Abstract
Background
Studies conflict on how acute versus chronic placental pathology impacts outcomes after neonatal encephalopathy from presumed hypoxic-ischemic encephalopathy (HIE). We examine how outcomes after presumed HIE vary by placental pathology categories.
Methods
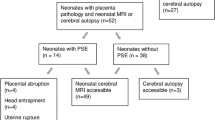
We performed retrospective chart review for neonates with presumed HIE, regardless of severity, focusing on 50 triads for whom placental specimens were available for re-review. Placentas were categorized as having only acute, any chronic, or no lesions. Primary outcomes included in-hospital morbidity/mortality and long-term neurodevelopmental symptoms. Secondary outcomes assessed neonatal MRI and EEG.
Results
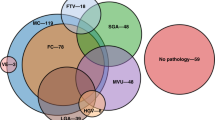
Demographics did not differ between groups. Forty-seven neonates were treated with therapeutic hypothermia. Placental acuity category was not associated with primary or secondary outcomes, but clinical and/or histopathological chorioamnionitis was associated with abnormal EEG background and post-neonatal epilepsy (16.7%, n = 3 with chorioamnionitis versus 0%, n = 0 without chorioamnionitis, p = 0.04).
Conclusions
When grouped by acute, chronic, or absent placental lesions, we observed no association with in-hospital, neurodevelopmental, MRI, or EEG outcomes. When reanalyzed by the presence of chorioamnionitis, we found that chorioamnionitis appeared to be associated with a higher risk of EEG alterations and post-neonatal epilepsy. Despite our limited sample size, our results emphasize the critical role of placental examination for neuroprognostication in presumed HIE.
Impact
-
Neonatal encephalopathy presumed to result from impaired fetal cerebral oxygenation or blood flow is called hypoxic ischemic encephalopathy (HIE).
-
Prior studies link placental pathology to various outcomes after HIE but disagree on the impact of acute versus chronic pathology.
-
Our study determines that neurodevelopmental outcomes, in-hospital outcomes, injury on MRI, and EEG findings in patients with HIE are not differentially associated with acute versus chronic placental pathology.
-
Chorioamnionitis is associated with an increased risk of abnormal EEG patterns and post-neonatal epilepsy.
-
Histopathologic chorioamnionitis without clinical symptoms is common in HIE, emphasizing the crucial role of placental pathology for neuroprognostication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Acun, C. et al. Trends of neonatal hypoxic-ischemic encephalopathy prevalence and associated risk factors in the United States, 2010 to 2018. Am. J. Obstet. Gynecol. S0002-9378(22)00443-4 (2022).
Badawi, N. et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ 317, 1554–1558 (1998).
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Parker, S. J., Kuzniewicz, M., Niki, H. & Wu, Y. W. Antenatal and intrapartum risk factors for hypoxic-ischemic encephalopathy in a Us birth cohort. J. Pediatr. 203, 163–169 (2018).
Russ, J. B., Simmons, R. & Glass, H. C. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neoreviews 22, e148–e162 (2021).
Fox, A., Doyle, E., Geary, M. & Hayes, B. Placental pathology and neonatal encephalopathy. Int. J. Gynaecol. Obstet. 160, 22–27 (2022).
Martinez-Biarge, M., Diez-Sebastian, J., Wusthoff, C. J., Mercuri, E. & Cowan, F. M. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics 132, e952–e959 (2013).
Nasiell, J., Papadogiannakis, N., Lof, E., Elofsson, F. & Hallberg, B. Hypoxic ischemic encephalopathy in newborns linked to placental and umbilical cord abnormalities. J. Matern. Fetal Neonatal. Med. 29, 721–726 (2016).
Nelson, K. B. et al. Antecedents of neonatal encephalopathy in the vermont oxford network encephalopathy registry. Pediatrics 130, 878–886 (2012).
Penn, A. A. et al. Placental contribution to neonatal encephalopathy. Semin. Fetal. Neonatal. Med. 26, 101276 (2021).
Redline, R. W. & O'Riordan, M. A. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch. Pathol. Lab. Med. 124, 1785–1791 (2000).
