Abstract
Obesity, left ventricular hypertrophy (LVH) and peripheral artery disease are frequently noted in patients with chronic kidney disease (CKD), thereby suggesting a close and causal relationship among them. This study was designed to assess whether the combination of an increased body mass index (BMI) and left ventricular mass index (LVMI) are independently associated with abnormally low and high ABI in patients with CKD stages 3–5. A total of 566 patients were included in the study and were classified into four groups according to sex-specific median BMIs and LVMIs. The ABI was measured using an ABI-form device. Abnormally low and high ABI was defined as ABI <0.9 or ⩾1.3 in either leg. The combination of high BMI and LVMI (vs. the combination of low BMI and LVMI) was significantly associated with abnormally low and high ABI in an unadjusted model (odds ratio (OR), 2.107; P=0.015) and in a multivariable model after adjustment for demographic, clinical and biochemical characteristics and medications (OR, 4.219; P=0.008). In addition, the interaction between BMI and LVMI in abnormally low and high ABI was statistically significant in the unadjusted (OR, 1.002; P=0.001) and multivariate models (OR, 1.004; P=0.003). Our findings show that the combination of high BMI and LVMI was associated with abnormally low and high ABI in patients with CKD stages 3–5. Patients with high BMI and LVMI might be at a high risk of abnormally low and high ABI.
Similar content being viewed by others
Introduction
The ankle-brachial index (ABI) is reported to be an accurate marker of atherosclerosis, and an ABI<0.9 is useful in the diagnosis of peripheral artery occlusive disease.1, 2, 3 In addition, an ABI ⩾1.3 is considered to indicate medial artery calcification.4 A high prevalence of peripheral artery occlusive disease and increased medial artery calcification are frequently noted in patients with chronic kidney disease (CKD).5, 6 Moreover, an abnormally low and high ABI is associated with high cardiovascular morbidity and mortality in patients with CKD.7, 8 Therefore, identifying patients with abnormally low and high ABI for aggressive treatment interventions is essential.
Obesity, LVH and peripheral artery disease are highly prevalent in CKD patients.7, 9, 10 Previous studies have reported that a high body mass index (BMI) is associated with either an abnormally low or high ABI.11, 12 In addition, recent studies have reported that an increased left ventricular mass was independently associated with an abnormally low or high ABI in various populations.13, 14, 15 Hence, there may be a close and causal relationship between obesity, left ventricular hypertrophy (LVH) and abnormally low and high ABI in patients with CKD. However, no studies have evaluated the combined effects of the BMI, left ventricular mass index (LVMI) and abnormally low and high ABI in patients with CKD. We hypothesize that obesity and LVH might have a synergetic effect on abnormally low and high ABI. The aim of this study was to assess the association of BMI and LVMI with abnormally low and high ABI in patients with CKD stages 3–5.
Patients and methods
Study patients and design
This study was conducted in a regional hospital in Southern Taiwan. The study consecutively recruited 627 predialysis patients with CKD stages 3–5 according to the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines,16 from January 2007 to October 2009. The study classified patients with evidence of kidney damage lasting longer than 3 months into CKD stages 3, 4 and 5, based on estimated glomerular filtration rates (eGFRs) of 30–59, 15–29 and <15 ml per min per 1.73 m2, respectively. The study patients were followed-up regularly at the Outpatient Department of Internal Medicine. They were selected to participate in this study if they agreed to receive echocardiographic examination. Patients with significant aortic or mitral valve disease (n=25), atrial fibrillation (n=20) or inadequate image visualization (n=16) were excluded. We did not include patients consecutively because ABI must be measured within 10 min of completing an echocardiograph. A total of 566 patients (mean age 65.8±12.7 years, 310 men and 256 women) were included. All the patients were in sinus rhythm. The protocol was approved by our institutional review board and all the enrolled patients submitted written, informed consent.
