Abstract
Chronic pulmonary obstructive disease (COPD) is the fourth leading cause of death worldwide, however, the pathogenic factors and mechanisms are not fully understood. Pulmonary emphysema is one of the major components of COPD and is thought to result from oxidative stress, chronic inflammation, protease–antiprotease imbalance and lung epithelial (LE) cell apoptosis. In our previous studies, COPD patients were noted to have higher levels of placenta growth factor (PlGF) in serum and bronchoalveolar lavage fluid than controls. In addition, transgenic mice overexpressing PlGF developed pulmonary emphysema and exposure to PlGF in LE cells induced apoptosis. Furthermore, intratracheal instillation of porcine pancreatic elastase (PPE) on to PlGF wild type mice induced emphysema, but not in PlGF knockout mice. Therefore, we hypothesized that PPE generates pulmonary emphysema through the upregulation of PlGF expression in LE cells. The elevation of PlGF then leads to LE cell apoptosis. In the present study, we investigated whether PPE induces PlGF expression, whether PlGF induces apoptosis and whether the downstream mechanisms of PlGF are related to LE cell apoptosis. We found that PPE increased PlGF secretion and expression both in vivo and in vitro. Moreover, PlGF-induced LE cell apoptosis and PPE-induced emphysema in the mice were mediated by c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) pathways. Given these findings, we suggest that the increase in PlGF and PlGF-induced JNK and p38 MAPK pathways contribute to PPE-induced LE cell apoptosis and emphysema. Regulatory control of PlGF and agents against its downstream signals may be potential therapeutic targets for COPD.
Similar content being viewed by others
Main
Chronic pulmonary obstructive disease (COPD) is the fourth leading cause of death worldwide with high mortality and morbidity rates even under advanced medical care.1 Emphysema is a major component of COPD and is characterized by destruction and enlargement of the alveolar region through chronic inflammation, oxidative stress and protease–antiprotease imbalance.2, 3 However, the factors and pathogenic mechanisms that cause COPD are not fully understood.4 Apoptosis of lung epithelial (LE) cells is one of the potential factors involved in the pathogenesis of COPD.5 Tsao et al.6 demonstrated that exogenous placenta growth factor (PlGF) induced LE cell apoptosis and inhibited cellular proliferation, and that the transgenic mice with over-expressed PlGF developed emphysema phenotype, suggesting that PlGF has an important role in the pathogenesis of emphysema.
PlGF belongs to a family of vascular endothelial growth factors (VEGF) and the major biological function of PlGF is promoting angiogenesis through VEGF receptor-1 (VEGFR-1) signaling.7, 8 PlGF expresses in the placenta, heart, lung, thyroid, brain and skeletal muscles.9 Increasing evidence suggest that PlGF expressed in LE cells may contribute to the progression of COPD. Cheng et al.10 reported that patients with COPD had higher levels of PlGF in serum and bronchoalveolar lavage (BAL) fluid, and that the level of PlGF was inversely related to lung function. Moreover, cigarette smoke, a major factor related to COPD, has been found to increase PlGF expression in rat lungs.11 Furthermore, ablation of PlGF has been found to protect mice from porcine pancreatic elastase (PPE)-induced emphysema.12 However, the detailed mechanisms underlining PlGF-induced LE cell apoptosis and the pathogenesis of emphysema remain to be elucidated.
PPE is a protease that can induce protease–antiprotease imbalance and has been applied in an animal model of emphysema and COPD.13 Previous studies have demonstrated that leukocyte elastase increases lung and intestinal epithelial cells apoptosis.14, 15 Neutrophil elastase (NE) has been shown to increase the expression and secretion of elafin in type II alveolar epithelial cells.16 Moreover, NE has also been shown to increase the expression of MUC4 and MUC5AC17, 18 and promote the secretion of mucin and expression of interleukin-8 (IL-8) in human bronchial epithelial cells.19, 20 Other than LE cells, NE has also been shown to upregulate cathepsin B and matrix matelloprotease-2 (MMP-2) in macrophages.21 To date, the mechanisms by which elastase induces LE cell apoptosis have not been established. In this study, we hypothesized that PPE generates LE cell apoptosis and pulmonary emphysema through the expression of PlGF and PlGF-mediated downstream signaling.
We found that PPE increased PlGF mRNA and protein expressions in vivo and in vitro. PPE stimulates PlGF promoter activity by early growth response-1 (Egr-1) and Egr-1 response element. Moreover, PlGF induced LE cell apoptosis in a dose-dependent manner, and chronic treatment of PlGF increased LE cell apoptosis. Furthermore, PlGF activated c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways, both of which were involved in PlGF-induced LE cell apoptosis and PPE-induced pulmonary emphysema in mice. These novel findings may provide possible therapeutic strategies for emphysema and COPD.
