Abstract
Reversible posterior leukoencephalopathy syndrome (RPLS) is a critical maternal complication in preeclampsia or eclampsia during pregnancy. However, studies regarding the clinicoradiological and outcome differences between RPLS and non-RPLS pregnancies are scarce. We aimed to explore the incidence of RPLS, and summarize the clinicoradiological characteristics and pregnancy outcomes. We consecutively collected a total of 100 patients who were diagnosed with preeclampsia or eclampsia, and examined via magnetic resonance imaging (MRI) among 21 872 women between 2013 and 2016. All patients were grouped into RPLS (n=49) and non-RPLS (n=51) groups according to their MRI results. Information about clinicoradiological features and pregnancy outcomes was collected retrospectively to explore the differences between the groups. The incidence of RPLS in pregnant women was 0.22% (49/21 872). The frequency of clinical symptoms, such as headache, vision change, seizure and consciousness disorders, and blood pressure conditions, such as severely elevated hypertension, systolic and diastolic pressure and mean arterial pressure levels, was increased in the RPLS group compared with that in the non-RPLS group (P<0.05). The occipital lobe was the most frequently affected area (93.88%) in RPLS patients. The cesarean section rate in RPLS group was higher than the non-RPLS group (P<0.05), whereas the 1 min Apgar score was lower (P<0.05). These results suggest that the incidence of RPLS was high. Information about clinical symptoms and blood pressure was useful in predicting RPLS. In addition, RPLS was significantly associated with the delivery mode and pregnancy outcomes. The most frequently affected area was the occipital lobe.
Similar content being viewed by others
Introduction
Reversible posterior leukoencephalopathy syndrome (RPLS) is a clinicoradiological syndrome that was first described by Hincheyet al.1 in 1996 and is associated with a multitude of symptoms, such as headaches, visual changes, seizures, consciousness impairment, mental disorders and focal neurologic deficits. To date, the incidence of RPLS and the pathogenic mechanism remain unclear. The diagnosis mainly relies on imaging examination owing to the lack of specificity of clinical symptoms. Although the prognosis of RPLS is good, especially in pregnant women, RPLS is critical in the acute phase and even dangerous, and the maternal and neonatal lives are at higher risk. Thus, early diagnosis and treatment are particularly important. The present study summarized the incidence of RPLS, and examined the general information, clinical symptoms, radiological characteristics and outcomes of patients and neonates to explore the characteristics of RPLS in preeclampsia or eclampsia.
Methods
This retrospective study was approved by the institutional ethics committee of The Third Affiliated Hospital of Guangzhou Medical University. There were 21 872 women who delivered at Guangzhou Medical Center for Critical Pregnant Women between January 2013 and March 2016. Among them, 100 patients met the inclusion criteria: (a) patients diagnosed with preeclampsia or eclampsia after 20 weeks of gestation or within 6 weeks post partum and (b) patients who underwent magnetic resonance imaging (MRI) examination after giving their informed consent by clinician. The general information, clinical symptoms, radiological characteristics and pregnancy outcomes were collected retrospectively.
Preeclampsia was defined as a woman who was complicated with hypertension, proteinuria and edema between the 20th week of gestation and the sixth post-partum week. Eclampsia was defined as preeclampsia plus seizures unrelated to other cerebral conditions.2, 3
All 100 patients were examined via whole-brain MRI (Achieva 3.0 T, PHILIPS, Amsterdam, The Netherlands), including T1-weighted, T2-weighted, T2 FLAIR and diffusion-weighted imaging, MRI diagnosis or confirmation was made by two neuroradiologists independently. The neuroradiologists then tried to reach a consensus. Typical MRI performance involved hyper intensities of T2WI and FLAIR in the occipital and parietal lobes. The diagnosis of RPLS was made based on a combination of clinical syndrome (headache, vision change, seizure, consciousness disorders or hypertension) and standard radiological criteria (hyper intensities of T2WI and FLAIR in the subcortex and gyrus as focal vasogenic edema).
Regular prenatal examination means that the examination times are consistent in terms of the time interval (gestation <28 weeks, 4-week interval; 28–36 weeks of gestation, 2-week interval; 36–40 weeks of gestation, 1-week interval; gestation >40 weeks, 3- day interval). Irregular prenatal examination was noted when the times of prenatal examination during pregnancy did not correspond to up to 2/3 of the recommended times.