Evaluation of cardiac structure and function
The echocardiographic examination was performed by an experienced cardiologist using a VIVID 7 (General Electric Medical Systems, Horten, Norway), with the participant respiring quietly in the left decubitus position. The cardiologist was blind to the other data. Two-dimensional and two-dimensionally guided M-mode images were recorded in the standardized views. The echocardiographic measurements included the left ventricular internal diameter in diastole (LVIDd), left ventricular posterior wall thickness in diastole (LVPWTd), interventricular septal wall thickness in diastole (IVSTd), E-wave deceleration time, transmitral E-wave velocity and transmitral A-wave velocity. The left ventricular ejection fraction was measured using the modified Simpson’s method. The left ventricular relative wall thickness (LVRWT) was calculated as the ratio of 2 × LVPWTd/LVIDd. The left ventricular mass was calculated using the Devereux-modified method (that is, left ventricular mass=1.04 × [(IVSTd+LVIDd+LVPWTd)3 – LVIDd3] – 13.6 g).17 The LVMI was calculated by dividing the left ventricular mass by the body surface area.
Assessment of ABI and definition of abnormally low and high ABI
The ABIs were measured using an ABI-form device (VP1000; Colin Co. Ltd, Komaki, Japan), which automatically and simultaneously measured blood pressure at the four extremities through an oscillometric method.18, 19, 20 The ABI was calculated as the ratio of the ankle systolic blood pressure divided by the arm systolic blood pressure. The ABI measurement was performed once for each patient. Abnormally low and high ABI was defined as ABI<0.9 or ⩾1.3 in either leg.
Collection of demographic, medical and laboratory data
Demographic and medical data, including age, sex, smoking history (ever vs. never) and comorbid conditions, were gathered from medical records or patient interviews. The BMI was calculated as the ratio of the weight in kg divided by the square of the height in m. Blood samples were obtained within 1 month of enrollment. Laboratory data were measured from fasting blood samples by using an autoanalyzer (D-68298 Mannheim COBAS Integra 400, Roche Diagnostics GmbH, Mannheim, Germany). Serum creatinine was measured using the compensated Jaffé (kinetic alkaline picrate) method in a Roche/Integra 400 Analyzer (Roche Diagnostics GmbH, Mannheim, Germany) with a traceable calibrator to perform isotope-dilution mass spectrometry.21 The eGFR was calculated using the four-variable equation in the Modification of Diet in Renal Disease study.22 In addition, data related to antihypertensive medications, including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), β-blockers, calcium channel blockers and diuretics use during the study period, were obtained from medical records.
Statistical analysis
Statistical analysis was performed using SPSS 15.0 for Windows (SPSS Inc. Chicago, IL, USA). Data were expressed as percentages, mean±s.d., or median (25th–75th percentile) for triglyceride. Multiple comparisons among the study groups were performed using one-way analysis of variance followed by a Boneferroni-adjusted post hoc test. Multiple logistic regression analysis was used to identify the factors associated with abnormally low and high ABI. The association between the study groups and abnormally low and high ABI was assessed using a modified stepwise procedure in a two-step model. The first model consisted of age, diabetes mellitus, coronary artery disease, systolic blood pressure, and pulse pressure. The second model consisted of biomarkers and medications including albumin, fasting glucose, log triglyceride, total cholesterol, hematocrit, eGFR, ACEIs and/or ARBs, β-blockers, calcium channel blockers and diuretics. A group of patients with low BMI and LVMI was treated as the reference group, which was at the lowest risk of abnormally low and high ABI. A difference was considered significant at P<0.05.
Results
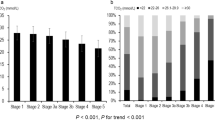
A total of 566 nondialyzed CKD patients were included. The mean age was 65.8±12.7 years with 310 men and 256 women. The patients were stratified into four groups according to sex-specific median BMIs (men: 25.4; women: 25.7 kg m−2) and LVMIs (men: 140.2; women: 134.3 g m−2). A comparison of the clinical characteristics among these study groups is shown in Table 1. The BMIs for the four groups were 22.9±1.9, 28.5±2.5, 22.5±2.0 and 29.7±3.3 kg m−2. In addition, the LVMIs for the four groups were 106.7±22.1, 111.3±18.7, 183.4±41.9 and 182.0±38.5 g m−2. The prevalences of abnormally low and high ABI for the four groups were 14.0, 15.1, 24.5 and 25.5% (P for trend: 0.020).
