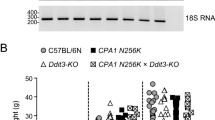
Abstract
Background:
The proprotein convertase 1/3 (PC1/3), encoded by proprotein convertase subtilisin/kexin type 1 ( PCSK1), cleaves and hence activates several orexigenic and anorexigenic proproteins. Congenital inactivation of PCSK1 leads to obesity in human but not in mice. However, a mouse model harboring the hypomorphic mutation N222D is obese. It is not clear why the mouse models differ in phenotype.
Methods:
Gene expression analysis was performed with pancreatic islets from Pcsk1N222D/N222D mice. Subsequently, biosynthesis, maturation, degradation and activity were studied in islets, pituitary, hypothalamus and cell lines. Coimmunoprecipitation of PC1/3-N222D and human PC1/3 variants associated with obesity with the endoplasmic reticulum (ER) chaperone BiP was studied in cell lines.
Results:
Gene expression analysis of islets of Pcsk1N222D/N222D mice showed enrichment of gene sets related to the proteasome and the unfolded protein response. Steady-state levels of PC1/3-N222D and in particular the carboxy-terminally processed form were strongly reduced in islets, pituitary and hypothalamus. However, impairment of substrate cleavage was tissue dependent. Proinsulin processing was drastically reduced, while processing of proopiomelanocortin (POMC) to adrenocorticotropic hormone (ACTH) in pituitary was only mildly impaired. Growth hormone expression and IGF-1 levels were normal, indicating near-normal processing of hypothalamic proGHRH. PC1/3-N222D binds to BiP and is rapidly degraded by the proteasome. Analysis of human PC1/3 obesity-associated mutations showed increased binding to BiP and prolonged intracellular retention for all investigated mutations, in particular for PC1/3-T175M, PC1/3-G226R and PC1/3-G593R.
Conclusions:
This study demonstrates that the hypomorphic mutation in Pcsk1N222D mice has an effect on catalytic activity in pancreatic islets, pituitary and hypothalamus. Reduced substrate processing activity in Pcsk1N222D/N222D mice is due to enhanced degradation in addition to reduced catalytic activity of the mutant. PC1/3-N222D binds to BiP, suggesting impaired folding and reduced stability. Enhanced BiP binding is also observed in several human obesity-associated PC1/3 variants, suggesting a common mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet 1997; 16: 303–306.
Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997; 387: 903–908.
Seidah NG, Sadr MS, Chrétien M, Mbikay M . The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions. J Biol Chem 2013; 288: 21473–21481.
Creemers JWM, Khatib A-M . Knock-out mouse models of proprotein convertases: unique functions or redundancy? Front Biosci 2008; 13: 4960–4971.
Lindberg I . Evidence for cleavage of the PC1/PC3 pro-segment in the endoplasmic reticulum. Mol Cell Neurosci 1994; 5: 263–268.
Farooqi IS, Volders K, Stanhope R, Heuschkel R, White A, Lank E et al. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab 2007; 92: 3369–3373.
Strawbridge RJ, Dupuis J, Prokopenko I, Barker A, Ahlqvist E, Rybin D et al. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes 2011; 60: 2624–2634.
Corpeleijn E, Petersen L, Holst C, Saris WH, Astrup A, Langin D et al. Obesity-related polymorphisms and their associations with the ability to regulate fat oxidation in obese Europeans: the NUGENOB study. Obesity (Silver Spring) 2010; 18: 1369–1377.
Goossens GH, Petersen L, Blaak EE, Hul G, Arner P, Astrup et al. Several obesity- and nutrient-related gene polymorphisms but not FTO and UCP variants modulate postabsorptive resting energy expenditure and fat-induced thermogenesis in obese individuals: the NUGENOB study. Int J Obes (Lond) 2009; 33: 669–679.
Gjesing AP, Vestmar MA, Jørgensen T, Heni M, Holst JJ, Witte DR et al. The effect of PCSK1 variants on waist, waist-hip ratio and glucose metabolism is modified by sex and glucose tolerance status. PLoS One 2011; 6: e23907.
