) as evident from histological examination of lung tissue sections (Fig. 3a, b). The inflammation comprised an eosinophil-dominated response as revealed by differential cell counts in the BAL fluid (Fig. 3c) suggesting induction of a robust type 2 immune response. Increase in neutrophil numbers, albeit lower in abundance than eosinophils, was also evident in these analyses. In contrast, airway tolerance was induced in the lungs of WT animals that were exposed to 0.5 µg HDM at 2 weeks of age followed by repeated exposure at 6 weeks of age to the same high HDM challenge dose of 25 μg each time that the mice in the Infl group were exposed to (WT-Tol group). Tolerance was suggested by suppression of all of the inflammatory parameters detected in the Infl group (Fig. 3a, b, c). MCAT mice repeatedly challenged by the high HDM dose at 6 weeks of age without any HDM pre-exposure (MCAT-Infl group) also showed inflammation around the airways and blood vessels (Fig. 3a, b). However, MCAT mice under the same tolerance-inducing conditions as the WT mice (MCAT-Tol group) failed to actively suppress airway inflammation as evident by a marked increase in lung pathology (Fig. 3a, b) and eosinophil-rich cell recovery in the BAL fluid comparable to that detected in the group immunized for inflammation (Fig. 3c). Therefore, loss of mitochondrial H2O2 led to a more pro-inflammatory status in MCAT mice under conditions that normally would induce tolerance suggesting that mitochondrial H2O2 plays an important role in facilitating tolerance establishment using a low dose of allergen. As another hallmark of the type 2 immune response, the level of total IgE in the serum was high in the WT mice under conditions of inflammation but was significantly suppressed under the tolerance condition (Fig. 3d). Similarly, MCAT mice displayed high levels of IgE under both inflammation- and tolerance-inducing conditions (Fig. 3d). We next assessed airway hyperreactivity (AHR) to determine if depletion of mitochondrial H2O2 was sufficient to alter lung function in response to allergen provocation. WT and MCAT mice from the inflammation condition (WT-Infl and MCAT-Infl groups) showed AHR in response to increasing doses of methacholine (MCh). WT-Tol mice showed lower AHR relative to those in the Infl group. However, MCAT mice subjected to the same tolerizing condition (MCAT-Tol group) mounted AHR at the same level as those in the MCAT-Infl group (Fig. 3e). Taken together, our data showed that mitochondrial H2O2 plays an important role in inducing stable tolerance that suppresses allergic airway inflammation with associated protective effects on lung function when repeatedly exposed to a high dose of HDM.
Analysis of lung tissue isolated from MCAT or WT littermate mice sensitized with low-dose HDM (0.5 µg, referred to here as Tol) at 2 weeks of age, followed by challenges with 25 µg HDM at 6 weeks of age. Mice were sacrificed 24 h after the last challenge. Mice without HDM pre-sensitization at 2 weeks (PBS exposure) but only subjected to HDM challenges at 6 weeks were considered as inflammation group (referred to here as Infl) while those without any exposure were considered as naïve controls. a, b PAS staining of lung sections showing cellular infiltration around blood vessels and airways (black arrow) and mucus productions in the small airways (red arrow). Scale bar: 100 µm. Data shown in (a) are representative results and in (b) are median ± IQR summarized from three independent experiments with 6–12 mice per group. c Frequency and absolute number of the inflammatory cells based on analysis of BAL fluid. d Assay of total serum IgE levels. Data shown are median ± IQR combined from two independent experiments with 5–10 mice per group. e Assessment of airway hyperresponsiveness (AHR) in mice. Rn Newtonian resistance. MCh methacholine. Data shown in (c) are median ± IQR and in (e) are mean ± SEM combined from three independent experiments with 6–10 mice per group. Kruskal–Wallis with Dunn’s post hoc test for multiple comparisons (b, c, d). Two-way ANOVA with Tukey’s multiple comparisons test (e). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; nsP > 0.05.
Depletion of mitochondrial H2O2 abolishes active suppression of Th2 response in a tolerization program
We next studied the CD4+ T-cell response in WT and MCAT mice subjected to the tolerizing condition. Under inflammation-inducing conditions, the lungs of WT and MCAT mice showed comparable Th1 response (T-bet+IFN-γ+) (Fig. 4a, b). In contrast, both the Th2 (GATA-3+IL-13+) (Fig. 4c, d) and the Th17 responses (RORγt+IL-17+) (Fig. 4e, f) were attenuated in the Tol group in the WT mice as compared to that in the Infl group which was not the case in the MCAT mice. Numbers of Foxp3+ CD4+ T cells (Tregs) were higher but comparable under both inflammation- and tolerance-inducing conditions in the lungs of both WT and MCAT mice as compared to that detected in naive mice (see Supplementary Fig. 2 online). In aggregate, these data demonstrated a role for mitochondrial H2O2 in suppressing both Th2 and Th17 responses to HDM in mice that were young adults that was achieved by repeated exposure to a low dose of the allergen in early life.
