Abstract
The structure of a new pentacecilide congener, pentacecilide D, produced by Penicillium cecidicola FKI-3765-1 was elucidated by various NMR experiments. The absolute stereochemistry of pentacecilides was elucidated by using the modified Mosher method for pentacecilide C. The inhibitory activity of all pentacecilides against lipid droplet formation and acyl-CoA:cholesterol acyltransferase isozymes was compared.
Similar content being viewed by others
Introduction
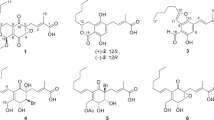
As we reported previously, pentacecilides A to C (Figure 1) were isolated from the whole culture of Penicillium cecidicola FKI-3765-1 as inhibitors of lipid droplet formation in mouse macrophages.1, 2 From the biochemical study,1 pentacecilides inhibited lipid droplet formation in mouse macrophages, probably due to the blockade of acyl-CoA:cholesterol acyltransferase (ACAT) activity. Furthermore, pentacecilides appeared as dual inhibitors of ACAT1 and ACAT2 isozymes.1, 3, 4, 5, 6, 7 The structures including the relative stereochemistry were also elucidated;2 however, the indication of the relative stereochemistry at C-2 was revised8, 9 because erroneous structures of pentacecilides had been shown in our previous reports.1, 2 Further precise analysis of metabolites in the whole culture led to the discovery of a new congener named pentacecilide D (Figure 1). In this study, the structure of the new pentacecilide congener was elucidated. Furthermore, the absolute stereochemistry of pentacecilides was determined by using the modified Mosher method10 for pentacecilide C.
Results
Isolation of pentacecilide D
The 13-day-old whole culture (1000 g) was extracted with 2.0 l of acetone. After the acetone extracts were filtered and concentrated to remove acetone, the aqueous solution was extracted with ethyl acetate. The extracts were dried over Na2SO4 and concentrated in vacuo to dryness to yield a red–brown material (1.7 g). The material was dissolved in 30% CH3CN, applied to an octadecyl silyl (ODS) column chromatography (100 g), and eluted stepwise with 30, 50, 70, 100% CH3CN containing 0.050 % trifluoroacetic acid (250 ml × 2 tubes for each solvent). Pentacecilides A and B were present in the first tube of 100% CH3CN. The first tube of 70% CH3CN containing pentacecilides C and D was concentrated in vacuo to dryness to give a red–brown material. The material (172 mg) was finally purified by preparative HPLC (column, PEGASIL ODS, 20 × 250 mm; Senshu Scientific, Tokyo, Japan; solvent, 55% CH3CN containing 0.050% trifluoroacetic acid; detection, UV at 210 nm; flow rate, 8.0 ml min−1). Under these conditions, pentacecilides C and D were eluted as peaks with retention times of 15.8 and 25.8 min, respectively. The respective fractions were concentrated in vacuo to dryness to give pure pentacecilides C (56.6 mg) and D (5.1 mg) as white crystals.
Structural elucidation of pentacecilide D
The physicochemical properties of pentacecilide D are summarized in Table 1. Pentacecilide D showed UV absorption maxima at 219, 276 and 310 nm, similar to pentacecilides A to C.2 IR absorption at 3426 and 1712–1617 cm−1 suggested the presence of hydroxy and carbonyl groups in the structure.
The molecular formula was determined to be C25H32O7 on the basis of HRESI-TOF-MS measurement, indicating that pentacecilide D was smaller than pentacecilide C.2 The 13C NMR spectrum (in CDCl3) showed 25 resolved signals, which were classified into five methyl carbons, four methylene carbons, two sp3 methine carbons, one sp2 methine carbon, three oxygenated sp3 methine carbons, two sp3 quaternary carbons, one oxygenated sp3 quaternary carbon, three sp2 quaternary carbons, two oxygenated sp2 quaternary carbons and two carbonyl carbons by analysis of the DEPT and HSQC spectra. The 1H NMR spectrum (in CDCl3) displayed 30 proton signals, one of which was suggested to be a hydroxy proton (δ 11.09). Taking the molecular formula into consideration, the presence of two hydroxy protons was suggested. The connectivity of proton and carbon atoms was established by the 13C-1H HSQC spectrum (Table 2). These spectral data from NMR and physicochemical properties showed that pentacecilide D has the same skeleton as pentacecilide C.2 Comparison of the 1H NMR spectra between pentacecilides C and D indicated that the methyl proton signals (C-17, δ 2.17) derived from the acetyl group in pentacecilide C disappeared from pentacecilide D. In addition, the chemical shift of C-2 (δ 69.9) and the molecular formula showed the presence of a hydroxy group at C-2. These data led to the conclusion that the structure of pentacecilide D was 2-deacetyl pentacecilide C (Figure 1). The structure satisfied the degree of unsaturation and the molecular formula.