Stijnen P, Tuand K, Varga TV, Franks PW, Aertgeerts B . Creemers JWM. The association of common variants in PCSK1 with obesity: a HuGE review and meta-analysis. Am J Epidemiol 2014; 180: 1051–1065.
Philippe J, Stijnen P, Meyre D, De Graeve F, Thuillier D, Delplanque J et al. A nonsense loss-of-function mutation in PCSK1 contributes to dominantly inherited human obesity. Int J Obes (Lond) 2014; 39: 295–302.
Creemers JWM, Choquet H, Stijnen P, Vatin V, Pigeyre M, Beckers S et al. Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity. Diabetes 2012; 61: 383–390.
Martín MG, Lindberg I, Solorzano-Vargas RS, Wang J, Avitzur Y, Bandsma R et al. Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other endocrinopathies in a pediatric cohort. Gastroenterology 2013; 145: 138–148.
Jackson RS, Creemers JWM, Farooqi IS, Raffin-Sanson M-L, Varro A, Dockray GJ et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest 2003; 112: 1550–1560.
Bandsma RHJ, Sokollik C, Chami R, Cutz E, Brubaker PL, Hamilton JK et al. From diarrhea to obesity in prohormone convertase 1/3 deficiency: age-dependent clinical, pathologic, and enteroendocrine characteristics. J Clin Gastroenterol 2013; 47: 834–843.
Pickett LA, Yourshaw M, Albornoz V, Chen Z, Solorzano-Vargas RS, Nelson SF et al. Functional consequences of a novel variant of PCSK1. PLoS One 2013; 8: e55065.
Frank GR, Fox J, Candela N, Jovanovic Z, Bochukova E, Levine J et al. Severe obesity and diabetes insipidus in a patient with PCSK1 deficiency. Mol Genet Metab 2013; 110: 191–194.
Wilschanski M, Abbasi M, Blanco E, Lindberg I, Yourshaw M, Zangen D et al. A novel familial mutation in the PCSK1 gene that alters the oxyanion hole residue of proprotein convertase 1/3 and impairs its enzymatic activity. PLoS One 2014; 9: e108878.
Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci USA 2002; 99: 10293–10298.
Lloyd DJ, Bohan S, Gekakis N . Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet 2006; 15: 1884–1893.
Strausberg SL, Alexander PA, Gallagher DT, Gilliland GL, Barnett BL, Bryan PN . Directed evolution of a subtilisin with calcium-independent stability. Biotechnology (N Y) 1995; 13: 669–673.
Lemaire K, Ravier MA, Schraenen A, Creemers JWM ¸ Van de Plas R, Granvik M et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA 2009; 106: 14872–14877.
Brouwers B, de Faudeur G, Osipovich AB, Goyvaerts L, Lemaire K, Boesmans L et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab 2014; 20: 979–990.
Blanco EH, Peinado JR, Martín MG, Lindberg I . Biochemical and cell biological properties of the human prohormone convertase 1/3 Ser357Gly mutation: a PC1/3 hypermorph. Endocrinology 2014; 155: 3434–3447.
Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One 2009; 4: e6529.
Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JWM, Tsukamoto K et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med 2005; 11: 757–764.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Merico D, Isserlin R, Stueker O, Emili A, Bader GD . Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One 2010; 5: e13984.
Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 2003; 34: 374–378.
Janky R, Verfaillie A, Imrichová H, Van de Sande B, Standaert L, Christiaens V et al. iRegulon: from a gene list to a gene regulatory network using large motif and track collections. PLoS Comput Biol 2014; 10: e1003731.
Andersen CL, Jensen JL, Ørntoft TF . Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 2004; 64: 5245–5250.
Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J . qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 2007; 8: R19.
Benzinou M, Creemers JWM, Choquet H, Lobbens S, Dina C, Durand E et al. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet 2008; 40: 943–945.
Kondo S, Saito A, Asada R, Kanemoto S, Imaizumi K . Physiological unfolded protein response regulated by OASIS family members, transmembrane bZIP transcription factors. IUBMB Life 2011; 63: 233–239.
Hebert DN, Bernasconi R, Molinari M . ERAD substrates: which way out? Semin Cell Dev Biol 2010; 21: 526–532.