Results
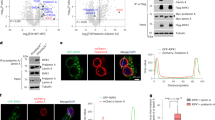
PPE treatment increased PlGF expression and secretion in LE cells
Previous study indicated 100 mU/ml NE increases elafin gene expression in A549 cells16 and PlGF is majorly secreted by alveolar type II epithelial cells.6, 22 Therefore, the mouse LE cells, mouse alveolar type II epithelial cells (MLE-15), were treated with 0–60 mU/ml PPE for 24 h to test whether PPE could induce PlGF expression. The secretion of PlGF into the medium was promoted by 60 mU/ml PPE treatment (Figure 1a). In MLE-15 cells, the mouse PlGF promoter region (2.5 kb)-driven luciferase activity indicated that 60 mU/ml PPE increased the mouse PlGF promoter activity by about threefold (Figure 1b). The results of reverse transcriptional (RT)-PCR and western blot demonstrated that 60 mU/ml PPE upregulated the expressions of PlGF mRNA and protein in the MLE-15 cells (Figures 1c and d). To study the transcriptional regulatory mechanism of PPE on PlGF expression, we analyzed the sequence of human PlGF promoter region and identified a speculated Egr-1 response element (CCCGCCCC) within 100 bp from transcriptional start site.23, 24 Western blot demonstrated that 60 mU/ml PPE transiently increased the expressions of Egr-1 in MLE-15 cells (Figure 1e). The data of chromatin immunoprecipitation assay showed that the exposure to 60 mU/ml PPE for 1 h would promote the association of Egr-1 protein and PlGF promoter region (Figure 1f). These findings confirmed that PPE increased PlGF expression and secretion in mouse LE cells.
Treatment of porcine pancreatic elastase (PPE) increased the expression and secretion of placenta growth factor (PlGF) in lung epithelial (LE) cells. Mouse alveolar type II epithelial cells, MLE-15, were washed twice with PBS then incubated in the medium without serum and treated with 0–60 mU/ml PPE for 24 h (a–d), 4 h (e) or 1 h (f). The level of PlGF in the culture medium was detected by ELISA (a). The activity of PlGF promoter in the cellular lysate was evaluated by luciferase activity (b). The mRNA level of PlGF was determined by reverse transcriptional PCR with primer sets for PlGF and GAPDH cDNA (c). Cellular lysates were also subjected to western blot analysis with antibodies for PlGF (d) or Egr-1 (e) and β-actin. The association of Egr-1 and PlGF promoter was evaluated by chromatin immunoprecipitation assay (f). Data are presented as mean±S.E.M. *P<0.05 versus vehicle-treated group
Exogenous PlGF induced LE cell apoptosis
Previous study indicated that 100 ng/ml PlGF induced MLE-15 cell apoptosis.6 In this study, MLE-15 cells were treated with 0–100 ng/ml mouse recombinant PlGF for 24 h. Exogenous PlGF dose-dependently increased the level of apoptosis in the MLE-15 cells according to the results of the caspase-3 activity assay (Figure 2a), trypan blue inclusion analysis (Figure 2b), flow cytometric assay (Figure 2c) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Figure 2d). Because the level of cells with trypan blue stain is similar to FITC-positive cell and a few annexin V-positive cells in cytomertic assay, PlGF majorly induced apoptotic cellular death rather than necrotic cellular death in LE cells. In addition, S cells were treated with 0–100 ng/ml human recombinant PlGF for 24 h. The results of the caspase-3 activity assay demonstrated that PlGF also increased human LE cell apoptosis in a dose-dependent manner (Figure 2e). Our previous study indicated co-treatment of 50 pg/ml PlGF, 200 pg/ml interleukin-8 (IL-8) and 200 pg/ml tumor necrosis factor-α (TNFα) for 14 days induced S-cell apoptosis.10 To mimic the chronic damage conditions in COPD, the S cells were treated with 800 pg/ml human recombinant PlGF for 14 days. The dose of PPE was dependent on PPE-induced PlGF secretion in S cells (data not shown). Chronic treatment with 800 pg/ml PlGF resulted in a similar apoptotic level as Figure 2e (Figure 2f). Taken together, these results confirmed that both 24 h and 14 days of treatment of PlGF increased LE cell apoptosis.
Exogenous PlGF induced LE cell apoptosis. MLE-15 cells were treated with 0–100 ng/ml recombinant mouse PlGF for 24 h (a–d). S cells were treated with 0–100 ng/ml (e) or 800 pg/ml (f) recombinant human PlGF for 24 h (e) or 14 days (f). The PlGF-induced apoptosis was evaluated by relative signals in a caspase-3 activity assay that are presented as relative fluorescence units (RFU)/min per mg protein (a, e and f), blue-stained cells in trypan blue inclusion assay (b), annexin V–FITC-stained cells in flow cytometric assay (c) and fluorescent cells in terminal deoxynucleotidyl transferase dUTP nick end label (TUNEL) assay (d). Scale bar=100 μm. Data are presented as mean±S.E.M. *P<0.05 versus vehicle-treated group
Exogenous PlGF activated JNK and p38 MAPK pathways in LE cells
To elucidate the detailed mechanism involved in PlGF-induced LE cell apoptosis, we treated MLE-15 cells with 100 ng/ml PlGF for 0–24 h. According to the results of western blot, PlGF induced prolonged and enhanced phosphorylation of JNK and p38 MAPK after 6 h of treatment (Figures 3a and b). But PlGF had no significant effect on other signaling pathways like Akt and NFκB (Supplementary Figure 1S). The 14-day treatment of S cells with 800 pg/ml of PlGF also increased the activation of JNK and p38 MAPK (Figures 3c and d). These results suggest that the JNK and p38 MAPK pathways may have crucial roles in PlGF-induced mouse and human LE cell apoptosis.