The blood pressure for the patients was obtained immediately at the onset of symptoms, and the measurements were classified into four categories according to the systolic pressure: normal (<140 mm Hg), mildly elevated (140–159 mm Hg), moderately elevated (160–179 mm Hg) and severely elevated (⩾180 mm Hg). Mean arterial pressure was defined as 2/3 diastolic pressure+1/3 systolic pressure.
The indicators for pregnancy outcomes included gestational weeks, delivery mode, length of hospital stay, neurological sequelae and death. Variables for neonatal prognosis included stillbirth, fetal growth restriction, premature delivery and Apgar scores. Fetal growth restriction was defined as an ultrasound-based fetal weight (estimated fetal weight (EFW)) <10th percentile plus Doppler abnormalities or birth weight <3rd percentile.
Statistical analyses
Descriptive statistics for continuous variables were presented as the mean±s.d., and categorical variables were presented as frequencies and percentages. We used Student’s t-test to examine the two groups of patients for the continuous variables and χ2-test for the categorized variables. The threshold for statistical significance was set at P<0.05. We analyzed our data using the SPSS 13.0 statistical software package (SPSS Inc., Chicago, IL, USA).
Results
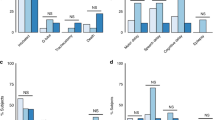
The incidence of RPLS in pregnant women was 0.22%. All 100 patients were divided into two groups: 49 patients were included in the RPLS group and 51 were in the non-RPLS group according to the imaging results. General information, clinical symptoms and blood pressure are presented in Table 1. The RPLS group included 26 women with preeclampsia and 23 women with eclampsia, for whom the mean age was 28.71±7.19 years old and the mean body mass index was 22.85±4.13 kgm−2. The non-RPLS group included 51 women with preeclampsia. Their mean age was 28.71±7.19 years old, and their mean body mass index was 22.03±3.19 kgm−2. There were a total of 29 primiparous patients in the RPLS group and 25 in the non-RPLS group. RPLS patients exhibited a higher proportion of irregular prenatal examination than the non-RPLS group (P=0.009). No statistically significant differences were noted in terms of normal blood pressure, mildly elevated and moderately elevated pressure. However, the proportion of severely elevated pressure in the RPLS group was increased compared with that in the non-RPLS group (38.78% vs. 13.73%; P=0.006). Furthermore, systolic pressure (P=0.003), diastolic pressure (P=0.029) and mean arterial pressure (P=0.005) were increased compared with those in the non-RPLS group.
Headache was the most common symptom in RPLS group, whereas visual changes were most frequently observed in the non-RPLS group (71.43% vs. 29.41%). The occurrence rates of headache (P<0.001), visual changes (P=0.045), seizures (P<0.001) and consciousness impairment (P<0.001) were increased in patients with RPLS compared with the non-RPLS group. In addition, dizziness was quite a common symptom for all the patients, and there was no significant difference between the two groups. The imaging features were notable in RPLS patients. For example, T1WI of the MRI exhibited a slightly low signal, while T2WI and FLAIR produced high signals. In addition, the diffusion-weighted imaging sequence exhibited a slightly lower signal, whereas the apparent diffusion coefficient (ADC) exhibited a higher signal that indicated the lesion was vascular edema (Figure 1). For the RPLS patients, the occipital lobe was the most frequently affected area (93.88%), followed by the parietal lobe (65.31%), basal ganglia (32.65%), temporal lobe (30.61%), frontal lobe (26.53%), brainstem (8.16%) and cerebellum (6.12%, Table 2).
(a) MRI on axial T1WI showing low signal predominantly in the occipital lobes, while (b) Axial T2WI showing hyperintensive signal. (c) MRI on axial DWI demonstrates both hyperintensive signal (small arrow) and low signal (thick arrow), while (d) Axial ADC showing hyperintensive signal indicates the lesion was vascular edema. (e) MRI on axial FLAIR showing hyperintensive signal (arrows). ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging.
Among all patients, one died due to RPLS, and the remaining patients exhibited no severe sequelae in either group. For the RPLS group, the mean gestational length was 30.95±5.14 weeks, and the mean length of hospital stay was 17.65±28.95 days. For the non-RPLS group, the mean gestational length was 31.36±4.69 weeks, and the mean hospitalization time was 10.11±5.44 days. There was no significant difference between the groups. However, there was a significant difference in the cesarean section rate between the two groups (P=0.015). The rates of stillbirth and fetal growth restriction in newborns were similar between the two groups (P=0.271 and P=0.212, respectively), but the rate of preterm delivery in the non-RPLS group was increased compared with that in the RPLS group (P=0.035). The differences in 5 min and 10 min Apgar score between the two groups were not statistically significant (P=0.250 and P=0.255, respectively), whereas the 1 min Apgar score in the RPLS group was reduced compared with the non-RPLS group (P=0.047, Table 3).