Määttänen P, Gehring K, Bergeron JJM, Thomas DY . Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol 2010; 21: 500–511.
Zandberg WF, Benjannet S, Hamelin J, Pinto BM, Seidah NG . N-glycosylation controls trafficking, zymogen activation and substrate processing of proprotein convertases PC1/3 and subtilisin kexin isozyme-1. Glycobiology 2011; 21: 1290–1300.
Shental-Bechor D, Levy Y . Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc Natl Acad Sci USA 2008; 105: 8256–8261.
Prabhu Y, Blanco EH, Liu M, Peinado JR, Wheeler MC, Gekakis N et al. Defective transport of the obesity mutant PC1/3 N222D contributes to loss of function. Endocrinology 2014; 155: 2391–2401.
Zhou Y, Lindberg I . Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. J Biol Chem 1994; 269: 18408–18413.
Geva Y, Schuldiner M . The back and forth of cargo exit from the endoplasmic reticulum. Curr Biol 2014; 24: R130–R136.
Hoshino A, Kowalska D, Jean F, Lazure C, Lindberg I . Modulation of PC1/3 activity by self-interaction and substrate binding. Endocrinology 2011; 152: 1402–1411.
Arias a E, Vélez-Granell CS, Mayer G, Bendayan M . Colocalization of chaperone Cpn60, proinsulin and convertase PC1 within immature secretory granules of insulin-secreting cells suggests a role for Cpn60 in insulin processing. J Cell Sci 2000; 113: 2075–2083.
Creemers JW, van de Loo JW, Plets E, Hendershot LM, Van De Ven WJ . Binding of BiP to the processing enzyme lymphoma proprotein convertase prevents aggregation, but slows down maturation. J Biol Chem 2000; 275: 38842–38847.
Gyamera-Acheampong C, Sirois F, Denis NJ, Mishra P, Figeys D, Basak et al. The precursor to the germ cell-specific PCSK4 proteinase is inefficiently activated in transfected somatic cells: evidence of interaction with the BiP chaperone. Mol Cell Biochem 2011; 348: 43–52.
Cakir I, Cyr NE, Perello M, Litvinov BP, Romero A, Stuart RC et al. Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. J Biol Chem 2013; 288: 17675–17688.
Zhu J, Bultynck G, Luyten T, Parys JB, Creemers JWM, Van de Ven WJM et al. Curcumin affects proprotein convertase activity: Elucidation of the molecular and subcellular mechanism. Biochim Biophys Acta 2013; 1833: 1924–1935.
Liew CW, Assmann A, Templin AT, Raum JC, Lipson KL, Rajan S et al. Insulin regulates carboxypeptidase E by modulating translation initiation scaffolding protein eIF4G1 in pancreatic β cells. Proc Natl Acad Sci USA 2014; 111: E2319–E2328.
Shen FS, Loh YP . Intracellular misrouting and abnormal secretion of adrenocorticotropin and growth hormone in cpefat mice associated with a carboxypeptidase E mutation. Proc Natl Acad Sci USA 1997; 94: 5314–5319.
Acknowledgements
PS designed the study, researched data and wrote the manuscript. BB researched data and reviewed the manuscript. ED and LV researched data, BR, FS, LT and JD reviewed the manuscript. JWMC wrote the manuscript and is guarantor of this article. This work was supported by IWT-Vlaanderen, personal fellowship PS, FWO Vlaanderen and KU Leuven grant GOA2008/16. We wish to thank Sandra Meulemans for technical assistance, Fred Van Leuven for advice and Robert Day, Ann White and Iris Lindberg for providing materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Stijnen, P., Brouwers, B., Dirkx, E. et al. Endoplasmic reticulum-associated degradation of the mouse PC1/3-N222D hypomorph and human PCSK1 mutations contributes to obesity. Int J Obes 40, 973–981 (2016). https://doi.org/10.1038/ijo.2016.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.3
This article is cited by
-
The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits
Nature Reviews Endocrinology (2022)
-
Glucose-regulated protein 78 binds to and regulates the melanocortin-4 receptor
Experimental & Molecular Medicine (2018)
-
Role of the ubiquitin/proteasome system on ACTH turnover in rat corticotropes
Endocrine (2018)