Flow analysis of effector T-cell subsets in the lungs of mice analyzed in Fig. 3. Note in data presented in Fig. 3, differential cell counts were not available for a few mice due to inadvertent leakage during BAL retrieval process. Representative flow plots (a, c, e) and quantifications of the percentages (b, d, f left panel) and absolute numbers (b, d, f right panel) of (a, b) Th1 (T-bet+IFN-γ+), (c, d) Th2 (GATA-3+IL-13+), and (e, f) Th17 cells (RORγt+IL-17+) (all gated on live CD4+TCRβ+FoxP3−) in the lungs. Data shown are median ± IQR combined from three independent experiments with 6–12 mice per group. Kruskal–Wallis with Dunn’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; nsP > 0.05.
Tolerance reduces lung DC and macrophage numbers and constrains upregulation of cDC2-specific genes that is impaired in MCAT mice
We next asked whether the loss of tolerance in MCAT mice was also mediated by an effect on lung DCs, as suggested by the results shown in Fig. 2 when DCs exposed to a low dose of HDM were particularly inefficient in inducing a Th2 response. Toward this end, we adopted the strategy described recently to distinguish cDC1 and cDC2 subsets and macrophages in the lungs of mice.28 Based on expression characteristics of specific molecules in DC subsets, we first sorted total DCs (devoid of monocyte-derived DCs), cDC1 and cDC2 subsets and macrophages from the lungs of naïve mice (gating strategy of the cells shown in Supplementary Fig. S3a, see online). RNA isolated from the DCs was analyzed for expression of the cDC1-specific gene, Irf8, and the cDC2-specific genes Irf4 and S100a4. As shown in Supplementary Fig. S3b, we confirmed subset-specific expression of the genes in sorted DCs showing that each of these genes faithfully reported cell type-specific expression as previously described29,30,31,32,33,34,35 and these genes were not expressed by lung macrophages. We next exposed 2-week-old WT and MCAT mice to the tolerizing regimen of repeated low-dose HDM exposure and examined the status of total DCs and cDC1 and cDC2 subsets in the lungs using the specific markers to identify DCs and the subsets. We observed a distinct profile of DC numbers in the two groups. While WT-Tol mice showed a 50% decrease in total DC numbers as compared to numbers in naive mice, in contrast, MCAT mice not only did not show this decrease but instead showed a slight increase in DC numbers (Fig. 5). Correspondingly, a decrease in both cDC1 and cDC2 subsets was evident in the WT mice although the cDC2:cDC1 ratio of 2:1 was maintained, this relative abundance of cDC2 to cDC1 subsets having been recently reported.28 In MCAT mice, there was no decrease in DC numbers but rather we detected an increase in both total and cDC1 and cDC2 subsets. However, the cDC2:cDC1 ratio was slightly decreased to 1.7. Overall, the lungs of MCAT-Tol mice harbored ~3x more total DCs as well as cDC1s and cDC2s as compared to those of WT-Tol mice, the difference reaching statistical significance. A similar trend was also evident in the case of lung macrophages which can also behave as antigen-presenting cells (Fig. 5).
Analysis of DCs and macrophages (Macs) isolated from the lungs of WT or MCAT littermate mice. Mice were sensitized with low-dose HDM (0.5 µg, referred to here as Tol) at 2 weeks of age (Fig. 1a). Mice were sacrificed 24 h after the last sensitization. WT or MCAT mice without any HDM exposure were used as naïve controls. Cell numbers per mouse corresponding to total DCs, cDC1, and cDC2 subsets and macrophages in WT and MCAT mice under naive or tolerized conditions. Data shown are mean ± SEM combined from two independent experiments with DCs pooled from 3 to 4 mice in each group in each experiment. Kruskal–Wallis with Dunn’s post hoc test. *P < 0.05.
Given that MCAT mice failed tolerance and mounted a strong Th2 response upon allergen challenge, the next question we asked was whether in addition to DC numbers being impacted by loss of mitochondrial H2O2, there was also any effect on the expression of genes that are associated with cDC1 and cDC2 phenotype and function. We resorted to sorting the total DC population comprising cDC1s and cDC2s from each group of mice rather than purifying the subsets individually to assess gene expression given the low abundance of DCs in the lungs. We computed the ratio of cDC2- to cDC1-specific gene expression in RNA isolated from the DC mix to derive a better sense of the overall response. The fold change in ratio of expression between each pair was adjusted based on the ratio of cDC2:cDC1 in the cell mix. Analysis of RT-qPCR data revealed 2× more S100a4:Irf8 and 1.5× more Irf4:Irf8 expression in the DCs from Tol MCAT mice compared to those in DCs from Tol WT mice (see Supplementary Fig. S4 online) suggesting enrichment of both S100a4 and Irf4 expression in cDC2s relative to that of Irf8 in cDC1s in H2O2-depleted DCs.28,36 Collectively, these data reaffirmed that induction of tolerance in the airways is not just passive ignorance of antigen but involves an active response that decreases DC and macrophage numbers in the lungs that requires mitochondrial H2O2.