Discussion
RPLS is a clinicoradiological syndrome that was first described by Hinchey et al.1 in 1996. It features a combination of symptoms, including headaches, visual changes, seizures, consciousness impairment, mental disorders and focal neurologic deficits. The incidence rate was 0.01%4 as previously reported in the literature, whereas the incidence of 0.22% in our study was higher than those in previous studies. Our increased rate was likely due to the admission of more intensive patients to our medical center for critical pregnant women. However, taking into account missed diagnoses and misdiagnosis, the actual incidence rate could be even higher.
RPLS exhibits various pathogenies, such as severe hypertension, preeclampsia or eclampsia, organ and bone marrow transplantation, renal dysfunction, autoimmunity, sepsis and chemotherapy.5, 6 Pregnant women exhibit special physiological changes, and the risk factors for preeclampsia and eclampsia, in some sense, were similar to the risk factors for RPLS, including primiparity, diabetes, repeated pregnancy, hypercoagulable state, history of preeclampsia, body mass index ⫺35 kgm−2, metabolic syndrome, acephalocystis racemosa, and maternal age ⫺40 or <17 years.7 The pathological and physiological changes in RPLS are potentially a result of numerous factors due to various pathogenies. Although the pathophysiology is not clear, the same pathogenic mechanisms are shared,8 including vasogenic edema caused by breakdown of cerebral autoregulation, disruption of blood–brain barrier or endothelial dysfunction due to cytotoxicity. Several theories have been proposed in recent years. The first theory is that severe hypertension is associated with failed autoregulation, leading to vasogenic edema caused by vasodilatation, breakthrough of the blood–brain barrier, hyperperfusion and increased vascular permeability.9 The second theory is that endothelial dysfunction is caused by cytotoxicity, such as drugs and bacterial endotoxins, which induce cytokine release or direct injury of endothelial cells, and thus lead to vasoconstriction and edema, with subsequent hypoperfusion.10, 11 In our study, severely elevated pressure, systolic or diastolic pressure and mean arterial pressure were increased in the RPLS group compared with those in the non-RPLS group, thus suggesting that the increase in blood pressure is an important factor for RPLS. However, 20–30% of RPLS patients exhibit normal or mildly elevated blood pressure, suggesting that hypertension is not essential for brain edema. Similarly, a study was performed to explore the relationship between increased blood pressure and basic blood pressure, indicating that RPLS can even develop in patients with normal blood pressure.6 Therefore, it is important to maintain the integrity of vascular endothelial because it is the basis of cerebral autoregulation, and RPLS can develop even in women with normal pressure.
RPLS onset typically occurs acutely or subacutely. Various clinical symptoms are observed and lack specificity, including common symptoms, such as headaches, visual changes, seizures, consciousness impairment, mental disorders, focal neurologic deficits, nausea and vomiting, and rare symptoms, such as tinnitus and vertigo.12, 13, 14 The incidence of headache and visual changes was high in RPLS patients with preeclampsia or eclampsia (up to 60%) In addition, the single symptom of headache also occurred in ~50% patients according to several studies,15, 16 whereas another study indicated that seizure was the most frequent symptom in RPLS patients with preeclampsia/eclampsia.17 In our study, headache (71.43%) was the most frequent symptom in the RPLS group, and visual changes (29.41%) were the most frequent symptom in the non-RPLS group. The incidences of headache (P<0.001), visual changes (P<0.001), seizures (P<0.001) and consciousness impairment (P<0.001) in the RPLS group were increased compared with those in the non-RPLS group. In addition, dizziness was also a common symptom in RPLS. However, there was no significant difference between the two groups (P=0.144).