Regarding the relative stereochemistry of pentacecilide D, NOESY experiments were carried out showing coupling constants in 1H NMR similar to pentacecilide C.2, 8, 9 Accordingly, the relative stereochemistry of pentacecilide D was elucidated as shown in Figure 1.
Absolute stereochemistry of pentacecilides
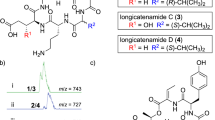
To elucidate the absolute stereochemistry of pentacecilides, the (S)- (1) and (R)- α-methoxy-α-(trifluoromethyl)phenylacetyl (MTPA) esters (2) were prepared by treatment of pentacecilide C with (R)-(−)- and (S)-(+)- MTPA chloride in the presence of 4-(dimethylamino) pyridine and triethylamine (in CH2Cl2, at room temperature, 80.6 and 61.5% yield, respectively) (Scheme 1). The 1H NMR spectra of 1 and 2 were completely assigned. As shown in Figure 2, the calculated Δδ values (Δδ=δS−δR) of H2-11, H3-12, H-5′ and H-8′ were positive, whereas those (Δδ=δS−δR) of H2-1, H-2, H-5 H2-6, H-9, H3-13, H3-14, H3-15 and H3-17 were negative, indicating that the absolute stereochemistry of C-7 was elucidated to be 7S. Accordingly, the absolute stereochemistry of the seven chiral centers in pentacecilide C was concluded to be 2R5R7S8R9R10S8′S, as shown in Figure 1. It is plausible that all pentacecilides are biosynthesized by the same pathway; therefore, the absolute stereochemistry of the other pentacecilides was deduced to be the same as that of pentacecilide C (Figure 1).
Biological properties
Inhibition of CE synthesis in macrophages and ACAT1- and ACAT2-CHO cells
In the previous study, pentacecilides inhibited cholesteryl ester (CE) synthesis in mouse macrophages and ACAT1- and ACAT2-CHO cells, strongly suggesting that they were dual inhibitors of ACAT1 and ACAT2 isozymes.1 Pentacecilide D and semisynthetic 3S*-hydroxy pentacecilide A (3) were evaluated in these three cell-based assays, and the IC50 values of CE synthesis were compared. Pentacecilide A is the most potent inhibitor of CE synthesis in the three assays, followed by pentacecilide B.1 Unfortunately, pentacecilides D and 3 showed almost no inhibitory activity at 22.5–24.2 μM.
No pentacecilides showed any cytotoxic effects on macrophages and ACAT1- and ACAT2-CHO cells, even at 20.6–24.3 μM.
Discussion
As described above, from various spectral analyses, the structure of a new congener, pentacecilide D, was elucidated to be 2-deacetyl pentacecilide C with the same relative stereochemistry as shown in Figure 1.
To determine the absolute stereochemistry of pentacecilides, the modified Mosher method10 was adapted to pentacecilide C. Calculation of the Δδ values led to the clear conclusion that pentacecilide C has the absolute stereochemistry of 2R5R7S8R9R10S8′S (Figure 1). Furthermore, assuming that all pentacecilides are generated through the same biosynthetic pathway, pentacecilides A, B and D have the same absolute stereochemistry as pentacecilide C. This conclusion was supported by evidence that all pentacecilides have negative optical rotations.2
In a cell-based assay using mouse macrophages, ACAT1- and ACAT2-CHO cells,11, 12, 13 pentacecilides A and B showed more potent inhibition of CE synthesis than pentacecilides C and D1 (Table 3). Furthermore, 3 showed no inhibition of CE synthesis in mouse macrophages, and ACAT1- and ACAT2-CHO cells, even at 24.2 μM (Table 3). Taking the structural differences into consideration (Figure 1), the presence of a hydroxy group at C-7 is unfavorable for inhibiting lipid droplet formation and ACAT activity. Regarding the C-3 position, 3 with a hydroxy group at C-3 showed weaker inhibitory activity than pentacecilide A, indicating that the presence of a carbonyl group at C-3 is important for activity. Regarding the C-2 position, introduction of acethoxy and hydroxy groups resulted in decreased activity. Namely, no modification at this position is preferable for inhibitory activity. Among these pentacecilides, it was concluded that pentacecilide A has the most favorable structure for showing potent inhibitory activity against lipid droplet formation and ACAT isozymes. Regarding the structure–activity relationships of the hydroxy group at C-4′, synthesis of acyl derivatives at this position is now in progress.
Methods
Materials
(S)-(+)- and (R)-(−)-MTPA chlorides, and sodium borohydride (NaBH4) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Acetic anhydride (Ac2O) and 4-(dimethylamino) pyridine were purchased from Kanto Chemical (Tokyo, Japan). Triethylamine was purchased from Nacalai Tesque (Kyoto, Japan).