Exogenous PlGF activated c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) in LE cells. MLE-15 cells were treated with 100 ng/ml recombinant mouse PlGF for 0–24 h (a and b). S cells were treated with 800 pg/ml recombinant human PlGF for 14 days (c and d). Cellular lysates were subjected to western blot analysis with antibodies for phosphorylated JNK (p-JNK), phosphorylated p38 MAPK (p-p38 MAPK), JNK and p38 MAPK (a and c). The quantitative data are indicated in (b) and (d). Data are presented as mean±S.E.M. *P<0.05 versus vehicle-treated group
Inhibition of the JNK and p38 MAPK pathways abrogated PlGF-induced LE cell apoptosis
To evaluate the roles of JNK and p38 MAPK in PlGF-induced LE cell apoptosis, MLE-15 and S cells were pretreated with inhibitors of JNK and p38 MAPK, SP 600125 and SB 203580, respectively, to block the JNK and p38 MAPK pathways. According to the results of the caspase-3 activity assay (Figure 4a), trypan blue inclusion analysis (Figure 4b), flow cytometric assay (Figure 4c) and TUNEL assay (Figure 4d), blockage of the JNK and p38 MAPK pathways caused attenuation of apoptosis in the PlGF-treated MLE-15 cells (100 ng/ml for 24 h). In addition, inhibition of the JNK and p38 MAPK signaling decreased the level of apoptosis in PlGF-treated MLE-15 (100 ng/ml for 24 h) and S cells (800 pg/ml for 14 days) as shown in caspase-3 activity assay (Figures 4e and f). These results strongly confirmed that JNK and p38 MAPK signaling are involved in PlGF-stimulated mouse and human LE cell apoptosis.
Inhibition of the JNK and p38 MAPK pathways abrogated PlGF-induced apoptosis in LE cells. MLE-15 (a–d) or S cells (e) were pretreated with SP 600125 and SB 203580 (JNK and p38 MAPK inhibitors) for 2 h, and treated with or without 100 ng/ml recombinant mouse (a–d) or human (e) PlGF for 24 h. S cells were treated with 800 pg/ml human PlGF, SB 203580 and SP 600125 daily for 14 days (f). The PlGF-induced apoptosis was evaluated by relative signals in a caspase-3 activity assay that are presented as RFU/min per mg protein (a, e and f), blue-stained cells in trypan blue inclusion assay (b), annexin V–FITC-stained cells in flow cytometric assay (c) and fluorescent cells in TUNEL assay (d). Scale bar=100 μm. Data are presented as mean±S.E.M. *P<0.05 versus vehicle-treated group, †P<0.05 versus PlGF-treated group
Intratracheal PPE instillation increased PlGF expression and secretion and downstream JNK and p38 MAPK pathway activation
The role of PlGF in PPE-induced LE cell apoptosis and emphysema was evaluated in an animal model. C57BL/6 mice were intratracheally instillated with saline (CON) or 100 mU/ml PPE (PPE) weekly for one month. The pulmonary cells of the PPE-treated mice displayed more expression of PlGF than the control mice, and the induced-PlGF majorly presented in LE cells (arrowheads in Figure 5a). The induction of PPE on PlGF expression as well as activation of JNK and p38 MAPK were further confirmed by western blot analysis (Supplementary Figure 2S). Moreover, PPE-treated mice displayed more phosphorylated JNK and p38 MAPK levels than the control mice (arrowheads in Figure 5a). In addition, the BAL fluid of the PPE-treated mice had higher PlGF levels than that of the control mice (Figure 5b). These results demonstrated that PPE instillation increase PlGF expression, activation of JNK and p38 MAPK, and PlGF secretion in the pulmonary cells of mice.
