Abstract
Hearing impairment caused by ototoxic insults, such as noise or gentamicin is a worldwide health problem. As the molecular circuitries involved are not yet resolved, current otoprotective therapies are rather empirical than rational. Here, immunohistochemistry and western blotting showed that the cytoprotective protein survivin is expressed in the human and guinea pig cochlea. In the guinea pig model, moderate noise exposure causing only a temporary hearing impairment transiently evoked survivin expression in the spiral ligament, nerve fibers and the organ of Corti. Mechanistically, survivin upregulation may involve nitric oxide (NO)-induced Akt signaling, as enhanced expression of the endothelial NO synthase and phosphorylated Akt were detectable in some surviving-positive cell types. In contrast, intratympanic gentamicin injection inducing cell damage and permanent hearing loss correlated with attenuated survivin levels in the cochlea. Subsequently, the protective activity of the human and the guinea pig survivin orthologs against the ototoxin gentamicin was demonstrated by ectopic overexpression and RNAi-mediated depletion studies in auditory cells in vitro. These data suggest that survivin represents an innate cytoprotective resistor against stress conditions in the auditory system. The pharmacogenetic modulation of survivin may thus provide the conceptual basis for the rational design of novel therapeutic otoprotective strategies.
Similar content being viewed by others
Main
Hearing impairment is a multifactorial disorder, caused by both genetic and environmental factors, including noise and ototoxic drugs, such as aminoglycoside antibiotics.1, 2 In developing countries, low cost and high efficacy make gentamicin the common choice for treatment of bacterial infections. Although ototoxicity is an important dose-limiting side effect, the benefits provided by gentamicin often outweigh the risks.1 Also, transtympanic injection of gentamicin at low concentrations is widely used for the treatment of Ménière's disease.1 Ototoxicity is often the consequence of irreversible drug-induced cell death in the cochlea, affecting also auditory and vestibular sensory cells. Although the detailed molecular mechanism underlying gentamicin-induced cytotoxicity have not been resolved in detail, the generation of free radicals by iron-gentamicin complexes has received strong support because anti-oxidants attenuate aminoglycoside-induced hearing loss.1, 3, 4
Similarly, acoustic trauma can induce temporary or permanent hearing impairment. In particular, a temporary auditory threshold shift caused by moderate noise exposure is clinically relevant for industry and other environments where such sound levels produce physical and psychological stress. Besides mechanical damage, acoustic trauma causes excitotoxicity, followed by metabolic disturbances.4, 5 Noise exposure not only affects hair cells, but also auditory nerve fibers and the stria vascularis, critical for the maintenance of cochlear homeostasis. At the molecular level, acoustic trauma inter alia results in the formation of reactive oxygen/nitrogen species and a calcium overload, which may culminate in triggering apoptotic and/or necrotic pathways.4, 5
Hence, important similarities in the ototoxic effects induced by noise and aminoglycosides exist, and both appear to converge in affecting pro- and anti-apoptotic cellular responses.5, 6, 7 Unfortunately, the molecular effectors mediating these biological responses remain poorly understood. Several pro-apoptotic mechanisms, including stress-activated protein kinase pathways, have been suggested to contribute to hearing impairment.5, 6, 7 In contrast, the knowledge on cytoprotective cellular responses counteracting permanent damage is sparse. These gaps in the existent knowledge have limited the research on otoprotective strategies mainly to prevention and to an empirical matter of trial and error.7, 8, 9
In general, modulation of programmed cell death (PCD) can be achieved by the dynamic expression of pro- and anti-apoptotic BCL-2 protein family members and apoptosis inhibitor proteins, such as survivin.10, 11, 12 Survivin's dual function as an apoptosis inhibitor and a mitotic regulator protect cancer cells against caspase-dependent as well as independent death pathways.11, 12 Recent evidence suggests that survivin may also be expressed in non-malignant differentiated tissues, potentially executing cytoprotective functions against various stress conditions.13, 14, 15
As neither survivin expression in the inner ear nor its potential clinical relevance for the pathophysiology of hearing impairment has been investigated so far, we here used comprehensive experimental approaches to investigate survivin's role in the auditory system. Our study provides the first evidence that the physical and chemical stress-induced modulation of survivin may represent an innate mechanism to protect the inner ear against ototoxic insults.
Results
Survivin expression in the human cochlea
To provide a rationale for an otoprotective role of survivin as well as its potential clinical relevance, we first examined survivin expression in mid-modiolar cross-sections of human cochlea by immunohistochemistry (IHC) (Figure 1). Employing our established IHC protocol used to study survivin's expression and function of,16, 17, 18, 19 survivin was specifically detectable as a cytoplasmic and nuclear protein in the organ of Corti, the lateral wall, interdental cells of the Limbus, the spiral ganglion, the Schwann cells, as well as in nerve fibers of the osseous spiral lamina regions (Figure 1a–d, and data not shown). No immunoreactivity was observed in cells of the inner and outer sulcus and the Reissner's membrane. Survivin was also detectable in the same cell types in the guinea pig cochlea (Figure 1a′–d′). As staining specificity is highly critical for immuno(histo)logical studies, we verified that the used antibodies and staining protocols reliably detect the survivin orthologs. No IHC signal was detectable upon omission of the primary α-survivin Ab or preabsorption of the α-survivin Ab with recombinant human survivin-GFP protein (data not shown). A single band with the molecular weight predicted for survivin was detectable by immunoblot analysis in whole cell lysates from proliferating mouse and guinea pig fibroblasts as well as from a human tumor, which served as the positive control (data not shown).