Relationship of study groups to abnormally low and high ABI
Table 2 lists the odds ratio (OR) estimates for abnormally low and high ABI with and without adjustment for demographic, clinical and biochemical characteristics and medications. The combination of high BMI and LVMI (vs. the combination of low BMI and LVMI) was significantly associated with abnormally low and high ABI in the unadjusted model (OR, 2.107; 95% confidence interval (CI), 1.154–3.848; P=0.015) and in the multivariable model after adjustment for age, diabetes mellitus, coronary artery disease, systolic blood pressure and pulse pressure (hazard ratio, 1.941; 95% CI, 1.032–3.652; P=0.040). This relationship remained significant after further adjustment for biomarkers, including albumin, fasting glucose, log triglyceride, total cholesterol, hematocrit and eGFR, and medications, including ACEIs and/or ARBs, β-blockers, calcium channel blockers and diuretics (OR, 4.219; 95% CI, 1.449–12.285; P=0.008).
The interaction between BMI and LVMI (per 10 kg m−2 × g m−2) in abnormally low and high ABI was statistically significant in the unadjusted model (OR, 1.002; 95% CI, 1.001–1.004; P=0.001) and multivariable model after adjustment for age, comorbidities, systolic blood pressure and pulse pressure (OR, 1.003; 95% CI, 1.001–1.004; P=0.001) and after further adjustment for biomarkers and medications (OR, 1.004; 95% CI, 1.00–1.007; P=0.003).
Discussion
A high prevalence of peripheral artery disease has been frequently noted in patients with CKD.9 In the present study, we evaluated the association of BMI and LVMI with abnormally low and high ABI in patients with CKD stages 3–5. Compared with the combination of low BMI and LVMI, the combination of high BMI and LVMI was associated with abnormally low and high ABI. In addition, the association between BMI and LVMI in abnormally low and high ABI was statistically significant, thus suggesting that obesity and LVH might have a synergetic effect on abnormally low and high ABI.
One critical finding of our study was that when comparing the two groups with high LVMI, only the group with high BMI was associated with abnormally low and high ABI (vs. low BMI and LVMI). Niu et al.12 investigated the risk factors for abnormal ABI in 799 residents without a history of cardiovascular disease and found that the BMI was higher in the abnormally low (<1.0) and the high (>1.3) ABI group than in the normal ABI group. Moreover, Doza et al.11 observed a high BMI in men with type 2 diabetes mellitus and abnormally low (<1.0) and high (>1.2) ABIs. In addition, Zhang et al.23 evaluated the relationship between ABI and metabolic syndrome in patients without CKD and determined that the BMI was higher in the high ABI group than in the normal ABI group. A high BMI indicated an increase in adipose tissue. In obesity, adipose tissue is a major endocrine and paracrine organ producing numerous proteins such as adiponectin, leptin, resistin, tumor necrosis factor-alpha, interleukin-6, plasminogen activator inhibitor-1, atrial natriuretic peptide and angiotensinogen, which were collectively termed adipokines.24, 25 Several experimental and clinical studies have demonstrated that adipokines directly affected endothelial function, vascular homeostasis and atherogenesis and indirectly affected atherosclerosis by modulating insulin resistance and metabolism of fats and glucose.24, 25, 26, 27 Furthermore, an exponentially raised BMI increased the risk of elevated blood pressure, elevated triglyceride and cholesterol, insulin resistance, metabolic syndrome and atherosclerosis.28, 29 Our study consistently demonstrated that high BMI was associated with an abnormally low and high ABI in CKD patients. However, BMI is limited to differentiate the fat, lean mass or fluid. BMI may be misleading in the presence of edema, which occurs commonly in patients with advanced CKD. Therefore, body composition analysis using dual-energy X-ray absorption or body composition monitor is of importance to elucidate this issue in the future studies.
The prevalence of abnormally low and high ABI was comparable between the group with lower BMI and higher LVMI (24.5%) and the group with higher BMI and LVMI (25.5%). It seemed like that LVH had an important role in the association with abnormal ABI. Furthermore, further analysis showed that only the group with high BMI and LVMI was associated with abnormally low and high ABI, suggesting that adding information of BMI to LVMI may provide additional benefit for risk stratification of abnormal ABI. LVH has been reported to be affected by or linked to various risk factors for an abnormally low and high ABI, such as arterial stiffness, hypertension and coexisting atherosclerosis.30, 31 Previous studies have demonstrated that the ABI in the LVH group was significantly lower than that in the non-LVH group.32, 33 In addition, Ix et al.14 evaluated the association between an abnormally low or high ABI and LVMI in 4972 MESA (multiethnic study of atherosclerosis) participants without clinical cardiovascular disease. The results reveal that either an abnormally low or high ABI was significantly associated with increased LVMI. These results suggest that a low and high ABI might be related to LVH. Atherosclerosis directly caused a decrease in blood perfusion in the lower extremities and an increase in arterial wall stiffness, contributing to arterial distensibility, which progressed to LVH.6, 34, 35 By contrast, LVH caused left ventricular systolic and diastolic dysfunction and a decrease in cardiac output, which deteriorated the blood perfusion of the extremities and promoted the progression of peripheral artery disease.