Intratracheal PPE instillation increased PlGF expression and secretion, and downstream JNK and p38 MAPK pathway activation. C57BL/6 mice were intratracheally instilled with saline (CON) and 100 mU/ml PPE (PPE) weekly for one month. Paraffin-embedded lung tissue sections were used for IHC analysis and incubated with antibodies of PlGF, p-JNK and p-p38 MAPK (a). Arrowheads indicated positive stain of PlGF, p-JNK and pP38 MAPK in LE cells. The mice BAL fluid was analyzed for the PlGF level by ELISA (b). Scale bar=100 μm. Data are presented as mean±S.E.M. *P<0.05 versus vehicle-treated group
PlGF and PlGF-activated JNK and p38 MAPK pathways were involved in PPE-induced apoptosis and emphysema in mice
To evaluate the roles of PlGF, JNK and p38 MAPK signaling in PPE-induced emphysema, 50 mg/kg of SP 600125 (JNK inhibitor), 50 mg/kg SB 603580 (p38 MAPK inhibitor), 3 mg/kg scramble siRNA and 3 mg/kg PlGF siRNA were co-treated with PPE instillation (PPE SP, PPE SB, PPE Sc-si and PPE Pl-si) weekly for one month. Both RT-PCR and western blot analysis confirmed the attenuation of PlGF expressions by PlGF siRNA (Supplementary Figure 3S). TUNEL assay indicated apoptotic cells in pulmonary tissue (Figure 6a), and the PPE-treated mice had a higher level of pulmonary apoptosis than the control mice (Figure 6c). Moreover, the PPE-treated mice had the emphysema phenotype with enlargement of the alveolar space (Figure 6b) as evaluated by mean linear intercept (MLI) (Figure 6d). In addition, blockage of the JNK and p-38 MAPK pathways (PPE SP and PPE SB) and silencing of PlGF (PPE Pl-si) attenuated the levels of PPE-induced pulmonary apoptosis (Figures 6a and c) and the air space enlargement in PPE-treated mice (Figures 6b and d). Collectively, our in vivo experiments further confirmed that the PPE-increased pulmonary PlGF and the JNK and p38 MAPK signaling pathways were involved in PPE-induced pulmonary apoptosis and emphysema in vivo.
Increased-PlGF and activation of the JNK and p38 MAPK pathways were involved in PPE-induced emphysema in mice. C57BL/6 mice were intratracheally instilled with saline (CON), 100 mU/ml PPE (PPE), 100 mU/ml PPE with 50 mg/kg SP 600125 (PPE SP), 50 mg/kg SB 203580 (PPE SB), 3 mg/kg scramble siRNA (PPE Sc-si) and 3 mg/kg PlGF siRNA (PPE Pl-si) weekly for one month. The frozen lung tissue sections were used for TUNEL assay (a) and paraffin-embedded lung tissue sections were used for H&E stain (b). Apoptotic cells in TUNEL assay were quantified (c) and the mean linear intercepts (MLI) from five independent sections were evaluated (d). Scale bar=200 μm. Data are presented as mean±S.E.M. *P<0.05 versus vehicle-treated group, †P<0.05 versus PPE-treated group
Discussion
Several studies have reported that elastase, a serine protease, induces epithelial cell apoptosis. Leukocyte elastase damages the intestinal epithelial cells via loss of mitochondrial integrity, and decreasing Akt and increasing JNK signaling.15 Moreover, the leukocyte elastase-induced LE cell apoptosis has been shown to be mediated by protease-activated receptor-1, and retinoic acid has been shown to abrogate leukocyte elastase-induced LE cell apoptosis.14, 25 Different from previously established mechanisms, our results showed for the first time that PPE resulted in LE cell apoptosis through PPE-induced PlGF expression and PlGF-mediated downstream JNK and p38 MAPK signaling pathways.
Our study demonstrated that treatment of 60 mU/ml PPE for 24 h increased PlGF expression and secretion in LE cells. Tsao et al.6 had shown that treatment of 100 ng/ml PlGF for 24 h triggered MLE-15 cell apoptosis at the same level as our finding in this study. According to this condition, we indentified JNK and p38 MAPK signaling pathways are involved in PlGF-induced LE apoptosis. Moreover, treatment of PPE induced secretion of ∼600 pg/ml PlGF in culture medium from LE cells and instillation of PPE induced secretion of ∼100 pg/ml PlGF in BAL from mice. The difference in the level of PlGF secretion may result from difference in treatment interval and environmental complexity. Our data showed that PPE-induced PlGF expression and secretion, and PlGF-mediated apoptosis occurred, at least in part, in LE cells. The results of this study offer an explanation that COPD patients contain higher levels of PlGF in serum and BAL than non-COPD ones. Furthermore, the knock down of PlGF by siRNA attenuated PPE-induced emphysema and pulmonary apoptosis directly proved that upregulated PlGF has vital role in pathogenesis of emphysema and COPD.
The pathogenesis of COPD is chronic airway inflammation due to neutrophil and macrophage recruitment26, 27 followed by the secretion of NE and matrix metalloprotease. A protease–antiprotease imbalance may modify gene expression profiles in LE cells to overcome the extracellular stimuli. Previous studies have indicated that NE increases the expressions of elafin, MUC4 and MUC5AC, and the secretion of elafin and mucin in LE cells.16, 17, 18, 19 In bronchial epithelial cells, NE has also been shown to upregulate the expression of IL-8 through Toll-like receptor-4 signaling.20 Using mouse LE cells, PPE was shown to induce the expression of PlGF mRNA and protein in a dose-dependent manner in the present study. According to previous studies, the expression of PlGF in lung tissue was upregulated by hypoxic stimuli and lung stretch23, 28 and 1.5-kb upstream promoter region was sufficient for hypoxia induction.23 We also validated that PPE-induced PlGF mRNA expression was mediated by the interaction of Egr-1 protein and Egr-1 response element in PlGF promoter.