Survivin expression in various regions of the human (a–d) and the guinea pig (a′–d′) cochlea visualized by IHC. (a/a′) Overview. (b/b′) Survivin is detectable in all cell types in the organ of Corti, except the cuticular plates of pillar cells and in Claudius cells. (c/c′) In the lateral wall, survivin immunoreactivity was observed in the spiral ligament and the stria vascularis. (d/d′) Survivin expression in the spiral ganglions. id, interdental cells; is, inner sulcus; lw, lateral wall; nf, nerve fibers; oC, organ of Corti; os, outer sulcus; Rm, Reissner's membrane; sg, spiral ganglion; sl, spiral ligament; SM, scala media; ST, scala tympany; sv, stria vascularis; SV, scala vestibuli. Scale bars, 50 μm
Survivin is transiently upregulated upon noise exposure in the guinea pig cochlea
The guinea pig is used as an accepted animal model to study the effects of ototoxic insults and otoprotective strategies. As we hypothesized that survivin may represent an innate otoprotective mechanism, we analyzed the correlation of noise-induced temporary hearing loss with survivin levels.
As there is an ongoing discussion, whether immunohistochemical detection methods allow a quantitative analysis of protein levels in situ, we first verified that our computer-assisted quantification of the immunoreactivity is suitable to detect changes in protein levels. For this purpose, we microinjected increasing amounts of an autofluorescent recombinant GFP–GST protein into cells. Subsequently, we used α-GFP Ab to immunohistochemically visualize the levels of injected protein. When comparing protein levels based on the quantification of GFP-fluorescence and on IHC-based analysis, we found that both measurements correlated with the amounts of ectopically applied protein (data not shown).
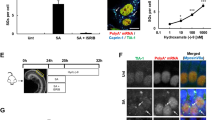
Survivin expression was examined in mid-modiolar cochlea cross-sections (Figure 2d) from unexposed animals (group NI), 1 h (70/90 dB for 1 h, group NII/III) or 2 h after noise exposure (90 dB for 1 h, group NIV) (Figure 2a). In addition, we verified the physiological effect of acoustic trauma by recording the auditory brainstem responses (ABRs) before and 2 h after noise exposure, revealing a mean hearing impairment of 33 dB (Figure 2b). Notably, treatment resulted in a temporary threshold shift only, and the animal's ABR fully recovered 7 days post exposure (data not shown).
Survivin induction by noise exposure. (a) Overview of treatment and animal groups. Guinea pigs were either unexposed (NI) or exposed to 70 dB (NII) or 90 dB (NIII/IV) SPL for 1 h. Bullae were removed at the indicated time points (arrows). (b) ABR recording revealed a mean hearing impairment of 33 dB in animals 2 h after noise exposure (90 dB SPL for 1 h, n=6, group NVI) compared to unexposed controls (n=8, group NI). Columns, mean; bars, ±S.D. ***P<0.005. (c) Noise-induced survivin expression in whole cochleae lysates shown by immunoblot analysis using the α-survivin Ab. Actin served as loading control. (d) Schematics of a cochlear mid-modiolar section. The cochlea is divided into three fluid compartments: the scala vestibuli (SV), the scala media (SM) and the scala tympany (ST), seperated by the Reissner's (Rm) and the basal membrane (bm), respectively. The outer wall of the SM is lined by the lateral wall (lw). The organ of Corti (oC) contains the sensory epithelia comprised of the auditory hair cells, which are innervated by the nerve fibers (nf), and the spiral ganglion neurons (sg). (e/f) Survivin expression before and after acoustic trauma analyzed by IHC. Relative immunoreactivity is indicated for the first cochlea turn (t1). (e) Enhanced survivin levels were evident in the spiral ligament as well as in the nerve fibers already 1 h after acoustic trauma, and further increased 2 h after 90 dB SPL exposure. Columns, mean; bars, ±S.D. **P<0.01. (f) Representative IHC-micrograph demonstrating enhanced survivin levels in the spiral ligament following acoustic trauma. Scale bar, 50 μm
Analysis of the data obtained by computer-assisted IHC indicated a noise-induced upregulation of survivin expression in the spiral ligament (P<0.005) as well as in the nerve fibers (P<0.005) (Figure 2e, f and Table 1). This increase of survivin was evident already 1 h after noise exposure in the cell types of the lateral wall for all three cochlea turns, and further increased 2 h after 90 dB noise exposure (Figure 2e and Table 1). In addition, increased survivin expression was observed in the organ of Corti, interdental cells of the limbus, ganglion cells as well as in the stria vascularis, although without reaching statistical significance (Table 1). No significant differences in expression were found when comparing the different cochlea turns (Table 1). The noise-induced survivin expression was confirmed by an independent method, as enhanced survivin levels were detectable by immunoblot analysis of lysates from macro-dissected whole cochleae (Figure 2c).
