Abstract
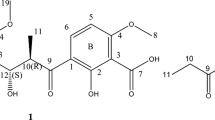
Longicatenamides A–D, two diastereomeric pairs of new cyclic hexapeptides, were isolated from the combined-culture of Streptomyces sp. KUSC_F05 and Tsukamurella pulmonis TP-B0596. Their planar structures were determined by spectroscopic analysis including extensive 2D NMR and MS analysis. The absolute configurations of their component amino acids were determined by the use of highly sensitive reagents we recently developed; the highly sensitive-advanced Marfey’s method (HS-advanced Marfey’s method), which led us to reduce the sample loss and prevent incorrect structural determination. Particularly, the Cβ-stereochemistry of hyGlu in longicatenamides A and C was assigned without any use of Cβ-Marfey’s methods. Longicatenamide A exhibited weak but preferential antimicrobial activity against Bacillus subtilis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bérdy J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J Antibiot. 2012;65:385–95.
Cimermancic P, Medema MH, Claesen J, Kurita K, Wieland Brown LC, Mavrommatis K, et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell. 2014;158:412–21.
Pye CR, Bertin MJ, Lokey RS, Gerwick WH, Linington RG. Retrospective analysis of natural products provides insights for future discovery trends. Proc Natl Acad Sci USA. 2017;114:5601–6.
Onaka H, Mori Y, Igarashi Y, Furumai T. Mycolic acid-containing bacteria induce natural-product biosynthesis in Streptomyces species. Appl Environ Microbiol. 2011;77:400–6.
Hshino S, Onaka H, Abe I. Activation of silent biosynthetic pathway and discovery of novel secondary metabolites in actinomycetes by co-culture with mycolic acidcontaining bacteria. J Ind Microbiol Biotechnol. 2019;46:363–74.
Kakeya H. Natural products-prompted chemical biology: phenotypic screening and a new platform for target identification. Nat Prod Rep. 2016;33:648–54.
Liu C, Kakeya H. Cryptic chemical communication: Secondary metabolic responses revealed by microbial co-culture. Chem Asian J. 2020;15:327–37.
Sugiyama R, Nishimura S, Ozaki T, Asamizu S, Onaka H, Kakeya H. 5-Alkyl-1,2,3,4-tetrahydroquinolines, new membrane-interacting lipophilic metabolites produced by combined culture of Streptomyces nigrescens and Tsukamurella pulmonis. Org Lett. 2015;17:1918–21.
Sugiyama R, Nishimura S, Ozaki T, Asamizu S, Onaka H, Kakeya H. Discovery and total synthesis of streptoaminals: Antimicrobial [5,5]-spirohemiaminals from the combined-culture of Streptomyces nigrescens and Tsukamurella pulmonis. Angew Chem Int Ed. 2016;55:10278–82.
Ozaki T, Sugiyama R, Shimomura M, Nishimura S, Asamizu S, Katsuyama Y, et al. Identification of the common biosynthetic gene cluster for both antimicrobial streptoaminals and antifungal 5-alkyl-1,2,3,4-tetrahydroquinolines. Org Biomol Chem. 2019;17:2370–8.
Sugiyama R, Nakatani T, Nishimura S, Takenaka K, Ozaki T, Asamizu S, et al. Chemical interactions of cryptic actinomycete metabolite 5-Alkyl-1,2,3,4-tetrahydroquinolines through aggregate formation. Angew Chem Int Ed. 2019;58:13486–91.
Jiang Y, Lu S, Hirai G, Kato T, Onaka H, Kakeya H. Enhancement of saccharothriolide production and discovery of a new metabolite, saccharothriolide C2, by combined-culture of Saccharothrix sp. and Tsukamurella pulmonis. Tetrahedron Lett. 2019;60:1072–4.
Von Nussbaum F, Anlauf S, Freiberg C, Benet-Buchholz J, Schamberger J, Henkel T, et al. Total synthesis and initial structure–activity relationships of longicatenamycin A. ChemMedChem. 2008;3:619–26.
Kuranaga T, Minote M, Morimoto R, Pan C, Ogawa H, Kakeya H. Highly sensitive labeling reagents for scarce natural products. ACS Chem Biol. 2020;15:2499–506.