COPD is a major cause of death worldwide.29 The levels of airway inflammation and obstruction may insidiously progress, and the early diagnosis and treatment is usually difficult due to the lack of pathognomonic clinical symptoms. Effective biomarker for early detection and therapeutic agents for COPD remain elusive.30, 31 Cigarette smoke, the major factor related to COPD, has been proven to increase PlGF expression.11 In this study, we demonstrated that PPE-induced protease–antiprotease imbalance, the pathogenic mechanism for COPD, increased the expression of PlGF. In future studies, we will explore the mechanisms, such as DNA methylation, small nucleotide polymorphism and microRNA, associated with the regulation of PlGF expression. We hypothesize that PlGF is a prognostic or predictive biomarker for COPD.
Kinase inhibitors for JNK and p38 MAPK have been used as experimental therapeutic strategies in patients with COPD.32, 33 In the pathogenic processes of COPD, p38 MAPK signaling has been found to have roles in the activation of inflammation cascade and infiltration of immunocytes in vivo and in vitro.34 One clinical trial demonstrated a better clinical efficacy in COPD patients receiving p38 MAPK inhibitor therapy.35 Our results also demonstrated additional beneficial effects of using JNK and p38 MAPK inhibitors in COPD therapy. First, ablation of JNK and p38 MAPK signaling prevented the PlGF-induced LE cells apoptosis, which is a potential mechanism for COPD. Second, inhibition of JNK and p38 MAPK activity attenuated PPE-induced emphysema, which is the major component of COPD.
In conclusion, PPE increased PlGF expression and secretion both in LE cells and lung parenchyma of mice. The contribution of PlGF toward LE cell apoptosis and emphysema development were through JNK and p38 MAPK pathways. Downregulation of PlGF expression and agents inhibiting PlGF-associated downstream signals may be potential therapeutic targets for emphysema and COPD.
Materials and Methods
Reagents
Rabbit antibodies for phospho-JNK (p-JNK), p-p38 MAPK and p38 MAPK were obtained from Cell Signaling Technology (Beverly, MA, USA). The antibodies for PlGF, JNK and Egr-1, mouse PlGF siRNA, corresponding scramble siRNA and p38 MAPK inhibitor (SB 203580) were purchased from Santa Cruz (Santa Cruz, CA, USA). Trizol reagent, SuperScript III Reverse Transcriptase and Lipofectamine 2000 were obtained from Invitrogen (Carlsbad, CA, USA). Mouse antibody for β-actin was purchased from Genetex (Irvine, CA, USA). Human and mouse recombinant PlGF protein and an enzyme-linked immunosorbent assay (ELISA) kit were obtained from R&D Systems (Minneapolis, MN, USA). A dual-luciferase reporter assay system was obtained from Promega (Madison, WI, USA). Hematoxylin, eosin, caspase-3 activity assay kit, Chromatin immunoprecipitation (ChIP) Assay Kit and EZ-Zyme Chromatin Prep Kit were purchased from Merck-Millipore (Boston, MA, USA). An In Situ Cell Death Detection Kit was purchased from Roche (Mannheim, Germany). FITC annexin V apoptosis detection kit I was obtained from BD Biosciences (San Jose, CA, USA). The JNK inhibitor, SP600125, was obtained from Enzo Life Science (Plymouth Meeting, PA, USA). A SuperSensitive Polymer-HRP IHC Detection System was purchased from Biogenex (Fremont, CA, USA). PPE was obtained from Worthington Biochemical Corporation (Lakewood, NJ, USA).
Animals
This study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and all animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Laboratory Animal Center, College of Medicine and Public Health, National Taiwan University. Eight-week-old male C57BL/6 WT mice were purchased from the Laboratory Animal Center, College of Medicine and College of Public Health, National Taiwan University.
Cell culture
MLE-15 cells were kindly provided by Dr. Tsao. (National Taiwan University, Taiwan). The MLE-15 cells were cultured in DMEM supplemented with 10% FBS, 100 unit/ml penicillin and 100 μg/ml streptomycin in a humidified 95% air–5% CO2 incubator at 37 °C. Human bronchial epithelial cells, S cells (ATCC number CRL-9609), were cultured in F12 nutrient mixture (Carlsbad, CA, USA) with 0.5 ng/ml recombinant epidermal growth factor, 500 ng/ml hydrocortisone, 0.005 mg/ml insulin, 0.035 mg/ml bovine pituitary extract, 500 nM ethanolamine, 500 nM phosphoethanolamine, 0.01 mg/ml transferrin, 6.5 ng/ml 3,3′,5-triiodothyronine, 500 ng/ml epinephrine, 0.1 ng/ml retinoic acid, 10% FCS 100 unit/ml penicillin and 100 μg/ml streptomycin in a humidified 95% air–5% CO2 incubator at 37 °C.
Plasmids
Mouse genomic DNA was extracted from the MLE-15 cells by a Quick-gDNA MiniPrep kit (Zymo Research, Irvine, CA, USA). The 2.5-kb mouse PlGF promoter region was amplified from mouse genomic DNA by PCR that was performed with Hi Fi Taq DNA polymerase (Geneaid, Taipei, Taiwan) as follows: 2 min at 94 °C, then 15 s at 94 °C, 30 s at 59 °C, and 2 min and 30 s at 72 °C for 35 cycles. The primers for 2.5-kb mouse PlGF promoter region were 5′-ATG GTA CCC TCA AGA TAG TCA GGA TAC C-3′ (forward primer; underlined, KpnI site) and 5′-ATA AGC TTT GCA GTC TGC CTG AGC ATC C-3′ (reverse primer; underlined, HindIII site). The amplified DNA fragments were cloned into pGL3 vectors (Promega). The sequences were confirmed by DNA sequence analysis.