eNOS and NO levels are enhanced by auditory trauma
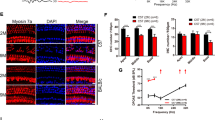
Reactive oxygen and nitrogen radicals are involved in acoustic trauma.4 Thus, we analyzed the levels of endothelial NO-synthase (eNOS) and NO concentrations following noise exposure. Notably, eNOS was found in several survivin-expressing cell populations, such as the organ of Corti, the nerve fibers and the lateral wall (Figure 3a–e). As the eNOS-mediated generation of NO appears to be an early event prerequisite for the induction of survivin,17, 19 we studied controls (group NI) and animals after noise exposure (90 dB for 1 h, group NIII). Enhanced eNOS was observed in the organ of Corti (P<0.005), the nerve fibers (P<0.05) as well as in both parts of the lateral wall, the stria vascularis (P<0.05) and the spiral ligament (P<0.05), mostly in all three cochlea turns (Figure 3e, Table 1). In addition, increased NO concentrations were found in organ culture supernatants from the noise-exposed animals for the organ of Corti (NO2− (562 nM±231) versus (331 nM±128)) and for the lateral wall (NO2− (737 nM±192) versus (356 nM±136)) (NIII versus NI) (Figure 3f).
eNOS and NO levels are enhanced by acoustic trauma. Columns, mean; bars, ±S.D. *P<0.05; **P<0.01; ***P<0.005. (a–e) eNOS expression in the guinea pig cochlea visualized by IHC. (a) Overview. (b–d) eNOS was detectable in all cell types in the stria vascularis, the organ of Corti, nerve fibers and spiral ganglion. Scale bars, 50 μm. (e) eNOS expression before (NI) and after noise (NIII) in the indicated cell types analyzed by IHC. Relative immunoreactivity is indicated for the different cochlea turns. (f) Enhanced [NO2−] measured in the supernatant of organ cultures from noise exposed (NIII) versus control animals (NI). (g, h) p-Akt expression visualized by IHC in the organ of Corti, nerve fibers and spiral ganglion following noise exposure (1 h, 90 db SPL). Scale bars, 50 μm. (i, j) eNOS expression before (NI) and after noise (NIII) in the indicated cell types for the first cochlea turn
Previously, we have shown that low NO concentrations enhance survivin expression in tumor cells by stimulating Akt signaling.17, 19 Indeed, IHC analysis not only revealed increased levels of phosphorylated Akt (pAkt) in different cochlea regions (Figure 3g and h), but also in eNOS-expressing cells, such as the nerve fibers and ganglion cells (Figure 3j and k). Notably, these cell types also displayed increased survivin levels subsequent to acoustic trauma.
Hence, noise-induced stress results in elevated eNOS levels and increased NO concentrations in the cochlea, which subsequently may contribute to an unpregulation of survivin via Akt signaling.
Gentamicin-induced ototoxicity attenuates survivin expression
To further study the role of survivin also in aminoglycoside antibiotic-induced permanent hearing loss, we analyzed its expression following intratympanic gentamicin injection 7 days post treatment. This time point was selected because we showed that injection of low gentamicin concentrations resulted in a permanent hearing impairment 7 days post treatment.20 First, a gentamicin-induced permanent threshold shift was verified by ABR recording, revealing a mean hearing impairment of 24 dB in gentamicin (group GI) versus saline-injected control animals (group GII) (Figure 4a). Survivin levels were quantitated by IHC and data analysis indicated a reduction in survivin expression in the organ of Corti (P<0.005), the spiral ganglion (P<0.005), the stria vascularis (P<0.005) as well as in the spiral ligament (P<0.005) for the first cochlea turn (Figure 4b, c and Table 1). Hence, the gentamicin-induced permanent hearing impairment correlated with attenuated survivin levels.