Kishimoto S, Tsunematsu Y, Nishimura S, Hayashi Y, Hattori A, Kakeya H. Tumescenamide C, an antimicrobial cyclic lipodepsipeptide from Streptomyces sp. Tetrahedron. 2012;68:5572–8.
Takahashi N, Kaneko K, Kakeya H. Total synthesis and antimicrobial activity of tumescenamide C and its derivatives. J Org Chem. 2020;85:4530–5.
Shiba T, Mukunoki Y. The total structure of the antibiotic longicatenamycin. J Antibiot. 1975;28:561–6.
Zou B, Long K, Ma D. Total synthesis and cytotoxicity studies of a cyclic depsipeptide with proposed structure of palau’amide. Org Lett. 2005;7:4237–40.
Sugiyama H, Watanabe A, Teruya T, Suenaga K. Synthesis of palau’amide and its diastereomers: confirmation of its stereostructure. Tetrahedron Lett. 2009;50:7343–5.
Kuranaga T, Sesoko Y, Sakata K, Maeda N, Hayata A, Inoue M. Total synthesis and complete structural assignment of yaku’amide A. J Am Chem Soc. 2013;135:5467–74.
Kuranaga T, Mutoh H, Sesoko Y, Goto T, Matsunaga S, Inoue M. Elucidation and total synthesis of the correct structures of tridecapeptides yaku’amides A and B. Synthesis-driven stereochemical reassignment of the four amino acid residues. J Am Chem Soc. 2015;137:9443–51.
Son S, Hong Y-S, Jang M, Heo KT, Lee B, Jang J-P, et al. Nicrophorusamides A and B, antibacterial chlorinated cyclic peptides from a gut bacterium of the carrion beetle Nicrophorus concolor. J Nat Prod. 2017;80:2962–8.
Marfey P. Determination of D-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res Commun. 1984;49:591–6.
Kuranaga T, Matsuda K, Takaoka K, Tachikawa C, Sano A, Itoh K, et al. Total synthesis and structural revision of kasumigamide, and identification of a new analogue. Chem Bio Chem. 2020;21:3329–32.
Ishida K, Murakami M. Kasumigamide, an antialgal peptide from the cyanobacterium Microcystis aeruginosa. J Org Chem. 2000;65:5898–900.
Holm GE, Gortner KA. The humin formed by the acid hydrolysis of proteins VI The effect of acid hydrolysis upon tryptophane. J Am Chem Soc. 1920;42:2378–85.
Bhushan R, Brückner H. Use of Marfey’s reagent and analogs for chiral amino acid analysis: assessment and applications to natural products and biological systems. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3148–61.
Vijayasarathy S, Prasad P, Fremlin LJ, Ratnayake R, Salim AA, Khalil Z, et al. C3 and 2D C3 Marfey’s methods for amino acid analysis in natural products. J Nat Prod. 2016;79:421–7.
Acknowledgements
This work was financially supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan [17H06401 (H.K.), 18K14396 (T.K.), and 19H02840 (H.K.)], the Platform Project for Supporting Drug Discovery and Life Science Research from the Japan Agency for Medical Research and Development (AMED) [20am01092j0004 (H.K.)], Japan, and the Tokyo Biochemical Research Foundation (T.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jiang, Y., Matsumoto, T., Kuranaga, T. et al. Longicatenamides A–D, Two Diastereomeric Pairs of Cyclic Hexapeptides Produced by Combined-culture of Streptomyces sp. KUSC_F05 and Tsukamurella pulmonis TP-B0596. J Antibiot 74, 307–316 (2021). https://doi.org/10.1038/s41429-020-00400-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-00400-3
This article is cited by
-
Amoxetamide A, a new anoikis inducer, produced by combined-culture of Amycolatopsis sp. and Tsukamurella pulmonis
The Journal of Antibiotics (2024)
-
Total syntheses of surugamides and thioamycolamides toward understanding their biosynthesis
Journal of Natural Medicines (2023)
-
A bioprocess perspective on the production of secondary metabolites by Streptomyces in submerged co-cultures
World Journal of Microbiology and Biotechnology (2021)