ELISA
Cellular medium from treated or untreated MLE-15 and BAL fluid from the mice were analyzed by a PlGF ELISA kit (R&D Systems) according to the manufacturer’s instructions.
Luciferase reporter assay
MLE-15 cells were co-transfected with the pGL3-PlGF promoter and pRenilla for 24 h. Treated or untreated MLE-15 cells were collected and analyzed on a dual-luciferase reporter assay system (Promega) by a lumicounter Packard BL10000 according to the manufacturer’s instructions.
RT-PCR assay
The total RNA of treated or untreated MLE-15 cells was extracted by Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA (5 μg) was used in the RT reactions using a SuperScript III reverse transcriptase kit (Invitrogen) according to the manufacturer’s instructions to synthesize the corresponding cDNA. The 250 bp or 530 bp of PlGF or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA fragments were amplified from the cDNA by PCR, performed with Dream Taq DNA polymerase (Thermo Fisher Scientific Inc., Waltham, MA, USA) as follows: 5 min at 95 °C, then 30 s at 98 °C, 30 s at 59 °C and 1 min at 72 °C for 35 cycles. The primers for the 250-bp PlGF cDNA fragment were 5′-CAG CCA ACA TCA CTA TGC AG-3′ and 5′-GGG TGA CGG TAA TAA ATA CG-3′. The primers for the 530-bp GAPDH cDNA fragment were 5′-GGG CGC CTG GTC ACC AGG GCT G-3′ and 5′-GGG GCC ATC CAC AGT CTT CTG-3′.
Protein extraction and immunoblot analysis
Treated or untreated MLE-15 and S cells were lysed using RIPA lysis buffer (Genestar, Taipei, Taiwan), which contained 1% NP-40, 0.1% SDS, 150 mM sodium chloride, 0.5% sodium deoxycholate and 50 mM Tris with a protease inhibitor cocktail (Bionovas, Toronto, Canada) and PhosSTOP (Roche, Basilea, Switzerland). Cellular lysates were centrifuged at 12 000 r.p.m. for 5 min and the resulting supernatant was collected. The extracted protein was quantified by protein assay. Equal amounts of protein were separated using 10% SDS-polyacrylamide gel electrophoresis and transferred to Immobilon-P membranes (Millipore, MA, USA). After blocking with 5% skimmed milk, the membranes were incubated with various primary antibodies and then incubated with the corresponding secondary antibodies. The protein bands were detected using an Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA) and quantified by ImageQuant 5.2 software (Healthcare Bio-Sciences, Philadelphia, PA, USA).
Chromatin immunoprecipitation (ChIP)
Genomic DNA fragment from treated- or untreated-MLE-15 cells were prepared by the EZ-Zyme Chromatin Prep Kit (Millipore) and analyzed by the Chromatin immunoprecipitation (ChIP) Assay Kit (Millipore) to evaluate the associated level of Egr-1 and PlGF promoter region. The antibody of Egr-1 was used for immunoprecipitation and the primer set (5′-CAG AGG TCA CTT AAG TAC CCA GCC ATC T-3′ and 5′-ATA AGC TTT GCA GTC TGC CTG AGC ATC C-3′) were used for amplify of mouse PlGF promoter according to the manufacturer’s instructions.
TUNEL assay
Treated or untreated MLE-15 cells and OCT-embedded lung tissue from the mice were analyzed for the level of apoptosis using an In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s instructions, and the fluorescence-positive cells were photographed by a Leica DM 4000B microscope (Leica, Solms, Germany).
Trypan blue inclusion assay
Treated or untreated MLE-15 cells were treated with 0–100 ng/ml PlGF for 24 h then stained with trypan blue (1 : 1 in volume), and the cells that stained blue were counted using a hemacytometer counting chamber (Invitrogen).
Caspase-3 activity assay
Treated or untreated MLE-15 and S cells were analyzed for caspase-3 activity using an assay kit (Merck-Millipore) according to the manufacturer’s instructions with fluorescent ELISA reader (Molecular Devices, Sunnyvale, CA, USA).
Flow cytometric analysis
Treated or untreated MLE-15 cells were analyzed on an FITC Annexin V Apoptosis Detection Kit I (Becton Drive, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions, and the FITC-positive cells were analyzed by a FACS Calibur flow cytometer (Becton Drive).
Immunohistochemistry assay
Paraffin was removed from paraffin-embedded tissue sections from the mice by xylene, dehydrated by ethanol and rehydrated by PBS. After treatment of 3% H2O2, the sections were applied to a SuperSensitive Polymer-HRP IHC Detection System (Biogenex) and incubated with PlGF, p-JNK and p-p38 MAPK antibodies as the primary antibodies. The stained sections were analyzed by a Leica DM 4000B microscope (Leica).