Gentamicin treatment results in survivin downregulation. Columns, mean; bars, ±S.D. *P<0.05, ***P<0.005. (a) ABR recording revealed a permanent mean hearing impairment of 24 dB in gentamicin (group GI, n=7) versus saline-injected control animals (group GII, n=9) 7 days post treatment. (b) Survivin levels in GI and GII were quantitated by IHC for the first cochlea turn 7 day post injection. (c) Representative IHC micrograph illustrating reduced survivin expression in ganglion cells following gentamicin treatment. Scale bar, 50 μm
Survivin protects against ototoxin-induced cytotoxicity
Aminoglycosides contribute to hearing loss by triggering apoptosis through caspase-dependent as well as independent mechanism (Figure 5b).5, 7 Although the cytoprotective activity of survivin against chemotherapy-based anti-cancer treatments has been shown, its function against ototoxin-induced PCD has not been investigated. As the coding sequence of the guinea pig (Gp) survivin gene as well as its biological functions were not known, we cloned the respective cDNA, revealing an open reading frame encoding for a protein of 142 amino acids. The survivinGp protein displays a high homology to the human and murine ortholog (GenBank accession number: GQ496319). Ectopic expression of a survivinGp-GFP fusion protein confirmed the typical intracellular localization known for human (Hu) survivinHu (Figure 5a).11, 12
Survivin protects against ototoxin-induced cytotoxicity. Columns, mean; bars, ±S.D. from three independent experiments. *P<0.05, **P<0.01, ***P<0.005. (a) Localization of guinea pig survivinGp-GFP transfectants in interphase and mitotic HeLa cells analyzed by fluorescence microscopy. DNA was marked by Hoechst dye (blue). Scale bars, 10 μm. (b) Induction of apoptosis by gentamicin treatment. In untreated HEI-OC1 cells, mitochondrial integrity is shown by the presence of the mono- and dimeric form of the MitoCapture dye (upper panel). Gentamicin treatment (1 μM, 24 h) causes loss of mitochondrial integrity resulting in loss of dimeric MitoCapture dye staining (lower panel). Scale bar, 10 μm. (c) Gentamicin-induced PCD is reduced in survivinGp- and survivinHu-GFP expressing transfectants. Cells transfected with the indicated plasmids were treated with gentamicin (1 μM), and analyzed 24 h later. Fragmented nuclei were visualized by staining with Hoechst dye and the percentage of apoptotic cells counted in at least 500 cells. (d) RNAi-mediated ablation of survivin following transfection with survivin- (surv) or a scrambled control-siRNA (scr) confirmed by immunoblot analysis. Actin served as loading control. (e) RNAi-mediated attenuation of survivin enhanced gentamicin-induced apoptosis. Cells were transfected with the indicated siRNAs and treated with gentamicin (1 μM, for 24 h) 24 h later. Fragmented nuclei were visualized by staining with Hoechst dye, and the percentage of apoptotic cells counted in at least 500 cells
To demonstrate the protective activity of survivin against ototoxin-induced cell damage, HeLa and the auditory cell line HEI-OC1, expressing survivinGp-GFP, survivinHu-GFP or GFP, respectively, were challenged with gentamicin (Figure 5c, and data not shown). In GFP-expressing control cells, treatment triggered apoptosis, whereas overexpression of survivin from both species significantly counteracted PCD as determined by quantitating apoptotic nuclei and caspase-3 activity (Figure 5c, and data not shown). Transgene expression was verified by fluorescence microscopy and immunoblot analysis, and similar results were obtained upon expression of murine survivin (data not shown). In addition, the cytotoxic effects of gentamicin were significantly enhanced by RNAi-mediated depletion of endogenous survivin (Figure 5d and e).
Discussion
Our study provides evidence for an otoprotective role of the apoptosis inhibitor protein survivin in the auditory system. We show that survivin is expressed in cell types critical for hearing perception in animal models currently employed to study hearing loss. Non-damaging noise resulted in a transient increase in survivin levels as well as in enhanced eNOS/NO concentrations, suggesting a functional correlation. In contrast, gentamicin-induced permanent hearing loss correlated with reduced survivin levels in the cochlea and survivin was cytoprotective against ototoxins in vitro. As the mammalian cochlea is unable to regenerate upon damage, these findings indicate that survivin appears to be part of a physiological protection mechanism against physical/chemical stress in the auditory system.
Despite several reports on cell death pathways involved in hearing loss and presbycusis, knowledge on antagonistic cytoprotective rescue mechanisms is limited.5, 6, 7 Ectopic overexpression of the X-linked inhibitor of apoptosis protein has been reported to counteract apoptosis in the cochlea, although the physiological protection mechanism remains to be demonstrated.21 For survivin, a large body of evidence has demonstrated that its function as an apoptosis inhibitor and member of the chromosomal passenger complex (CPC) protects cancer cells against chemo-radiation therapy-induced cell death.11, 12 Although recent data suggest that survivin may also be expressed in non-malignant tissues and stem cells, potentially executing cytoprotective functions against various stress conditions, the molecular details are not yet resolved.13, 22 Hence, it would be of interest to examine whether the other CPC members, AuroraB-kinase, Borealin and INCENP are also expressed and modulated by stress conditions in the auditory system. Here, we found survivin expression in important transducers of acoustic signals from and to the central nervous system, as well as in several non-neuronal cell populations, including the stria vascularis, critical for the maintenance of cochlear homeostasis and thus, hearing perception.