General experimental procedures
Fungal strain P. cecidicola FKI-3765-1 was used for the production of pentacecilides.1, 2 Pentacecilide D was from 13-day-old culture broth fermented as reported previously.1 For determination of the amounts of pentacecilide D in culture broths, samples (ethyl acetate extracts) dissolved in methanol were analyzed by the HP1100 system (Hewlett-Packard, Palo Alto, CA, USA) under the following conditions: column, Symmetry (2.1 × 150 mm; Waters, Milford, MA, USA); flow rate, 0.2 ml min−1; mobile phase, a 20-min linear gradient from 60% CH3CN to 100% CH3CN containing 0.050% H3PO4; detection, UV at 210 nm. Under these conditions, pentacecilide D was eluted with a retention time of 3.59 min, respectively.
SSC-ODS-7515-12 (Senshu Scientific) was used for ODS column chromatography. HPLC was carried out using the L-6200 system (Hitachi, Tokyo, Japan).
UV spectra were recorded on a spectrophotometer (8453 UV-Visible spectrophotometer; Agilent Technologies, Santa Clara, CA, USA). IR spectra were recorded on a Fourier transform infrared spectrometer (FT-710; Horiba, Kyoto, Japan). Optical rotations were measured with a digital polarimeter (DIP-1000; JASCO, Tokyo, Japan). ESI-TOF-MS and HRESI-TOF-MS spectra were recorded on a mass spectrometer (JMS-T100LP; JEOL, Tokyo, Japan). Various NMR spectra were measured with a spectrometer (XL-400; Varian, Palo Alto, CA, USA).
Preparation of the (S)-(+)- and (R)-(−)-MTPA ester derivatives of pentacecilide C
To a solution of pentacecilide C (5.0 mg, 0.010 mmol) in CH2Cl2 (500 μl), (R)-(−)-MTPA chloride (16.5 mg, 0.065 mmol), 4-(dimethylamino) pyridine (5.3 mg, 0.043 mmol) and triethylamine (10 μl, 0.073 mmol) were added. The reaction mixture was stirred at room temperature. After 10 h, the reaction mixture was diluted with 1.0 N HCl and the aqueous phase was extracted with EtOAc. The organic layer was recovered, dried over anhydrous Na2SO4, filtered and concentrated in vacuo to dryness. The product was purified by preparative HPLC (column; PEGASIL ODS, 20 × 250 mm; Senshu Scientific; solvent, 80% CH3CN; detection, UV at 210 nm; flow rate, 8.0 ml min−1) to give 1 (7.40 mg, 0.0081 mmol, 80.6%) as a white powder. Similarly, 2 (4.63 mg, 0.0050 mmol, 61.5%) was obtained using (S)-(+)-MTPA chloride.
7, 4′-(S)-(+)-α-methoxy-α-(trifluoromethyl)phenylacetoxy pentacecilide C (1): 1H NMR (400 MHz, CDCl3) δ 1.05 (s, 3H), 1.06 (s, 3H), 1.10 (s, 3H), 1.36 (s, 3H), 1.52 (d, 3H, J=7.0 Hz), 1.68, 2.28 (m, 2H), 1.85, 2.14 (m, 2H), 2.17 (s, 3H), 2.57 (m, 2H), 2.74, 2.82 (dd, 2H, J=17.0, 11.0 Hz, J=17.0, 3.5 Hz), 3.52 (s, 3H), 3.73 (s, 3H), 4.58 (m, 1H), 5.54 (m, 1H), 5.56 (m, 1H), 6.35 (s, 1H), 7.26 (m, 3H), 7.44 (m, 3H), 7.52 (m, 2H), 7.75 (m, 2H); HRESI-TOF-MS (m/z) found: 941.2935, calcd: 941.2948 [M+Na]+ for C47H48 F8NaO12.
7, 4′-(R)-(−)-α-methoxy-α-(trifluoromethyl)phenylacetoxy pentacecilide C (2): 1H NMR (400 MHz, CDCl3) δ 1.14 (s, 3H), 1.17 (s, 3H), 1.30 (s, 3H), 1.33 (s, 3H), 1.53 (d, 3H, J=7.0 Hz), 1.87, 2.16 (m, 2H), 2.31 (m, 2H), 2.17 (s, 3H), 2.50 (m, 2H), 2.74, 2.82 (dd, 2H, J=17.0, 11.0 Hz, J=17.0, 3.5 Hz), 3.53 (s, 3H), 3.76 (s, 3H), 4.57 (m, 1H), 5.60 (m, 1H), 5.61 (m, 1H), 6.31 (s, 1H), 7.32 (m, 3H), 7.44 (m, 3H), 7.52 (m, 2H), 7.74 (m, 2H); HRESI-TOF-MS (m/z) found: 941.2980, calcd: 941.2948 [M+Na]+ for C47H48 F8NaO12.