Discussion
Using a genetic approach that dissipates H2O2 specifically from mitochondria as it is generated, here we demonstrate the ability of mitochondrial H2O2 to blunt a Th2 response to allergens such as HDM in a sustained fashion if repeated exposure to a low dose of the allergen is instituted early in life at 2 weeks of age. The time point of 2 weeks (day 14) was chosen based on previous findings of a significant decrease in airway inflammation between mice immunized on day 3 vs. day 14 using the same dose of HDM.37 Correspondingly, this timeframe is also early enough in human life that measures can be implemented to ward off allergic disease later in life. We also show that the ability of lung DCs to mount a Th2 response is dependent on the dose of allergen pre-exposure with a low dose of HDM inducing a significantly attenuated Th2 response in comparison to a higher dose that endows the DCs with a far stronger Th2-inducing ability. While cDC2s were 2× more abundant than cDC1s in all mice-whether WT or MCAT, or naïve or Tol, Tol WT mice showed a reduction in the number of lung DCs, which was not observed in MCAT mice. Rather, there was a slight increase in the number of cDC2s in the lungs of Tol MCAT mice as compared to that in naive mice resulting in a difference of ~3× more cDCs in Tol MCAT mice compared to that in Tol WT mice. Similarly, lung macrophage numbers also showed a trend in reduction in WT mice after tolerization. In addition, our data suggest a propensity for increased expression of cDC2-specific genes, S100a4 and Irf4, relative to expression of the cDC1-specific gene, Irf8, in the DCs of Tol MCAT mice. Collectively, these results show that H2O2 production from lung DCs in response to repeated exposure to a low dose of allergen in early life helps to constrain DC numbers in the lungs of the mice.
Based on previous findings, it is possible that the level and species of ROS matters with an optimal level of H2O2 favoring suppression of NF-κB activation and inflammation, as observed in our studies and those of others16,38,39 but a relatively high level of ROS including O2- favoring inflammation.40 AIT is achieved using low doses of allergens and in our study a high dose of HDM failed to induce tolerance suggesting that low doses of allergens prevent accumulation of high levels of ROS.
Multiple studies published to date have examined the effect of lipopolysaccharide (LPS) on allergic inflammation. In earlier studies, a dose of ~100 ng was reported to promote allergic airway inflammation, whereas a higher dose (μg range) inhibited the same process.41,42 The study of Rodriguez et al. attributed the suppression to IFN-γ produced by Th1 cells.42 Along the same lines and relevant to our findings, a more recent study has shown that unlike adult mice, infant mice (18-day-old—we used 14-day-old mice) are resistant to a relatively low LPS dose of 5 μg in the context of HDM sensitization and do not mount appreciable high IFN-γ+ Th1 cells but a higher dose of 50 μg of LPS, Th1 cells are induced.43 Essentially, infant mice did not mount a Th1 response to a low LPS dose to the same degree as an adult mouse. In our experiments, we used infant mice (2 week old) who would be unresponsive to 5 μg or lower doses of LPS and we used HDM with low LPS content. For our low HDM dose of 0.5 μg/exposure, the average LPS content was 14.8 pg that amounted to only 148 pg for the 10× higher HDM dose used. Most importantly, and in agreement with the data of Bachus et al.43 our data showed a similar (but low) Th1 response elicited at both doses and therefore we do not believe LPS influenced the suppression of Th2 response caused by prior exposure to a low dose of HDM.
Conventional DCs (cDCs) are present in the lung and most tissues and have been classified into two major lineages, each of which selectively expresses a set of cell surface molecules and transcription factors that regulate the development and function of DCs.44,45 The cDC1 subset of cDCs promotes Type 1 immune response dependent on the transcription factors IRF8 and BATF3, whereas cDC2s, which promote Th2 and Th17 responses, require IRF4.29,30,31,32,33,34,35 A recent study has also described inflammatory cDC2s that show a mixed phenotype and mediate immune response against respiratory viruses.28 Single-cell RNA-sequencing data in this study identified S100a4 as another cDC2-specific gene. S100A4 was previously associated with allergy and a Type 2 immune response in humans and mice.36 A siRNA-based screen identified S100A4 as a candidate in a gene module that correlated with seasonal allergic rhinitis in humans and expression of the Th2-specific gene, IL-13.36 In addition, S100a4−/− mice failed to mount allergic inflammation. Notably, and relevant to our study, DCs from S100a4−/− mice showed attenuated capacity to stimulate T cells suggesting DCs to be an important cell type that conferred Th2 capacity on T cells. It is also worth noting that S100A4 is a secreted molecule that facilitates leukocyte adhesion and cell migration.46,47 Our data suggesting augmentation of expression of cDC2-specific genes in cDCs warrant a more comprehensive study in the future of differential gene programming in lung cDCs in response to tolerizing conditions in the presence or absence of mt H2O2.