As there is an ongoing debate whether survivin is indeed expressed in post-mitotic non-malignant cells, several control experiments were performed in this study that substantiated the specific detection and IHC-based quantification of survivin in the cochlea, such as the use of negative controls, preabsorption of Abs, as well as the analysis by immunofluorescence and western blot with appropriate controls. However, the concerns whether IHC-based approaches allow to quantify protein expression levels in situ are not yet resolved. Although we addressed this issue by using a computer-assisted quantification of the immunoreactivity, we feel that even this method may allow only a semi-quantitative rather than a quantitative determination of protein levels. Despite these limitations, we feel that our data justify the conclusion that stress conditions modulate survivin in the cochlea. Even though we showed that survivin is a critical otoprotective factor, this does not rule out the additional participation of other cytoprotective proteins.10
Although the detailed molecular pathways involved in modulating survivin levels remain to be identified, our data suggest a role of the eNOS/NO-axis for the noise-induced upregulation of survivin. The concept of a concentration-dependent biphasic effect of NO signaling is well accepted.17, 19, 23 As shown by others and us, low NO levels, which may be generated by the physiological expression of eNOS, can stimulate cellular survival programs inter alia by the Akt-mediated upregulation of survivin.17, 19, 23 Although we found increased levels of p-Akt in some eNOS/survivin-positive cell types, the functional role of NO-induced Akt-phosphorylation needs to be further substantiated. In particular, the IHC-based detection of phosphorylated proteins in situ following extensive fixation and processing steps such as required for the cochlea, needs to be interpreted with caution.
In contrast, high NO concentrations, which may be generated by high-energetic noise or aminoglycoside antibiotics,24 can induce cell death pathways by activating mitogen-activated protein-kinases (MAPK) resulting also in survivin downregulation.17, 19, 23 Although, the contribution of the individual MAPK members, such as the p38-MAPK, the extracellular signal-regulated kinase or the c-Jun N-terminal kinase (JNK) to gentamicin-induced survivin downregulation was not examined in our study, previous reports favor a critical role for the MAPK pathway in the auditory system.6, 17, 19, 23, 25, 26 Hence, blocking JNK activation by peptide inhibitors to counteract hearing impairment,27 might thus act by also stabilizing survivin pools.
Whether the induction of survivin by subtoxic stress conditions also have a functional role for the phenomenon of ‘sound conditioning’ remains to be clarified.28 Recently, nuclear receptors, such as the estrogen and glucocorticoid receptor have been implicated in modulating otoprotective cellular programs.28, 29, 30 As intratympanic dexamethasone injection is currently employed in the clinics to treat transient hearing impairment and glucocorticoid receptor response elements are present in the survivin promoter, clinical efficacy may also act via influencing survivin expression.31
Albeit the molecular details how different (noxious) stimuli affect survivin expression remain to be resolved, it is conceivable to speculate that survivin represents a nodal protein where different pathways involved in the induction or prevention of hearing loss converge. Enhancing survivin levels under ototoxic stress conditions may provide a microenvironment protecting the auditory system against trauma. Once the damage exceeds a certain threshold, this cytoprotective resistor breaks down. The cloning of the guinea pig survivin gene now allows to employ this animal model in translational hearing research to further dissect the molecular details and to target this cytoprotective resistor. Our data suggest that enhancing survivin levels by pharmacogenetic approaches may be exploited as a potential strategy, not only to counteract cell death but also to aid regenerative processes in the auditory system.
Materials and Methods
Assessment of hearing function and noise exposure
The auditory brainstem response (ABR) provides information regarding auditory function and hearing sensitivity. ABRs were assessed in an acoustically shielded chamber (Industrial Acoustics Company GmbH, Niederkrüchten, Germany) to biaural click stimuli (duration 100 μs, 300 averaged stimuli, from 90 dB to 10 dB sound pressure level (SPL) following anesthesia. ABRs were recorded using subdermal recording electrodes (Viasys Healthcare, San Diego, CA, USA). The hearing threshold was defined as the lowest intensity at which a visible ABR wave was seen in two averaged runs and was determined by discrimination of wave Jewitt I, III and V for both ears. Broadband noise exposure was provided at 70 or 90 dB SPL using plastic tubes positioned directly in both ear canals near the tympanic membrane. Stimulus presentation, data recording and analysis were performed using the Spirit2000 Evoked Potential System (Nicolet Biomedical Inc., Madison, WI, USA).
Animals and gentamicin administration
Male, 2-weeks-old BFA-pigmented guinea pigs (Charles River Laboratories, Wilmington, MA, USA) were used. Animals were kept at 22°C±1°C under a 12 : 12 h light–dark cycle, and fed chow and water ad libitum. Experimental protocols were approved by the local Animal Care and Use Committee. For treatments, guinea pigs were anesthetized by an intraperitoneal injection of ketamine (175 mg/kg) and xylazine (10 mg/kg) (BayerAG, Leverkusen, Germany), and the body temperature was maintained using light heating. Anesthetized animals received either 4 mg gentamicin/ear (Ratiopharm, Ulm, Germany) by injection through the anterior parts of the tympanic membrane or equivalent volumes (0.1 ml) of saline under microscopic control.