Preparation of 3S*-Hydroxy-pentacecilide A
To a solution of pentacecilide A (2.9 mg, 0.0070 mmol) in CH3OH (500 μl), NaBH4 (3.3 mg, 0.087 mmol) was added, and the resulting solution was stirred at room temperature. After 10 h, the reaction mixture was diluted with H2O and the aqueous phase was extracted with EtOAc. The organic layer was recovered, dried over anhydrous Na2SO4, filtered and concentrated in vacuo to dryness. The product was purified by preparative HPLC (column; PEGASIL ODS, 20 × 250 mm; Senshu Scientific; solvent, 80% CH3CN; detection, UV at 210 nm; flow rate, 8.0 ml min−1) to give 3 (2.5 mg, 0.0060 mmol, 85.7%) as a white powder.
3S*-Hydroxy-pentacecilide A (3): 1H NMR (400 MHz, CDCl3) δ 0.86 (s, 3H), 1.02 (s, 3H), 1.09 (s, 3H), 1.26 (m, 1H), 1.29 (s, 3H), 1.46, 1.56 (m, 2H), 1.55 (d, 3H, J=6.0 Hz), 1.66, 1.80 (m, 2H), 1.70 (m, 2H), 1.76 (m, 1H), 2.00, 2.08 (m, 2H), 2.40 (m, 2H), 2.68, 2.85 (dd, 2H, J=17.0, 11.0 Hz, J=17.0, 3.5 Hz), 3.26 (dd, J=10.0, 4.0 Hz, 1H), 4.63 (m, 1H), 6.26 (s, 1H), 11.0 (s, 1H); HRESI-TOF-MS (m/z) found: 437.2290, calcd: 437.2304 [M+Na]+ for C25H34NaO5.
Biological assays
An assay for the synthesis of CE, triacylglycerol and phospholipids by mouse macrophages was carried out according to the method described previously.11 An assay for ACAT1 and ACAT2 activities in ACAT1- and ACAT2-CHO cells was carried out by our established method.12, 13
References
Yamazaki, H. et al. Pentacecilides, new inhibitors for lipid droplet formation in mouse macrophages produced by Penicillium cecidicola FKI-3765-1:I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 62, 195–200 (2009).
Yamazaki, H., Ōmura, S. & Tomoda, H. Pentacecilides, new inhibitors for lipid droplet formation in mouse macrophages produced by Penicillium cecidicola FKI-3765-1:II. Structure elucidation. J. Antibiot. 62, 207–211 (2009).
Chang, C. C., Huh, H. Y., Cadigan, K. M. & Chang, T. Y. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem. 268, 20747–20755 (1993).
Anderson, R. A. et al. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 273, 26747–26754 (1998).
Cases, S. et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 273, 26755–26764 (1998).
Oelkers, P., Behari, A., Cromley, D., Billheimer, J. T. & Sturley, S. L. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem. 273, 26765–26771 (1998).
Parini, P. et al. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 110, 2017–2023 (2004).
Corrigenda. J. Antibiot. 62, 651 (2009).
Corrigenda. J. Antibiot. 62, 653–654 (2009).
Ohtani, I., Kusumi, T., Kashman, Y. & Kakisawa, H. High-field FT NMR application of Mosher's method. The absolute configuration of marine terpenoids. J. Am. Chem. Soc. 113, 4092–4096 (1991).
Namatame, I., Tomoda, H., Arai, H., Inoue, K. & Ōmura, S. Complete inhibition of mouse macrophage-derived foam cell formation by triacsin C. J. Biochem. 125, 319–327 (1999).
Lada, A. T. et al. Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. J. Lipid. Res. 45, 378–386 (2004).
Ohshiro, T., Rudel, L. L., Ōmura, S. & Tomoda, H. Selectivity of microbial acyl-CoA: cholesterol acyltransferase inhibitors toward isozymes. J. Antibiot. 60, 43–51 (2007).
Acknowledgements
This study was supported in part by a Sasakawa Scientific Research Grant (to HY) from The Japan Science Society. We express our thanks to Ms N Sato for NMR experiments, and Dr K Nagai and Ms A Nakagawa for measuring mass spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamazaki, H., Ugaki, N., Matsuda, D. et al. Absolute stereochemistry of pentacecilides, new inhibitors of lipid droplet formation in mouse macrophages, produced by Penicillium cecidicola FKI-3765-1. J Antibiot 63, 315–318 (2010). https://doi.org/10.1038/ja.2010.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2010.39
Keywords
This article is cited by
-
Characterization of a bioactive meroterpenoid isolated from the marine-derived fungus Talaromyces sp.
Applied Microbiology and Biotechnology (2022)
-
Exploration of marine natural resources in Indonesia and development of efficient strategies for the production of microbial halogenated metabolites
Journal of Natural Medicines (2022)