At relatively lower doses, H2O2 has been shown to cause cell cycle arrest of different cell types including fibroblasts48 and vascular smooth muscle cells49 although high doses induce cell death. It is unclear how repeated exposure to a low dose of allergen in newborn mice impacts cell viability and proliferation of lung DCs and macrophages. The decrease in cDC numbers in the lungs of WT-Tol mice is reminiscent of a previous finding of tolerance induced by apoptotic DCs due to suppression of DC maturation and activation after uptake of the apoptotic DCs with induction of antigen-specific Tregs.50 It seems plausible that the reduction in DC numbers consequent to tolerance induction by low dose allergen exposure imposes a similar restraint on immune activation which requires further investigation. We not only observed a decrease in the number of DCs in WT-Tol mice but also detected a trend towards reduction in the number of lung macrophages. This observation is in line with our previous study in which we showed a regulatory role for PPARγ expression in lung CD11c+ cells-both DCs and macrophages, in suppressing expression of pro-inflammatory cytokine genes in both cell types that was important for the induction of airway tolerance.51 Importantly, our recent study of human severe asthma has identified lung macrophages expressing the Type 2 cytokine IL-4 in a subset of these patients whose airways were enriched in IL-4+ innate immune cells.52 It stands to reason, therefore, that tolerance induces a contraction of both DC and macrophage numbers in the lungs.
An increase in the relative expression of cDC2-specific genes in MCAT-Tol DCs compared to that in WT-Tol DCs may be mediated by increased NF-κB activation given that our previous study showed the ability of H2O2, induced downstream of PPARγ activation, to inhibit NF-κB activation.16 A recent study of diabetes mellitus has also implicated mitochondrial H2O2 in inhibition NF-κB activation.53 A role for NF-κB in IRF4 gene expression was reported earlier.54 Similar to our findings, immune suppression by nasal DCs in response to inhaled antigen was attributed to ROS production in DCs55 suggesting that DCs in the upper airways may utilize a common mechanism of mitochondrial ROS/H2O2 to prevent unwarranted allergic responses to inhaled antigens.
Taken together, our experimental model suggests that induction of immune tolerance in the airways provides protection from allergic airway disease by downsizing cDCs in the lungs with a potential to regulate expression of cDC2-specific genes. The novel insights gained in our study highlight an opportunity to prevent allergic asthma in later life by exposure to low doses of allergens in early life.
Methods
Mice
B6.Cg-Tg(CAG-OTC/CAT)4033Prab/J, which are hemizygous transgenic mice for the human catalase gene in mitochondria (referred to here as MCAT mice), were purchased from Jackson Laboratories (catalog 016197). For all experiments, both female and male mice were used at the indicated age. The non-carrier (wild type) littermates were used as controls. B6.Thy1.1-OT-II TCR Tg (referred to here as OT-II mice) were a gift from Lauren Cohn (Yale University, New Haven, Connecticut, USA) and were used between 8 and 12 weeks of age. All animals were housed under pathogen-free conditions in the Department of Laboratory Animal Resources at University of Pittsburgh and were used according to all applicable and approved Institutional Animal Care and Use Committee protocols.
Mouse model of low-dose HDM-induced early-life airway tolerance
HDM extract (from Dermatophagoides Pteronyssinus) was purchased from Greer Labs (Cat # XPB70D3A2.5). We used HDM from three lots (317430, 348718, and 369446). For low HDM dose of 0.5 μg/exposure, the average LPS content was 14.8 pg that amounted to 148 pg for the 10× higher HDM dose used. The models involved sensitization of mice with a low or a higher dose of HDM. A third group was exposed to the vehicle (PBS) only and the fourth group was naïve mice that were used as negative controls. The protocol is depicted in Fig. 1a. Experiments aimed at examining DCs after the sensitization phase were terminated 24 h after the last HDM instillation (day 10). When the full model was run, mice were sacrificed 24 h after the last challenge.
Bronchoalveolar lavage (BAL)
BAL was performed on anesthetized mice by infusion and recovery of 1 mL sterile Ca2+- and Mg2+-free PBS. 1.5 × 105 BAL fluid cells were dispersed onto glass slides by cytospin followed by three-step Giemsa staining (Thermo Fisher Scientific) according to the manufacturer’s instructions. The percentages of eosinophils, neutrophils, lymphocytes, and macrophages were enumerated out of a total of ~300 cells by light microscopy. The absolute number of each cell type was calculated based on the total BAL cell recovery from each mouse.