Organ and primary cell culture
Animals were killed by pentobarbital-sodium (Narcoren) injection before tissues removal. For organ culture, the organs of Corti and lateral walls were separated, incubated in microtiter plates (6 h, 37°C), and the supernatant was stored at −80°C.20 For primary cell culture, lung tissue was disaggregated in fibroblast medium (199/Ham's F-12; 20% fetal calf serum, antibiotics, 10 μg/ml insulin; Gibco BRL, Invitrogen, Darmstadt, Germany) containing 0.1% collagenase III (Sigma Aldrich, Munich, Germany). Cells were seeded into tissue culture plates and further cultivated in fibroblast medium.
Nitrite measurement
NO production in organ culture supernatant was assessed by measuring the oxidation product nitrite by the Griess reaction as described.19
Antibodies (Ab), reagents and treatment
Ab were: Polyclonal rabbit and monoclonal mouse α (anti)-survivin (NB-500-201/NB-500-205, Novus Biologicals, Cambridge, UK); anti-β-Actin (A2066; Sigma Aldrich); monoclonal mouse α-eNOS Ab (BD Transduction Laboratories, San Jose, CA, USA); polyclonal α-phospho-AKT (9275, Cell Signaling, Danvers, MA, USA); appropriate HRP-conjugated secondary Ab (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cells were treated with gentamicin (1 μM) (Sigma Aldrich) for 24 h as described.17
Tissue preparation and immunohistochemistry
Cochleae were removed and fixed with 0.2% picric acid, 4% paraformaldehyde and 0.1% glutardialdehyde for 24 h at 4°C. For mid-modiolar cross-section preparation, cochlea were decalcified (EDTA for 3 weeks, 4°C), dehydrated by ethanol and xylene and embedded in paraffin.20 Tissues were processed for IHC as described.17, 19 Antigen retrieval was performed in a pressure cooker (survivin: EDTA buffer, 10 mM, pH 9.0; eNOS/p-Akt: citrate buffer, 10 mM, pH 6.0). For visualization of survivin (polyclonal α-survivin Ab-diluted 1 : 1250), p-Akt (polyclonal α-p-Akt Ab-diluted 1 : 50) or eNOS (monoclonal α-eNOS Ab-diluted 1 : 50), the EnVision detection system (Dako GmbH, Glostrup, Denmark) was used as described.17, 19 Sections were counterstained with hematoxylin. Negative control slides without primary Ab were included for each staining. Specificity of the α-survivin Ab was tested by preincubation of 5 μg α-survivin Ab with 100 μg immobilized recombinant GST-survivin-GFP protein (4 h, 4°C). The supernatant was subsequently tested by IHC. Recombinant GST-survivin-GFP protein was prepared as described.18 Staining procedures against a specific antigen were performed in a single experiment for all sections to minimize experimental variations.
Human tissue
Human cochleae were obtained from a female tumor patient and from a corpse (male, 50 years of age; preparation <3 h post mortem) at the ENT Department of the University Hospital of Mainz. Studies of human tissue biopsies were performed according to the requirements of the local ethics committee, and informed consent was obtained in accordance with the Declaration of Helsinki.
RNA extraction, cDNA cloning and survivin expression plasmids
Total RNA extraction, purification and cDNA synthesis were performed as described.32 The cDNA of the guinea pig survivin gene was cloned using PCR primers designed from highly conserved regions in the 5′- and 3′-UTRs of the mouse, rat and human survivin genes using the primers 5′conS (5′-AAAGGATCCACATGGGGGCCCCGTCGTTGCC-3′) and 3′conS (5′-AAAGCTAGCCTCCAAGGCGGCCAGCTGCTC-3′) or 3′conSstop (5′-AAAGCTAGCTCACTCCAAGGCGGCCAGCTGCTC-3′), respectively. PCR products were cloned into pGEM-T-easy (Promega, Madison, WI, USA), and the sequence of the survivinGp ORF determined by sequence analysis. For ectopic expression, the coding region of survivinGp was amplified by PCR and cloned into plasmid pc3-GFP as described,18 resulting in the pc3survivinGp and pc3survivinGp-GFP expression plasmids. Plasmids were verified by sequence analysis.18 Eukaryotic expression constructs for GFP and GFP-tagged versions of human and murine survivin were reported.16
Cells, transfection, microinjection, microscopy and image analysis
HeLa and Vero cells were maintained and transfected as described.17 The organ of Corti-derived cell line HEI-OC1 was cultured at 33°C.6 Observation, image analysis and quantification of protein localization were performed as described.18, 33 Preparation and microinjection of recombinant GST–GFP fusion proteins were performed as described in detail.18 DNA/cell nuclei were visualized by staining with Hoechst 33258 (Sigma Aldrich).18 At least 100 fluorescent cells in three independent experiments were examined.