H&E stain
Paraffin was removed from paraffin-embedded tissue sections from the mice by xylene, dehydrated by ethanol and rehydrated by PBS. The sections stained with hematoxylin and eosin were then analyzed by a Leica DM 4000B microscope (Leica).
PPE-induced emphysema
The intratracheal instillation of PPE into the C57BL/6 WT mice was performed as described previously.11 In brief, eight-week-old mice were instilled intratracheally with saline (CON), 100 mU/ml PPE (PPE), 100 mU/ml PPE with 50 mg/kg JNK inhibitor SP600125 (PPE SP), 50 mg/kg p38 MAPK inhibitor SB 203580 (PPE SB), 3 mg/kg scramble siRNA (PPE Sc-si) and 3 mg/kg mouse PlGF siRNA (PPE Pl-si) weekly for one month. The dose of siRNA instillation was according to previous study.36 There were five mice in each group. Lung tissue and BAL fluid collection were performed as described previously.10
Statistical analysis
The results are presented as mean±S.E.M. from five independent repeats and animals. The Mann–Whitney test was used to compare two independent groups. Kruskal–Wallis followed by Bonferroni post-hoc analysis was used for multiple testing. Statistical analyses were performed using SPSS version 8.0 (SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
Abbreviations
- COPD:
-
chronic pulmonary obstructive disease
- LE:
-
lung epithelial
- PlGF:
-
placenta growth factor
- PPE:
-
porcine pancreatic elastase
- JNK:
-
c-Jun N-terminal kinase
- MAPK:
-
mitogen-activated protein kinase
- VEGF:
-
vascular endothelial growth factor
- VEGFR1:
-
VEGF receptor-1
- BAL:
-
bronchoalveolar lavage
- NE:
-
neutrophil elastase
- IL-8:
-
interleukin-8
- MMP-2:
-
matrix matelloprotease-2
- Egr-1:
-
early growth response-1
- ELISA:
-
enzyme-linked immunosorbent assay
- ChIP:
-
chromatin immunoprecipitation
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- TUNEL:
-
terminal deoxynucleotidyl transferase dUTP nick end labeling
- IHC:
-
immunohistochemistry
References
Decramer M, Janssens W, Miravitlles M . Chronic obstructive pulmonary disease. Lancet 2012; 379: 1341–1351.
Fischer BM, Pavlisko E, Voynow JA . Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis 2011; 6: 413–421.
Abboud RT, Vimalanathan S . Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis 2008; 12: 361–367.
Agusti A, Sobradillo P, Celli B . Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am J Respir Crit Care Med 2011; 183: 1129–1137.
Sharafkhaneh A, Hanania NA, Kim V . Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc 2008; 5: 475–477.
Tsao PN, Su YN, Li H, Huang PH, Chien CT, Lai YL et al. Overexpression of placenta growth factor contributes to the pathogenesis of pulmonary emphysema. Am J Respir Crit Care Med 2004; 169: 505–511.
De Falco S . The discovery of placenta growth factor and its biological activity. Exp Mol Med 2012; 44: 1–9.
Dewerchin M, Carmeliet P . PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harb Perspect Med 2012; 2: pii: a011056 doi:10.1101/cshperspect.a011056.
DiPalma T, Tucci M, Russo G, Maglione D, Lago CT, Romano A et al. The placenta growth factor gene of the mouse. Mamm Genome 1996; 7: 6–12.
Cheng SL, Wang HC, Yu CJ, Yang PC . Increased expression of placenta growth factor in COPD. Thorax 2008; 63: 500–506.
Li PC, Tsai WH, Chien CT . Dietary monascus adlay supplements facilitate suppression of cigarette smoke-induced pulmonary endoplasmic reticulum stress, autophagy, apoptosis and emphysema-related PLGF in the rat. Food Chem 2013; 136: 765–774.
Cheng SL, Wang HC, Yu CJ, Tsao PN, Carmeliet P, Cheng SJ et al. Prevention of elastase-induced emphysema in placenta growth factor knock-out mice. Respir Res 2009; 10: 115.
Shapiro SD . Animal models for COPD. Chest 2000; 117: 223S–227S.
Suzuki T, Moraes TJ, Vachon E, Ginzberg HH, Huang TT, Matthay MA et al. Proteinase-activated receptor-1 mediates elastase-induced apoptosis of human lung epithelial cells. Am J Respir Cell Mol Biol 2005; 33: 231–247.
Ginzberg HH, Shannon PT, Suzuki T, Hong O, Vachon E, Moraes T et al. Leukocyte elastase induces epithelial apoptosis: role of mitochondial permeability changes and Akt. Am J Physiol Gastrointest Liver Physiol 2004; 287: G286–G298.
Reid PT, Marsden ME, Cunningham GA, Haslett C, Sallenave JM . Human neutrophil elastase regulates the expression and secretion of elafinn (elastase-specific inhibitor) in type II alveolar epithelial cells. FEBS Lett 1999; 457: 33–37.