Roescher, A. M., Timmer, A., Erwich, J. J. & Bos, A. F. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLoS One 9, e89419 (2014).
Bingham, A., Gundogan, F., Rand, K. & Laptook, A. R. Placental findings among newborns with hypoxic ischemic encephalopathy. J. Perinatol. 39, 563–570 (2019).
Chang, T. et al. Neonatal encephalopathy, sentinel events, and the placenta. J. Neonatal-Perinat. Med. 5, 41–48 (2012).
McDonald, D. G. et al. Placental fetal thrombotic vasculopathy is associated with neonatal encephalopathy. Hum. Pathol. 35, 875–880 (2004).
Espinoza, M. L. et al. Placental pathology as a marker of brain injury in infants with hypoxic ischemic encephalopathy. Early Hum. Dev. 174, 105683 (2022).
Johnson, C. T., Burd, I., Raghunathan, R., Northington, F. J. & Graham, E. M. Perinatal inflammation/infection and its association with correction of metabolic acidosis in hypoxic-ischemic encephalopathy. J. Perinatol. 36, 448–452 (2016).
Mir, I. N. et al. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am. J. Obstet. Gynecol. 213, 849 e841–847 (2015).
Harteman, J. C. et al. Placental pathology in full-term infants with hypoxic-ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. J. Pediatr. 163, 968–995.e962 (2013).
Hellwig, L. et al. Association of perinatal sentinel events, placental pathology and cerebral mri in neonates with hypoxic-ischemic encephalopathy receiving therapeutic hypothermia. J. Perinatol. 42, 885–891 (2022).
Kovatis, K. Z. et al. Relationship between placental weight and placental pathology with MRI findings in mild to moderate hypoxic ischemic encephalopathy. Cureus 14, e24854 (2022).
Wintermark, P., Boyd, T., Gregas, M. C., Labrecque, M. & Hansen, A. Placental pathology in asphyxiated newborns meeting the criteria for therapeutic hypothermia. Am. J. Obstet. Gynecol. 203, 579.e571–579 (2010).
Wu, Y. W. et al. Placental Pathology and Neonatal Brain Mri in a Randomized Trial of Erythropoietin for Hypoxic-Ischemic Encephalopathy. Pediatr. Res. 87, 879–884 (2020).
Benz, L. D. et al. Placental findings are not associated with neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy - an 11-year single-center experience. J. Perinat. Med. 50, 343–350 (2022).
Frank, C. M. et al. Placental pathology and outcome after perinatal asphyxia and therapeutic hypothermia. J. Perinatol. 36, 977–984 (2016).
Khong, T. Y. et al. Sampling and definitions of placental lesions: amsterdam placental workshop group consensus statement. Arch. Pathol. Lab. Med. 140, 698–713 (2016).
Redline, R. W. Classification of placental lesions. Am. J. Obstet. Gynecol. 213, S21–S28 (2015).
Chalak, L. et al. Acute and chronic placental abnormalities in a multicenter cohort of newborn infants with hypoxic-ischemic encephalopathy. J. Pediatr. 237, 190–196 (2021).
Almog, B. et al. Placenta weight percentile curves for singleton and twins deliveries. Placenta 32, 58–62 (2011).
Vik, T. et al. The placenta in neonatal encephalopathy: a case-control study. J. Pediatr. 20277–85.e73 (2018).
Natarajan, G., Pappas, A. & Shankaran, S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (Hie). Semin. Perinatol. 40, 549–555 (2016).
Azzopardi, D. et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med 371, 140–149 (2014).
Jacobs, S. E. et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch. Pediatr. Adolesc. Med. 165, 692–700 (2011).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Lee, B. L. et al. Long-term cognitive outcomes in term newborns with watershed injury caused by neonatal encephalopathy. Pediatr. Res. 92, 505–512 (2022).
Lee, B. L. & Glass, H. C. Cognitive outcomes in late childhood and adolescence of neonatal hypoxic-ischemic encephalopathy. Clin. Exp. Pediatr. 64, 608–618 (2021).