RNAi
Double-stranded siRNAs (Eurogenetec, Köln, Germany) directed against human survivin or a scrambled control were used to downregulate endogenous survivin as described.17
Protein extraction and immunoblot analysis
Preparation of cell lysates and tissue extraction from tumor biopsies or guinea pig cochleae were carried out as described.17 Protein concentrations were determined by the Bradford protein assay and 5 μg were used for each analysis. Immunoblotting was performed as described.18
Computer-assisted IHC quantification
Quantification of IHC staining was performed as described.34 Briefly, slides were scanned using brightfield imaging at 40-fold magnification with an Axiovert-200 microscope (Zeiss, Jena, Germany), and images were recorded with a 3-CCD-DXC-390P camera (Sony) keeping capture settings constant. Automated analysis of 24-bit RGB images with a resolution of 1 μm/pixel was performed using Photoshop.V7 (Adobe Systems, San Jose, CA, USA). To quantify protein expression levels in a specific cochlear region, the respective digitized images were imported and analyzed in a common PSD file. Immunoreactive areas were selected by color sampling using a color dropper tool with a tolerance level of 15, and pixel numbers and intensities were obtained from the color histogram. To determine the relative immunoreactive area (RIA), the number of selected pixel was divided by the pixel number representing the total surface of the specific cochlear region. The RIA was multiplied with the background-corrected mean pixel intensity (MPI), resulting in the relative immunoreactivity (RIR=RIA × MPI) expressed in arbitrary units (AU). Eight consecutive sections per cochlea turn were analyzed for each animal blindly with respect to treatment.
Statistics
To analyze the IHC quantification data, mean values and S.D. in all subgroups as well as for group comparisons were computed using the two sample t-tests. We fitted a mixed linear model, taking into account that each animal contributed several measurements to the analysis using PROC MIXED from SAS 9.2 (SAS Institute Inc., Cary, NC, USA). For all cell culture experiments, a paired Student's t-test was performed, representing data from three independent experiments done in triplicate. Data are presented as mean values with S.D. P-values <0.05 were considered significant.
Measurement of apoptosis and cell vitality
Assessment of apoptosis was performed by analysis of mitochondrial integrity using the MitoCapture dye (PromoCell) and quantitation of caspase-3 activity as described.17 Apoptotic, fragmented nuclei were visualized by staining with Hoechst dye, and their percentage determined.18
Accession codes
Abbreviations
- ABR:
-
auditory brainstem response
- BDNF:
-
brain-derived growth factor
- CPC:
-
chromosomal passenger complex
- eNOS:
-
endothelial NO-synthase
- GST:
-
glutathione S-transferase
- IHC:
-
immunohistochemistry
- PCD:
-
programmed cell death
- RGB:
-
red-yellow-blue
- SPL:
-
sound pressure level
References
Guthrie OW . Aminoglycoside induced ototoxicity. Toxicology 2008; 249: 91–96.
Eisen MD, Ryugo DK . Hearing molecules: contributions from genetic deafness. Cell Mol Life Sci 2007; 64: 566–580.
Sha SH, Qiu JH, Schacht J . Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med 2006; 354: 1856–1857.
Henderson D, Bielefeld EC, Harris KC, Hu BH . The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006; 27: 1–19.
Jiang H, Sha SH, Forge A, Schacht J . Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ 2006; 13: 20–30.
Kalinec GM, Fernandez-Zapico ME, Urrutia R, Esteban-Cruciani N, Chen S, Kalinec F . Pivotal role of Harakiri in the induction and prevention of gentamicin-induced hearing loss. Proc Natl Acad Sci USA 2005; 102: 16019–16024.
Cheng AG, Cunningham LL, Rubel EW . Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg 2005; 13: 343–348.
Atar O, Avraham KB . Therapeutics of hearing loss: expectations versus reality. Drug Discov Today 2005; 10: 1323–1330.
Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM . Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res 2007; 226: 22–43.
Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59.
Altieri DC . Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 2008; 8: 61–70.
Stauber RH, Mann W, Knauer SK . Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res 2007; 67: 5999–6002.
Kindt N, Menzebach A, Van de Wouwer M, Betz I, De Vriese A, Conway EM . Protective role of the inhibitor of apoptosis protein, survivin, in toxin-induced acute renal failure. FASEB J 2008; 22: 510–521.
Fukuda S, Pelus LM . Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 2006; 5: 1087–1098.
Johnson EA, Svetlov SI, Wang KK, Hayes RL, Pineda JA . Cell-specific DNA fragmentation may be attenuated by a survivin-dependent mechanism after traumatic brain injury in rats. Exp Brain Res 2005; 167: 17–26.
Engels K, Knauer SK, Metzler D, Simf C, Struschka O, Bier C et al. Dynamic intracellular survivin in oral squamous cell carcinoma: underlying molecular mechanism and potential as an early prognostic marker. J Pathol 2007; 211: 532–540.
Fetz V, Bier C, Habtemichael N, Schuon R, Schweitzer A, Kunkel M et al. Inducible NO synthase confers chemoresistance in head and neck cancer by modulating survivin. Int J Cancer 2009; 124: 2033–2041.