Obesity has reached epidemic proportions in the developed world36 and has increased the risk of atherosclerotic disease, heart disease, and CKD.10, 24, 25, 37 LVH is a frequent complication in obese individuals. Rider et al. had reported the positive association between BMI and LVH detected using echocardiography.38 The biological mechanisms linking the hemodynamic overload with the obesity-related myocardial geometric abnormalities are complex and not entirely clarified. Hemodynamic of obesity is characterized by a predominant volume overload and pressure overload, and the increased salt intake associated with food overload, causing increased water retention.39, 40, 41 Furthermore, human adipocytes also produce potent mineralocorticoid releasing factors,42 which are likely associated with development of arterial hypertension and with enhanced profibrotic activity.43, 44 In addition, visceral adiposity also generates insulin resistance45 and stimulates sympathetic drive,46 other potent stimuli for increasing left ventricular mass.47 These above factors further contributed to atherosclerosis.26, 27, 43, 45 In our study, the group with high BMI and LVMI showed an increased prevalence of risk factors for atherosclerosis, such as high prevalence of hypertension, high systolic blood pressure and pulse pressure and a low eGFR. Even after adjustment for these confounding factors, the group with high BMI and LVMI was still associated with abnormally low and high ABI. Obesity is affected by or associated with various risk factors for LVH, such as insulin resistance, metabolic syndrome and hypertension.28, 38, 48, 49 Hence, obesity and LVH might have a synergetic effect on abnormally low and high ABI.
There were several limitations to this study. First, this study was cross-sectional, so the causal relationship and long-term clinical outcomes could not be confirmed. Future prospective studies are needed to address the issues. Second, increasing studies have suggested that albuminuria might be an important risk factor for peripheral artery disease.50 However, the quantitative assessment of albuminuria was not performed in this study, so the impact of albuminuria could not be assessed. Finally, as the subjects of this study were already being evaluated for heart disease by echocardiography, it was susceptible to selection bias, making findings potentially less generalized.
In conclusion, the coexistence of high BMI and LVMI was independently associated with an abnormally low and high ABI in patients with CKD stages 3–5. Patients with increased BMI and LVMI might be at a high risk of abnormally low and high ABI.
References
Fishbane S, Youn S, Flaster E, Adam G, Maesaka JK . Ankle-arm blood pressure index as a predictor of mortality in hemodialysis patients. Am J Kidney Dis 1996; 27: 668–672.
Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ . Edinburgh artery study: Prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991; 20: 384–392.
Newman AB, Tyrrell KS, Kuller LH . Mortality over four years in shep participants with a low ankle-arm index. J Am Geriatr Soc 1997; 45: 1472–1478.
Orchard TJ, Strandness DE Jr . Assessment of peripheral vascular disease in diabetes. Report and recommendations of an international workshop sponsored by the american heart association and the american diabetes association 18-20 september 1992, new orleans, louisiana. Diabetes Care 1993; 16: 1199–1209.
de Vinuesa SG, Ortega M, Martinez P, Goicoechea M, Campdera FG, Luno J . Subclinical peripheral arterial disease in patients with chronic kidney disease: prevalence and related risk factors. Kidney Int Suppl 2005; 93: S44–S47.
Leskinen Y, Salenius JP, Lehtimaki T, Huhtala H, Saha H . The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: Requirements for diagnostics. Am J Kidney Dis 2002; 40: 472–479.
Guerrero A, Montes R, Munoz-Terol J, Gil-Peralta A, Toro J, Naranjo M, Gonzalez-Perez P, Martin-Herrera C, Ruiz-Fernandez A . Peripheral arterial disease in patients with stages IV and V chronic renal failure. Nephrol Dial Transplant 2006; 21: 3525–3531.
Ono K, Tsuchida A, Kawai H, Matsuo H, Wakamatsu R, Maezawa A, Yano S, Kawada T, Nojima Y . Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol 2003; 14: 1591–1598.