Radiology was of great importance to the diagnosis and evaluation of RPLS, and MRI was the gold standard for the diagnosis of RPLS.18 Typically, T1WI of the MRI exhibited a slightly low signal, and T2WI and FLAIR exhibited high signals. In addition, the diffusion-weighted imaging sequence showed a slightly low signal, and apparent diffusion coefficient showed a high signal to distinguish the lesion from vascular edema to cytotoxic edema.13 Typical features include the involvement of the posterior cerebral circulation, indicating bilateral and symmetrical cerebral edema in the subcortical white matter, especially in occipital lobe. However, there were also some uncommon RPLS cases reported, including hemorrhages, infarction and cytotoxic edema.19 The occipital and parietal lobes were the most frequently affected areas in RPLS patients. A recent study indicated the most frequently affected area in RPLS patients with preeclampsia/eclampsia was the occipital lobe (94–98.7%), followed by the frontal lobe (77–78.9%), temporal lobe (64–68%) and cerebellum (53%).20 In our study, the location of RPLS was generally consistent with the literature, although RPLS in the basal ganglia, brainstem and cerebellum was increasingly noted. Possible explanations might include a failure to treat vasogenic edema promptly when it occurred in the first, reversible stage or the continuous increase in blood pressure, which studies have shown to be associated with the involvement of deep brain white matter.21
Generally speaking, RPLS is reversible within several days to weeks, and the prognosis is good.1, 22 However, the symptoms develop very quickly during the first few hours and reach their worst levels within 12–48 h.22 The incidence of eclampsia was 0.28%. The maternal mortality rate was 3.66% in pregnant women with eclampsia and the mortality rate of RPLS with eclampsia was 4.8%.23 In our study, only one patient died, and the mortality rate was much lower than that reported in the literature. It was very likely that there was a connection with the experience of a long-term cure for critical pregnant women in our center. In addition, the hospitalization time and gestational weeks show no statistically significant differences, and no patients exhibited any severe sequelae, indicating that the short-term prognosis in RPLS with preeclampsia or eclampsia was better than that in RPLS with other pathogenies. Long-term prognosis, including the probability of suffering from cardiovascular and cerebrovascular diseases (such as hypertension, heart infarction, stroke and venous system disease), was considerably increased compared with normal pregnancy according to the literature, potentially as a result of endothelial damage.24
Pregnancy outcomes were closely related to the gestational week (gestational age) of the termination of pregnancy in general because the gestational age can affect the maturity of the fetus, which has an important influence on the perinatal outcomes. Therefore, although the positive termination of pregnancy can relieve patient conditions, it can increase the rate of premature birth by iatrogenic intervention, thus increasing perinatal mortality. In our study, the delivery mode, premature birth, stillbirth and fetal growth restriction rates in the RPLS group were increased compared with the non-RPLS group, and cesarean section (P=0.015) and premature delivery (P=0.035) exhibited statistically significant differences along with the Apgar score at 1 min (P=0.047). These findings may arise because iatrogenic intervention as a result of the continuous deterioration of the disease increases the cesarean section and premature delivery rates. The long-term effects on the offspring are unclear. Some large-cohort studies indicate that preeclampsia or eclampsia can reduce the risk of cerebral palsy in offspring and increase the risk of other diseases, such as respiratory system disease, endocrine disease, nutritional and metabolic diseases and hematological system diseases.25, 26 Whether RPLS in patients with preeclampsia or eclampsia influences the health of the offspring is worthy of further study.
Some limitations inherent in this study should be acknowledged. First, this was a retrospective study, and the results depend on medical records and are subject to their availability and accuracy. Second, the clinical difference before and after the disease was not analyzed due to the lack of medical records. Third, follow-up MRI was not performed on some RPLS patients. In addition, the mechanism of RPLS was not assessed in our study, so further prospective studies are needed.
In summary
The incidence of RPLS in preeclampsia or eclampsia patients was high, and the clinical symptoms and blood pressure were helpful for predicting RPLS. Furthermore, RPLS affects the delivery mode and pregnancy outcomes. The most frequently affected areas were the occipital and parietal lobes.
References
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LRA . Reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996; 334: 494–500.
SMFM Publications Committee. SMFM statement: benefit of antihypertensive therapy or mild-to-moderate chronic hypertension during pregnancy remains uncertain. Am J Obstet Gynecol 2015; 213: 3–4.
Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM . The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001; 20: IX–XIV.
Daniel S, Thampi B, Viswanathan M, Manoj P . Posterior reversible leukoencephalopathy syndrome: a rare complication with gestational hypertension and pre eclampsia. Int J Med Res 2014; 2: 50–53.
Fugate JE, Rabinstein AA . Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015; 14: 914–925.
Mueller-Mang C, Mang T, Pirker A, Klein K, Prchla C, Prayer D . Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology 2009; 51: 373–383.