Knauer SK, Bier C, Habtemichael N, Stauber RH . The Survivin-Crm1 interaction is essential for chromosomal passenger complex localization and function. EMBO Rep 2006; 7: 1259–1265.
Engels K, Knauer SK, Loibl S, Fetz V, Harter P, Schweitzer A et al. NO signaling confers cytoprotectivity through the survivin network in ovarian carcinomas. Cancer Res 2008; 68: 5159–5166.
Heinrich UR, Fischer I, Brieger J, Rumelin A, Schmidtmann I, Li H et al. Ascorbic acid reduces noise-induced nitric oxide production in the guinea pig ear. Laryngoscope 2008; 118: 837–842.
Chan DK, Lieberman DM, Musatov S, Goldfein JA, Selesnick SH, Kaplitt MG . Protection against cisplatin-induced ototoxicity by adeno-associated virus-mediated delivery of the X-linked inhibitor of apoptosis protein is not dependent on caspase inhibition. Otol Neurotol 2007; 28: 417–425.
Lechler P, Wu X, Bernhardt W, Campean V, Gastiger S, Hackenbeck T et al. The tumor gene survivin is highly expressed in adult renal tubular cells: implications for a pathophysiological role in the kidney. Am J Pathol 2007; 171: 1483–1498.
Fukumura D, Kashiwagi S, Jain RK . The role of nitric oxide in tumour progression. Nat Rev Cancer 2006; 6: 521–534.
Hong SH, Park SK, Cho YS, Lee HS, Kim KR, Kim MG et al. Gentamicin induced nitric oxide-related oxidative damages on vestibular afferents in the guinea pig. Hear Res 2006; 211: 46–53.
Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL . Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res 2004; 64: 9217–9224.
Wei X, Zhao L, Liu J, Dodel RC, Farlow MR, Du Y . Minocycline prevents gentamicin-induced ototoxicity by inhibiting p38 MAP kinase phosphorylation and caspase 3 activation. Neuroscience 2005; 131: 513–521.
Eshraghi AA, Wang J, Adil E, He J, Zine A, Bublik M et al. Blocking c-Jun-N-terminal kinase signaling can prevent hearing loss induced by both electrode insertion trauma and neomycin ototoxicity. Hear Res 2007; 226: 168–177.
Meltser I, Tahera Y, Canlon B . Glucocorticoid receptor and mitogen-activated protein kinases activity after restraint stress and acoustic trauma. J Neurotrauma 2009; 26: 1835–1845.
Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA et al. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest 2008; 118: 1563–1570.
Haake SM, Dinh CT, Chen S, Eshraghi AA, Van De Water TR . Dexamethasone protects auditory hair cells against TNFalpha-initiated apoptosis via activation of PI3K/Akt and NFkappaB signaling. Hear Res 2009; 255: 22–32.
Canlon B, Henderson D, Salvi R . Pharmacological strategies for prevention and treatment of hearing loss and tinnitus. Hear Res 2007; 226: 1–2.
Knauer SK, Bier C, Schlag P, Fritzmann J, Dietmaier W, Rodel F et al. The survivin isoform survivin-3B is cytoprotective and can function as a chromosomal passenger complex protein. Cell Cycle 2007; 6: 1502–1509.
Fetz V, Knauer SK, Bier C, Kriess JP, Stauber RH . Translocation biosensors – cellular system integrators to dissect CRM1-dependent nuclear export by chemicogenomics. Sensors 2009; 7: 5423–5445.
Selivanova O, Heinrich UR, Brieger J, Feltens R, Mann W . Fast alterations of vascular endothelial growth factor (VEGF) expression and that of its receptors (Flt-1, Flk-1 and Neuropilin) in the cochlea of guinea pigs after moderate noise exposure. Eur Arch Otorhinolaryngol 2007; 264: 121–128.
Acknowledgements
We thank S Friedl and K Backes for excellent technical assistance, and F Kalinec for supplying HEI-OC1 cells. Grant support provided by BIOMATICS, Head and Neck Cancer Foundation, Stiftung Rheinland-Pfalz für Innovation, DFG KN973/1-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Knauer, S., Heinrich, UR., Bier, C. et al. An otoprotective role for the apoptosis inhibitor protein survivin. Cell Death Dis 1, e51 (2010). https://doi.org/10.1038/cddis.2010.25
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2010.25
Keywords
This article is cited by
-
Expressional analysis of disease-relevant signalling-pathways in primary tumours and metastasis of head and neck cancers
Scientific Reports (2018)
-
Survivin expression pattern in the intestine of normoxic and ischemic rats
BMC Gastroenterology (2017)
-
Analysis of apoptosis methods recently used in Cancer Research and Cell Death & Disease publications
Cell Death & Disease (2012)
-
Aminoglycoside induced nephrotoxicity: molecular modeling studies of calreticulin-gentamicin complex
Journal of Molecular Modeling (2012)
-
Cell death in disease: from 2010 onwards
Cell Death & Disease (2011)