Lung histology
Right lobes of the lung were fixed in SafeFix II (Thermo Fisher Scientific) and were embedded in paraffin. Sectioned slides were stained with Periodic Acid-Schiff (PAS) reagent to assess airway mucus. Cells infiltrating the tissue around airways and blood vessels were also evaluated as an indication of airway inflammation. As described previously,56 a scoring system on a scale of 0 to 4 was used to grade the size of lung infiltrates around the bronchovascular bundles from multiple fields per slide in a blinded fashion. Briefly, a score of 0 signified a completely lack of inflammation and a score of 4 represented a large (>3 cells deep) widespread inflammatory infiltrate around the majority of bronchovasucular bundles. Approximately 20 consecutive ×20 fields were examined on each slide. Inflammation scores were derived as a mean value per animal and were compared between groups.
mRNA isolation and quantitative real-time PCR
Mouse lung mRNA isolation and cDNA synthesis were as described previously.56,57 qPCR was performed using TaqMan 2× Master Mix reagent (Applied Biosystems, catalog 4304437) and validated Taqman gene expression primer/probe sets (Life Technologies) that included: mouse Hprt1 (Mm03024075_m1), mouse Ifng (Mm01168134_m1), mouse Il-13 (Mm00434204_m1), mouse Il-17a (Mm00439618_m1), mouse Irf4 (Mm00516431_m1), mouse Irf8 (Mm00492567_m1) and mouse S100a4 (Mm00803372_g1). The PCR reactions were performed at the University of Pittsburgh Genomics Research Core using ABI PRISM 7700 Sequence System (Applied Biosystems). Results were analyzed using SDS 2.2.2 software. Relative gene expression was determined by calculating 2−□Ct normalized to Hprt1.
Lung cell isolation and flow cytometry
Lungs of anesthetized mice were perfused with PBS and dissected. Tissues were digested with 0.7 mg/mL collagenase (Roche, cat #10103578001) and 30 μg/mL DNase (Roche, Cat # 10104159001) in RPMI-1640 medium at 37 °C for 45 min then homogenized on a gentleMACS dissociator (Miltenyi Biotec) according to the manufacturer’s protocol. The digestion reaction was stopped by adding PBS containing 2% FBS and 0.5 M EDTA. Single-cell suspensions were prepared by passing the homogenate through a 70 μm cell strainer (Fisher Scientific). Red blood cells were lysed using BD Pharmlyse solution (BD Biosciences, Cat # 555899). For intracellular staining of cytokines and transcription factors, the resulting single cells were stimulated with 50 ng/ml PMA (Sigma-Aldrich, Cat # P8193) and 500 ng/ml ionomycin (Sigma-Aldrich, Cat # I3909) along with Golgi trafficking blocking reagents brefeldin A (BD Biosciences, Cat # 555029) and monensin (BD Biosciences, Cat # 554724) for 2.5 h. Cells were stained with viability dye FVD eFlour 780 (eBioscience, Cat # 65-0865-14) for exclusion of dead cells. Cells were fixed, permeabilized and stained using a Foxp3/Transcription Factor Staining Buffer Set (eBiosciences, Cat # 88-8824-00) as per manufacturer’s protocols. Cells were stained with fluorochrome-conjugated anti-mouse antibodies as follows: eFlour 450 FoxP3 (eBiosciences, clone FJK-16s), BV605 TCR-β (BD Biosciences, clone H57-597), BV605 CD25 (BD Biosciences, clone OX-39), Alexa Flour 488 GATA-3 (BD Biosciences, clone L50-823), PerCP-Cyanine5.5 IL-17A (eBiosciences, clone eBio 17B7), PE RORγt (eBiosciences, clone eBio B2D), PE IL-5 (Biolegend, clone TRFK5), PE-CF594 IFN-γ (BD Biosciences, clone XMG1.2), PE-Cyanine7 IL-13 (eBiosciences, clone eBio 13A), APC T-bet (Biolegend, clone 4B10), APC IL-10 (BD Biosciences, clone JES5-16E3), and Alexa Fluor 700 CD4 (Biolegend, clone RM4-5). Flow cytometry data were acquired on a five-laser BD Fortessa (BD Immunocytometry Systems) or five-laser Cytek Aurora (Cytek Biosciences) flow cytometer. Data were analyzed using FlowJo software (TreeStar). For cell surface markers, thresholds of positivity were determined using appropriate unstained samples. In the case of intracellular transcription factors (FoxP3, T-bet, GATA-3, and RORγt) fluorescence minus one (FMO) controls were used, which included staining for all other relevant markers. Lastly, for cytokine expression, full panel staining was performed on paired unstimulated and stimulated (PMA plus ionomycin) samples. Gating hierarchies were then developed, and thresholds of positivity were set based upon these criteria as appropriate.