Staff AC, Sibai BM, Cunningham FG. Prevention of preeclampsia and eclampsia. In: Chesleys Hypertensive Disorders in Pregnancy, Elsevier, 2015, pp 253–267..
Hobson EV, Craven I, Blank SC . Posterior reversible encephalopathy syndrome: a truly treatable neurologic illness. Perit Dial Int 2012; 32: 590–594.
Bartynski WS . Posterior reversible encephalopathy syndrome, Part 2: Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008; 29: 1043–1049.
Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G . Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypotheses 2014; 82: 619–622.
Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT . Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology 2000; 217: 371–376.
Singh RR, Ozyilmaz N, Waller S, U-King-Im JM, Lim M, Siddiqui A, Sinha MD . A study on clinical and radiological features and outcome in patients with posterior reversible encephalopathy syndrome (PRES). Eur J Pediatr 2014; 173: 1225–1231.
Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E . The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol 2012; 259: 155–164.
Granata G, Greco A, Iannella G, Granata M, Manno A, Savastano E, Magliulo G . Posterior reversible encephalopathy syndrome—Insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev 2015; 14: 830–836.
Marrone LC, Gadonski G, Diogo LP, Brunelli JP, Martins WA, Laguna Gde O, Bahlis LF, Filho JR, da Costa BE, Poli-de-Figueiredo CE, Marrone AC, da Costa JC . Posterior reversible encephalopathy syndrome: differences between pregnant and non-pregnant patients. Neurol Int 2014; 6: 5376–5388.
Kurdoglu Z, Cetin O, Sayın R, Dirik D, Kurdoglu M, Kolusarı A, Yıldızhan R, Guler Sahin H . Clinical and perinatal outcomes in eclamptic women with posterior reversible encephalopathy syndrome. Arch Gynecol Obstet 2015; 292: 1013–1018.
Roth C, Ferbert A . The posterior reversible encephalopathy syndrome: what’s certain, what’s new? Pract Neurol 2011; 11: 136–144.
Nielsen LH, Grøn BS, Ovesen PG . Posterior reversible encephalopathy syndrome postpartum. Clin Case Rep 2015; 3: 266–270.
Liman TG, Bohner G, Heuschmann PU, Scheel M, Endres M, Siebert E . Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol 2012; 19: 935–943.
Kutlesič MS, Kutlesič RM, Koratevič GP . Posterior reversible encephalopathy syndrome in eclamptic patients: neuroradiological manifestation, pathogenesis and management. Med Pregl 2015; 68: 53–58.
Lv C, Gao B . Can clinical and MRI findings predict the prognosis of variant and classical type of posterior reversible encephalopathy syndrome (PRES)? Still a challenge. Acta Radiol 2015; 56: NP3–NP4.
Lee VH, Wijdicks EF, Manno EM, Rabinstein AA . Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 2008; 65: 205–210.
Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP, Souza. JP . Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. Obstetric Anesthesia Digest 2015; 35: 14–24.
Bellamy L, Casas JP, Hingorani AD, Williams DJ . Preeclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007; 335: 974–977.
Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J . Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol 2009; 201: 269.e1–269.e10.
Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J . Diseases in children born to mothers with preeclampsia: a population-based sibling cohort study. Am J Obstet Gynecol 2011; 204: 157.e1–e5.
Acknowledgements
We acknowledge financial support from the Major Disease Prevention and Control Collaborative Innovation Center of Guangdong Province, Guangzhou Municipal Bureau of Education for Collaborative Innovation Major Project (13XT04), Guangzhou Municipal Bureau of Science and Technology Information for the Huimin Special Project (2014Y2-00182) and Guangdong Provincial Department of Science and Technology Social Development Planning Project (2014020212348).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fang, X., Liang, Y., Chen, D. et al. A study on clinicoradiological characteristics and pregnancy outcomes of reversible posterior leukoencephalopathy syndrome in preeclampsia or eclampsia. Hypertens Res 40, 982–987 (2017). https://doi.org/10.1038/hr.2017.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.76
Keywords
This article is cited by
-
Serum Neurofilament Light: a Potential Diagnostic and Prognostic Biomarker in Obstetric Posterior Reversible Encephalopathy Syndrome
Molecular Neurobiology (2021)
-
Effect of blood pressure on reversible posterior leukoencephalopathy syndrome in pre-eclampsia or eclampsia
Hypertension Research (2018)