Finder, M. et al. Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr. 174, 48–55 (2020).
Grossmann, K. R., Westblad, M. E., Blennow, M. & Lindstrom, K. Outcome at early school age and adolescence after hypothermia-treated hypoxic-ischemic encephalopathy: an observational, population-based study. Arch. Dis. Child Fetal Neonatal. Ed. 108, 295–301 (2023).
Scher, M. S. "The first thousand days" define a fetal/neonatal neurology program. Front. Pediatr. 9, 683138 (2021).
Scher, M. S., Trucco, G. S., Beggarly, M. E., Steppe, D. A. & Macpherson, T. A. Neonates with electrically confirmed seizures and possible placental associations. Pediatr. Neurol. 19, 37–41 (1998).
Hayes, B. C. et al. The placenta in infants >36 weeks gestation with neonatal encephalopathy: a case control study. Arch. Dis. Child Fetal Neonatal. Ed. 98, F233–F239 (2013).
Strand, K. M., Andersen, G. L., Haavaldsen, C., Vik, T. & Eskild, A. Association of placental weight with cerebral palsy: population-based cohort study in Norway. BJOG 123, 2131–2138 (2016).
Danko, I., Tanko, A., Kelemen, E. & Cserni, G. Placental pathology of preeclampsia from a clinical point of view: correlation between placental histopathology, clinical signs of preeclampsia and neonatal outcome. J. Obstet. Gynaecol. Res. 49, 1471–1480 (2023).
Helfrich, B. B. et al. Corrigendum to ‘maternal vascular malperfusion of the placental bed associated with hypertensive disorders in the boston birth cohort’ [Placenta 52 (2017) 106–113]. Placenta 86, 52–53 (2019).
Bear, J. J. & Wu, Y. W. Maternal infections during pregnancy and cerebral palsy in the child. Pediatr. Neurol. 57, 74–79 (2016).
Wu, Y. W. et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 290, 2677–2684 (2003).
Wu, Y. W. & Colford, J. M. Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 284, 1417–1424 (2000).
Liu, Z., Tang, Z., Li, J. & Yang, Y. Effects of placental inflammation on neonatal outcome in preterm infants. Pediatr. Neonatol. 55, 35–40 (2014).
Redline, R. W. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum. Pathol. 38, 1439–1446 (2007).
Giraud, A., Stephens, C. M., Boylan, G. B. & Walsh, B. H. The impact of perinatal inflammation on the electroencephalogram in preterm infants: a systematic review. Pediatr. Res. 92, 32–39 (2022).
Paz-Levy, D. et al. Inflammatory and vascular placental lesions are associated with neonatal amplitude integrated Eeg recording in early premature neonates. PLoS One 12, e0179481 (2017).
Tsamantioti, E., Lisonkova, S., Muraca, G., Ortqvist, A. K. & Razaz, N. Chorioamnionitis and risk of long-term neurodevelopmental disorders in offspring: a population-based cohort study. Am. J. Obstet. Gynecol. 227, 287 e281–287.e217 (2022).
Shellhaas, R. A. et al. Early-life epilepsy after acute symptomatic neonatal seizures: a prospective multicenter study. Epilepsia 62, 1871–1882 (2021).
Acknowledgements
The authors would like to thank the study subjects for helping to advance our understanding of this field.
Funding
No targeted funding was obtained to support this study. J.B.R. is supported by NINDS grant 5K12NS098482-05 and a Duke University School of Medicine Strong Start Award.
Author information
Authors and Affiliations
Contributions
A.C.S.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published. K.C.S.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published. D.T.T.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published. J.B.G.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published. M.E.L.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published. J.B.R.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
As determined by the Duke University Institutional Review Board, patient consent was not required for a retrospective chart review study utilizing deidentified patient data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stone, A.C., Strickland, K.C., Tanaka, D.T. et al. The association of placental pathology and neurodevelopmental outcomes in patients with neonatal encephalopathy. Pediatr Res 94, 1696–1706 (2023). https://doi.org/10.1038/s41390-023-02737-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02737-5