For cell sorting, single lung cell suspensions were prepared by enzymatic digestion as described above. We pooled lung cells from 3 to 4 2-week-old pups that did not differ by more than 1 day in age for these experiments. Pooling was necessary to obtain enough DCs from the lungs of the young mice at the same time maintaining the expected profile of cDC2:cDC1 ratio of 2:1 in the lungs of naïve WT mice.28 The cells were pre-enriched by positive selection via incubation with CD11c magnetic beads followed by passage over LS separation columns, both according to the manufacturer’s protocols (Miltenyi Biotec). CD11c-enriched cells were first incubated with Fc Block (Biolegend, clone 93) diluted 1:100 for 15 mins at 0oC, then stained with optimally diluted FITC anti-MHC Class II (Southern Biotec, clone NIMR-4), PE anti-CD26 (Biolegend, clone H194-112), and PE Dazzle 594 anti-CD64 (Biolegend, X54-5/7.1) for 30 min at 0 °C in the dark. The cells were washed and resuspended in PBS for sorting. The gating strategies for alveolar macrophages and total cDCs were as described by Bosteels et al.28 Macrophages were identified as CD11c+, autofluorescence high, and cDCs were CD11c+, MHC Class II high, CD26 high, CD64 low. Sorting was performed on a FACSAria II instrument (BD Immunocytometry Systems). Test sorts were done initially on a small number of cells to confirm the purity of the isolated populations, then the remaining cells were sorted directly into tubes containing RLT lysis buffer (Qiagen) for RNA isolation. For some experiments cDC1 and cDC2 were specifically purified by cell sorting. This was performed by adding PE-Cyanine7 anti-CD172a (Biolegend, clone P84), APC anti-XCR1 (Biolegend, clone ZET) and Alexa Fluor 700 anti-FcεRIα (Biolegend, clone MAR-1) to the panel above. After gating out monocyte-derived DCs (CD64+CD26low), within total DCs detected as CD11c+MHC Class IIhighCD26highCD64low, cDC1s were identified as XCR1+CD172− and cDC2s as XCR1−CD172a+ MAR-1− cells.28
In vitro DC and T-cell co-culture
Single-cell suspensions were prepared from whole lungs or lung draining mediastinal lymph nodes (mLNs). Lung DCs were enriched from single-cell suspensions using CD11c ultrapure magnetic beads (Miltenyi Biotec, catalog 130-125-835). For lung DCs, the enriched cells were further purified by fluorescence-activated cell sorting based on the phenotype MHC class II+ CD11c+ SiglecF- using a FACSAria cell sorter that yielded ≥94% purity. Anti-mouse antibodies used for DC sorting were as follows: FITC MHC class II (SouthernBiotech, clone NIMR-4), APC CD11c (Biolegend, clone N418) and PE SiglecF (BD Biosciences, E50-2440). Naive CD4+ T cells were isolated from spleens of OT-II mice, the OVA-responsive TCR transgenic mice, using a naive CD4+ T-cell isolation kit (Miltenyi Biotec, catalog 130-104-453). Isolated naïve CD4+ T cells (1 × 105 cells/well) were co-cultured with purified lung DCs (2 × 104 cells/well) in a U bottom 96-well plate in complete cell culture medium described before.56 OVA peptide (20 µg/ml) (OVA323-339) was added for the duration of the culture incubation. Cultures were incubated for 5 days and the cell culture supernatants were collected for cytokine assay using Bio-Plex multiplex immunoassay kit (Bio-Rad). Cells were stimulated with PMA/Ionomycin/GolgiStop, stained for live and dead cells, fixed and stained for surface markers, intracellular cytokines and transcription factors.
Assay of serum total IgE
Serum total IgE concentration was measured using mouse IgE ELISA kit (Invitrogen, catalog 88-50460) as previously described.56
Measurement of catalase activity
Single-cell suspensions were prepared from spleen and lung, and CD11c positive/negative cells were isolated as needed using CD11c ultrapure magnetic beads as described above. Cells were lysed by quick freeze/thaw followed by sonication using an Ultrasonic Liquid Processor (Misonix Inc, model XL2020) under the setting of 30% amplitude/30 s on/30 s off over 4 cycles. The resulting homogenate was centrifuged at 10,000 × g for 15 min at 4 °C. The supernatants were collected to measure catalase activity using a Catalase Colorimetric Activity Kit (Invitrogen, catalog EIACATC). Protein concentration in each sample was determined using a Protein Assay kit (Bio-Rad). Catalase activity was normalized to protein concentration (U/mg).
Assay of secreted H2O2
Cell culture medium after various treatments was collected, centrifuged to remove any residual cells, and stored at −80 °C until further use. The concentration of released H2O2 was determined using an Amplex® Red Hydrogen peroxide/Peroxidase Assay Kit (Invitrogen, Cat # A22188) as previously described.16
Assessment of AHR
For assessment of lung function, mice under anesthesia using xylazine (12/mg/kg), sodium pentobarbital (90 mg/kg) and pancuronium bromide (0.8 mg/kg) were subjected to the forced oscillation technique (FOT) using a flexiVent PFT apparatus (SCIREQInc). Perturbation with increasing doses of inhaled methacholine was made to measure the airway responsiveness.