Cioffi G, Tarantini L, Frizzi R, Stefenelli C, Russo TE, Selmi A, Toller C, Furlanello F, de Simone G . Chronic kidney disease elicits excessive increase in left ventricular mass growth in patients at increased risk for cardiovascular events. J Hypertens 2011; 29: 565–573.
Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS . Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144: 21–28.
Doza B, Kaur M, Chopra S, Kapoor R . Cardiovascular risk factors and distributions of the ankle-brachial index among type 2 diabetes mellitus patients. Int J Hypertens 2012; 2012: 485812.
Niu F, Zhang L, Wang X, Liu L, Wang H . The association between changes in urinary albumin-to-creatinine ratio and risk of abnormal ankle-brachial index in a community-based chinese population. J Atheroscler Thromb 2012; 19: 912–917.
Albuquerque PF, Albuquerque PH, Albuquerque GO, Servantes DM, Carvalho SM, Oliveira Filho JA . Ankle-brachial index and ventricular hypertrophy in arterial hypertension. Arq Bras Cardiol 2012; 98: 84–86.
Ix JH, Katz R, Peralta CA, de Boer IH, Allison MA, Bluemke DA, Siscovick DS, Lima JA, Criqui MH . A high ankle brachial index is associated with greater left ventricular mass mesa (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2010; 55: 342–349.
Su HM, Lin TH, Hsu PC, Chu CY, Lee WH, Chen SC, Lee CS, Voon WC, Lai WT, Sheu SH . Abnormally low and high ankle-brachial indices are independently associated with increased left ventricular mass index in chronic kidney disease. PLoS ONE 2012; 7: e44732.
Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, Johnson CA, Kausz A, Kimmel PL, Kusek J, Levin A, Minaker KL, Nelson R, Rennke H, Stettes M, Witten B . K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–266.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N . Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol 1986; 57: 450–458.
Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S . Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis 2003; 166: 303–309.
Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y . Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25: 359–364.
Yokoyama H, Shoji T, Kimoto E, Shinohara K, Tanaka S, Koyama H, Emoto M, Nishizawa Y . Pulse wave velocity in lower-limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb 2003; 10: 253–258.
Vickery S, Stevens PE, Dalton RN, van Lente F, Lamb EJ . Does the id-ms traceable mdrd equation work and is it suitable for use with compensated jaffe and enzymatic creatinine assays? Nephrol Dial Transplant 2006; 21: 2439–2445.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D . A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 1999; 130: 461–470.
Zhang Y, Chen J, Zhang K, Kong M, Wang T, Chen R, Wang L, Wang J, Huang H . Inflammation and oxidative stress are associated with the prevalence of high aankle-brachial index in metabolic syndrome patients without chronic renal failure. Int J Med Sci 2013; 10: 183–190.
Kershaw EE, Flier JS . Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548–2556.
Garruti G, Giusti V, Nussberger J, Darimont C, Verdumo C, Amstutz C, Puglisi F, Giorgino F, Giorgino R, Cotecchia S . Expression and secretion of the atrial natriuretic peptide in human adipose tissue and preadipocytes. Obesity (Silver Spring, Md) 2007; 15: 2181–2189.
Beddhu S, Pappas LM, Ramkumar N, Samore MH . Malnutrition and atherosclerosis in dialysis patients. J Am Soc Nephrol 2004; 15: 733–742.
Yamauchi T, Kuno T, Takada H, Nagura Y, Kanmatsuse K, Takahashi S . The impact of visceral fat on multiple risk factors and carotid atherosclerosis in chronic haemodialysis patients. Nephrol Dial Transplant 2003; 18: 1842–1847.
Kwan BC, Murtaugh MA, Beddhu S . Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol 2007; 2: 992–998.
Obesity: preventing and managing the global epidemic. Report of a who consultation. World Health Organ Tech Rep Ser 2000; 894: i-xii, 1–253.
Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH . The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg 2008; 48: 1197–1203.
Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J . Calcification of medial elastic fibers and aortic elasticity. Hypertension 1997; 29: 999–1006.
Devereux RB, Reichek N . Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977; 55: 613–618.
Fu W, Ye C, Mei C, Rong S, Wang W . Reverse correlation between ankle-brachial index and left ventricular hypertrophy in patients on maintenance haemodialysis. Nephrology (Carlton) 2006; 11: 9–14.
Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB . Impact of arterial stiffening on left ventricular structure. Hypertension 2000; 36: 489–494.
Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB . Association of carotid atherosclerosis and left ventricular hypertrophy. J Am Coll Cardiol 1995; 25: 83–90.
Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL . Increasing prevalence of overweight among us adults. The national health and nutrition examination surveys, 1960 to 1991. JAMA 1994; 272: 205–211.
Byers T . Body weight and mortality. N Engl J Med 1995; 333: 723–724.
Rider OJ, Lewandowski A, Nethononda R, Petersen SE, Francis JM, Pitcher A, Holloway CJ, Dass S, Banerjee R, Byrne JP, Leeson P, Neubauer S . Gender-specific differences in left ventricular remodelling in obesity: Insights from cardiovascular magnetic resonance imaging. Eur Heart J 2013; 34: 292–299.
Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, Devereux RB . Impact of obesity on cardiac geometry and function in a population of adolescents: the strong heart study. J Am Coll Cardiol 2006; 47: 2267–2273.
Woodruff SJ, Fryer K, Campbell T, Cole M . Associations among blood pressure, salt consumption and body weight status of students from south-western Ontario. Public Health Nutr 2014; 17: 1114–1119.
Kawamoto R, Kohara K, Katoh T, Kusunoki T, Ohtsuka N, Abe M, Kumagi T, Miki T . Effect of weight loss on central systolic blood pressure in elderly community-dwelling persons. Hypertens Res 2014; 37: 933–938.
Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR . Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA 2003; 100: 14211–14216.
Schrier RW, Masoumi A, Elhassan E . Aldosterone: role in edematous disorders, hypertension, chronic renal failure, and metabolic syndrome. Clin J Am Soc Nephrol 2010; 5: 1132–1140.
Fujii M, Ohnishi H, Saitoh S, Akasaka H, Miura T, Mori M . The combination of abdominal obesity and high-sensitivity C-reactive protein predicts new-onset hypertension in the general Japanese population: the Tanno-Sobetsu study. Hypertens Res 2015; 38: 426–432.
Bjorntorp P . Metabolic implications of body fat distribution. Diabetes Care 1991; 14: 1132–1143.
Tentolouris N, Liatis S, Katsilambros N . Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci 2006; 1083: 129–152.
Ferrara LA, Mancini M, de Simone G, Pisanti N, Capone D, Fasano ML, Mancini M . Adrenergic nervous system and left ventricular mass in primary hypertension. Eur Heart J 1989; 10: 1036e40.
Maggio AB, Aggoun Y, Marchand LM, Martin XE, Herrmann F, Beghetti M, Farpour-Lambert NJ . Associations among obesity, blood pressure, and left ventricular mass. J Pediatr 2008; 152: 489–493.
Dobrowolski P, Prejbisz A, Klisiewicz A, Florczak E, Rybicka J, Januszewicz A, Hoffman P . Determinants of concentric left ventricular hypertrophy in patients with resistant hypertension: RESIST-POL study. Hypertens Res 2015; 38: 545–550.
Cao JJ, Barzilay JI, Peterson D, Manolio TA, Psaty BM, Kuller L, Wexler J, Bleyer AJ, Cushman M . The association of microalbuminuria with clinical cardiovascular disease and subclinical atherosclerosis in the elderly: the Cardiovascular Health Study. Atherosclerosis 2006; 187: 372–377.
Acknowledgements
The research presented in this article is supported by the grant from Kaohsiung Municipal Hsiao-Kang Hospital (kmhk-103-002), Kaohsiung Medical University, Kaohsiung, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, SC., Lee, WH., Hsu, PC. et al. Association of body mass index and left ventricular mass index with abnormally low and high ankle-brachial indices in chronic kidney disease. Hypertens Res 39, 166–170 (2016). https://doi.org/10.1038/hr.2015.128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.128
Keywords
This article is cited by
-
Clinical significance of an elevated ankle-brachial index differs depending on the amount of appendicular muscle mass: the J-SHIPP and Nagahama studies
Hypertension Research (2018)
-
The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins
Hypertension Research (2017)
-
Association of Ankle-Brachial Index and Aortic Arch Calcification with Overall and Cardiovascular Mortality in Hemodialysis
Scientific Reports (2016)