Fischer BM, Cuellar JG, Diehl ML, deFreytas AM, Zhang J, Carraway KL et al. Neutrophil elastase increases MUC4 expression in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2003; 284: L671–L679.
Shao MX, Nadel JA . Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-α-converting enzyme. J Immunol 2005; 175: 4009–4016.
Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB . Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase C-mediated mechanism. Am J Pathol 2005; 167: 651–661.
Devaney JM, Greene CM, Taggart CC, Carroll TP, O'Neill SJ, McElvaney NG . Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett 2003; 544: 129–132.
Geraghty P, Rogan MP, Greene CM, Boxio RM, Poiriert T, O'Mahony M et al. Neutrophil elastase up-regulates cathepsin B and matrix metalloprotease-2 expression. J Immunol 2007; 178: 5871–5878.
Mura M, dos Santos CC, Stewart D, Liu M . Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol 2004; 97: 1605–1617.
Green CJ, Lichtlen P, Huynh NT, Yanovsky M, Laderoute KR, Schaffner W et al. Placenta growth factor gene expression is induced by hypoxia in fibroblasts: a central role for metal transcription factor-1. Cancer Res 2001; 61: 2696–2703.
Krämer B, Meichle A, Hensel G, Charnay P, Krönke M . Characterization of an Krox-24/Egr-1-responsive element in the human tumor necrosis factor promoter. Biochim Biophys Acta 1994; 1219: 413–421.
Nakajoh M, Fukushima T, Suzuki T, Yamaya M, Nakayama K, Sekizawa K et al. Retinoic acid inhibits elastase-induced injury in human lung epithelial cell lines. Am J Respir Cell Mol Biol 2003; 28: 296–304.
Roghanian A, Sallenave JM . Neutrophil elastase (NE) and NE inhibitors: canonical and noncanonical functions in lung chronic inflammatory diseases (cystic fibrosis and chronic obstructive pulmonary disease). J Aerosol Med Pulm Drug Deliv 2008; 21: 125–144.
Lagente V, Le Quement C, Boichot E . Macrophage metalloelastase (MMP-12) as a target for inflammatory respiratory diseases. Expert Opin Ther Targets 2009; 13: 287–295.
Mohammed KA, Nasreen N, Tepper RS, Antony VB . Cyclic stretch induces PlGF expression in bronchial airway epithelial cells via nitric oxide release. Am J Physiol Lung Cell Mol Physiol 2007; 292: L559–L566.
Mathers CD, Loncar D . Projections of global mortality and burden of disease from 2002 to 2030 p38 MAP kinase inhibitors: a future therapy for inflammatory diseases. PLoS Med 2006; 3: e442.
Agustí A, Barnes PJ . Update in chronic obstructive pulmonary disease 2011. Am J Respir Crit Care Med 2012; 185: 1171–1176.
Rosenberg SR, Kalhan R . Biomarkers in chronic obstructive pulmonary disease. Transl Res 2012; 159: 228–237.
Adcock IM, Caramori G . Kinase targets and inhibitors for the treatment of airway inflammatory diseases. Bio Drugs 2004; 18: 167–180.
Banerjee A, Koziol-White C, Panettieri R Jr . p38 MAPK inhibitors, IKK2 inhibitors, and TNFa inhibitors in COPD. Curr Opin Pharmacol 2012; 12: 287–292.
Renda T, Baraldo S, Pelaia G, Bazzan E, Turato G, Papi A et al. Increased activation of p38 MAPK in COPD. Eur Respir J 2008; 31: 62–69.
Mayer RJ, Callahan JF . p38 MAP kinase inhibitors: a future therapy for inflammatory diseases. Drug Discov Today Ther Strateg 2006; 3: 49–54.
Lomas-Neira JL, Chung CS, Wesche DE, Perl M, Ayala A . In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukoc Biol 2005; 77: 846–853.
Acknowledgements
This study was supported by grants NHRI-EX101-10150SI from the National Health Research Institute, Taiwan and FEMH-2013-C-109 from the Far Eastern Memorial Hospital, Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Agostini
Supplementary Information accompanies this paper on Cell Death and Disease website
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Hou, HH., Cheng, SL., Liu, HT. et al. Elastase induced lung epithelial cell apoptosis and emphysema through placenta growth factor. Cell Death Dis 4, e793 (2013). https://doi.org/10.1038/cddis.2013.329
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.329
Keywords
This article is cited by
-
Role of GSDM family members in airway epithelial cells of lung diseases: a systematic and comprehensive transcriptomic analysis
Cell Biology and Toxicology (2023)
-
OXR1 signaling pathway as a possible mechanism of elastase-induced oxidative damage in pulmonary cells: the protective role of ellagic acid
Molecular Biology Reports (2022)
-
An innate contribution of human nicotinic receptor polymorphisms to COPD-like lesions
Nature Communications (2021)
-
Suppression of PTPN6 exacerbates aluminum oxide nanoparticle-induced COPD-like lesions in mice through activation of STAT pathway
Particle and Fibre Toxicology (2017)
-
Immune-mediated inflammation in the pathogenesis of emphysema: insights from mouse models
Cell and Tissue Research (2017)