Statistical analysis
Unpaired Student’s t test, Mann–Whitney U test, Kruskal–Wallis, or two-way ANOVA were used for statistical analysis as appropriate. P < 0.05 was considered significant. The GraphPad Prism 9 software was used for the rest of statistical analyses.
References
Herxheimer, H. Bronchial hypersensitization and hyposensitization in man. Int. Arch. Allergy Appl. Immunol. 2, 40–59 (1951).
Du Toit, G. et al. Effect of avoidance on peanut allergy after early peanut consumption. N. Engl. J. Med. 374, 1435–1443 (2016).
Palmqvist, M., Cui, Z. H., Sjostrand, M., Linden, A. & Lotvall, J. Reduced late asthmatic response by repeated low-dose allergen exposure. Eur. Respir. J. 17, 872–880 (2001).
Noon, L. Prophylactic inoculation against hay fever. Lancet 177, 1572–1573 (1911).
Yanagida, N. et al. A single-center, case-control study of low-dose-induction oral immunotherapy with cow’s milk. Int. Arch. Allergy Immunol. 168, 131–137 (2015).
Maeta, A. et al. Low-dose oral immunotherapy using low-egg-allergen cookies for severe egg-allergic children reduces allergy severity and affects allergen-specific antibodies in serum. Int. Arch. Allergy Immunol. 175, 70–76 (2018).
Blumchen, K. et al. Efficacy, safety, and quality of life in a multicenter, randomized, placebo-controlled trial of low-dose peanut oral immunotherapy in children with peanut allergy. J. Allergy Clin. Immunol. Pract. 7, 479–491.e410 (2019).
Werfel, T. et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy 61, 202–205 (2006).
Rotiroti, G., Shamji, M., Durham, S. R. & Till, S. J. Repeated low-dose intradermal allergen injection suppresses allergen-induced cutaneous late responses. J. Allergy Clin. Immunol. 130, 918–924.e911 (2012).
Scadding, G. K. & Brostoff, J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite. Clin. Allergy 16, 483–491 (1986).
Marcucci, F. et al. Low-dose local nasal immunotherapy in children with perennial allergic rhinitis due to dermatophagoides. Allergy 57, 23–28 (2002).
Schubert, R. et al. Effect of n-3 polyunsaturated fatty acids in asthma after low-dose allergen challenge. Int Arch. Allergy Immunol. 148, 321–329 (2009).
Eifan, A. O., Calderon, M. A. & Durham, S. R. Allergen immunotherapy for house dust mite: clinical efficacy and immunological mechanisms in allergic rhinitis and asthma. Expert Opin. Biol. Ther. 13, 1543–1556 (2013).
Passalacqua, G. et al. Current insights in allergen immunotherapy. Ann. Allergy Asthma Immunol. 120, 152–154 (2018).
Reynolds, L. A. & Finlay, B. B. Early life factors that affect allergy development. Nat. Rev. Immunol. 17, 518–528 (2017).
Khare, A. et al. Mitochondrial H2O2 in lung antigen-presenting cells blocks NF-kappaB activation to prevent unwarranted immune activation. Cell Rep. 15, 1700–1714 (2016).
Thomas, W. R. House dust mite allergens: new discoveries and relevance to the allergic patient. Curr. Allergy Asthma Rep. 16, 69 (2016).
Dutta, S. & Sengupta, P. Men and mice: relating their ages. Life Sci. 152, 244–248 (2016).
Wang, S., Lai, X., Deng, Y. & Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 242, 117242 (2020).
He, K. et al. Blimp-1 is essential for allergen-induced asthma and Th2 cell development in the lung. J. Exp. Med. 217, e20190742 (2020).
Hammad, H. et al. Th2 polarization by Der p 1-pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood 98, 1135–1141 (2001).
Eickhoff, S. et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell 162, 1322–1337 (2015).
Roquilly, A. et al. Local modulation of antigen-presenting cell development after resolution of pneumonia induces long-term susceptibility to secondary infections. Immunity 47, 135–147.e135 (2017).
Plantinga, M. et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38, 322–335 (2013).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Santulli, G., Xie, W., Reiken, S. R. & Marks, A. R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl Acad. Sci. U.S.A. 112, 11389–11394 (2015).
Vicente-Gutierrez, C. et al. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat. Metab. 1, 201–211 (2019).
Bosteels, C. et al. Inflammatory type 2 cDCs acquire features of cDC1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity 52, 1039–1056.e1039 (2020).
Bajana, S., Roach, K., Turner, S., Paul, J. & Kovats, S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J. Immunol. 189, 3368–3377 (2012).
Gao, Y. et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity 39, 722–732 (2013).
Hambleton, S. et al. IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med. 365, 127–138 (2011).
Williams, J. W. et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 4, 2990 (2013).
Vander Lugt, B. et al. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat. Immunol. 15, 161–167 (2014).
Durai, V. et al. Cryptic activation of an Irf8 enhancer governs cDC1 fate specification. Nat. Immunol. 20, 1161–1173 (2019).
Kim, S. et al. High amount of transcription factor IRF8 engages AP1-IRF composite elements in enhancers to direct type 1 conventional dendritic cell identity. Immunity 53, 759–774.e759 (2020).
Bruhn, S. et al. A generally applicable translational strategy identifies S100A4 as a candidate gene in allergy. Sci. Transl. Med. 6, 218ra214 (2014).
Gollwitzer, E. S. et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat. Med. 20, 642–647 (2014).
Korn, S. H., Wouters, E. F., Vos, N. & Janssen-Heininger, Y. M. Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J. Biol. Chem. 276, 35693–35700 (2001).
Strassheim, D. et al. Modulation of bone marrow-derived neutrophil signaling by H2O2: disparate effects on kinases, NF-kappaB, and cytokine expression. Am. J. Physiol. Cell Physiol. 286, C683–C692 (2004).
Mitra, S. & Abraham, E. Participation of superoxide in neutrophil activation and cytokine production. Biochim. Biophys. Acta 1762, 732–741 (2006).
Eisenbarth, S. C. et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196, 1645–1651 (2002).
Rodriguez, D. et al. Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J. Immunol. 171, 1001–1008 (2003).
Bachus, H. et al. Impaired tumor-necrosis-factor-alpha-driven dendritic cell activation limits lipopolysaccharide-induced protection from allergic inflammation in infants. Immunity 50, 225–240 (2019). e224.
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578 (2014).
Murphy, K. M. Transcriptional control of dendritic cell development. Adv. Immunol. 120, 239–267 (2013).
Li, Z. H., Dulyaninova, N. G., House, R. P., Almo, S. C. & Bresnick, A. R. S100A4 regulates macrophage chemotaxis. Mol. Biol. Cell 21, 2598–2610 (2010).
Osterreicher, C. H. et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl Acad. Sci. U.S.A. 108, 308–313 (2011).
Chen, Q. & Ames, B. N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc. Natl Acad. Sci. U.S.A. 91, 4130–4134 (1994).
Deshpande, N. N. et al. Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid. Redox Signal. 4, 845–854 (2002).
Kushwah, R., Oliver, J. R., Zhang, J., Siminovitch, K. A. & Hu, J. Apoptotic dendritic cells induce tolerance in mice through suppression of dendritic cell maturation and induction of antigen-specific regulatory T cells. J. Immunol. 183, 7104–7118 (2009).
Khare, A., Chakraborty, K., Raundhal, M., Ray, P. & Ray, A. Cutting edge: dual function of PPARgamma in CD11c+ cells ensures immune tolerance in the airways. J. Immunol. 195, 431–435 (2015).
Camiolo, M. J. et al. High-dimensional profiling clusters asthma severity by lymphoid and non-lymphoid status. Cell Rep. 35, 108974 (2021).
Silva-Rodrigues, T. et al. Hyperglycemia in a type 1 Diabetes Mellitus model causes a shift in mitochondria coupled-glucose phosphorylation and redox metabolism in rat brain. Free Radic. Biol. Med. 160, 796–806 (2020).
Boddicker, R. L. et al. The oncogenic transcription factor IRF4 is regulated by a novel CD30/NF-kappaB positive feedback loop in peripheral T-cell lymphoma. Blood 125, 3118–3127 (2015).
Bedford, J. G. et al. Unresponsiveness to inhaled antigen is governed by conventional dendritic cells and overridden during infection by monocytes. Sci. Immunol. 5, eabb5439 (2020).
Oriss, T. B. et al. IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice. JCI Insight 2, e91019 (2017).
Das, S. et al. Respiratory syncytial virus infection of newborn CX3CR1-deficient mice induces a pathogenic pulmonary innate immune response. JCI Insight 2, e94605 (2017).
Acknowledgements
This work was supported by National Institutes of Health grants R01 AI048927 (A.R.), P01 AI106684 (A.R.), R01 HL113956 (A.R. and P.R.) and P01 HL114453 (P.R.). We thank Ayan Sinha for technical assistance, Kun He for helpful discussions, and Marc Gauthier for advice on statistical analysis of data.
Author information
Authors and Affiliations
Contributions
A.R. conceived the study. H.Y. and A.R. designed the experiments. H.Y., J.C., S.H., S.D., T.B.O. and S.L.K. performed the experiments. H.Y., A.R. and P.R. analyzed the data. S.M.N. helped with statistical analysis of data. H.Y. and A.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A.R. has a research agreement with Pieris Pharmaceuticals. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yuan, H., Chen, J., Hu, S. et al. Early life exposure to house dust mite allergen prevents experimental allergic asthma requiring mitochondrial H2O2. Mucosal Immunol 15, 154–164 (2022). https://doi.org/10.1038/s41385-021-00458-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-021-00458-8