Research Highlights |
Featured
-
-
Article |
 Enantioselective synthesis of tatanans A–C and reinvestigation of their glucokinase-activating properties
Enantioselective synthesis of tatanans A–C and reinvestigation of their glucokinase-activating properties
Tatanans A, B and C, members of a family of complex sesquilignan natural products, were recently reported to possess potent anti-diabetic, glucokinase-activating properties. Here, a convergent enantioselective total synthesis of these tatanans enabled by catalytic allylic dearomatization is described. Contrary to previous reports, biological assays show that tatanans A–C are not allosteric activators of glucokinase.
- Qing Xiao
- , Jeffrey J. Jackson
- & Armen Zakarian
-
News & Views |
 Diversifying complexity
Diversifying complexity
A synthetic strategy that uses a series of simple reactions to distort the core architecture of complex natural products could provide libraries of stereochemically rich compounds that will help in the search for new biological probes and drugs.
- Indrajeet Sharma
- & Derek S. Tan
-
Article |
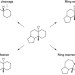 A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products
A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products
An approach for the construction of complex and diverse compound libraries is described, whereby natural products are altered through a series of ring system distortion reactions. The compounds produced have markedly different physiochemical properties from those in standard screening collections and thus could offer advantages in the search for lead molecules that can be developed into drug candidates.
- Robert W. Huigens III
- , Karen C. Morrison
- & Paul J. Hergenrother
-
Article |
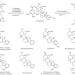 A divergent approach to the synthesis of the yohimbinoid alkaloids venenatine and alstovenine
A divergent approach to the synthesis of the yohimbinoid alkaloids venenatine and alstovenine
A hydrindanone-based approach to yohimbinoid natural products has been developed. A judicious choice of reaction conditions — inspired by prior work by the Stork group — allows effective control of the stereochemistry at C3 of the yohimbinoid skeleton. This approach has resulted in the first total syntheses of the C3 epimeric natural products venenatine and alstovenine.
- Terry P. Lebold
- , Jessica L. Wood
- & Richmond Sarpong
-
-
Article |
 Natural-product-derived fragments for fragment-based ligand discovery
Natural-product-derived fragments for fragment-based ligand discovery
Natural products populate areas of chemical space not occupied by average synthetic molecules. Here, an analysis of more than 180,000 natural product structures results in a library of 2,000 natural-product-derived fragments, which resemble the properties of the natural products themselves and give access to novel inhibitor chemotypes.
- Björn Over
- , Stefan Wetzel
- & Herbert Waldmann
-
Editorial |
 Take aim
Take aim
A collection of articles in this issue focuses on the ability to selectively perform a reaction at just one specific site in a complex molecule that contains many other similarly reactive sites.
-
Article |
 Selective transformations of complex molecules are enabled by aptameric protective groups
Selective transformations of complex molecules are enabled by aptameric protective groups
Selective modifications of structurally complex molecules bearing multiple reactive functional groups often require cumbersome multistep synthetic efforts. Here, aptameric protective groups based on short RNA sequences are described — they bind to neamine antibiotics, simultaneously protecting several functionalities and enabling regio- and chemoselective functionalizations.
- Andreas A. Bastian
- , Alessio Marcozzi
- & Andreas Herrmann
-
News & Views |
 Tackling tunicamycin
Tackling tunicamycin
The tunicamycins, secondary metabolites of various Streptomyces species, are invaluable tools in glycobiology. It has now been shown that their biosynthesis involves an unusual exo-glycal intermediate produced by previously unknown short-chain dehydrogenase/reductase activity.
- Ethan D. Goddard-Borger
- & Stephen G. Withers
-
-
News & Views |
 Welwitindolinone is well worth it
Welwitindolinone is well worth it
Recent syntheses of the natural product 3-hydroxy-N-methylwelwitindolinone C isothiocyanate are taken as examples to answer an oft-raised question about the value of total synthesis.
- John L. Wood
-
News & Views |
 Naturally inspired oligomers
Naturally inspired oligomers
The design of a small-molecule library for drug discovery attempts to combine the favourable diversity of natural product structures with the modularity of peptide synthesis.
- Jeffrey Aubé
-
Article |
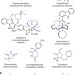 Enantioselective construction of quaternary N-heterocycles by palladium-catalysed decarboxylative allylic alkylation of lactams
Enantioselective construction of quaternary N-heterocycles by palladium-catalysed decarboxylative allylic alkylation of lactams
Nitrogen-containing heterocycles are ubiquitous in natural products, pharmaceuticals and in materials science. Here, the stereoselective synthesis of a wide array of structurally diverse, functionalized lactams by palladium-catalysed enantioselective enolate alkylation is described.
- Douglas C. Behenna
- , Yiyang Liu
- & Brian M. Stoltz
-
Article |
 Remodelling of the natural product fumagillol employing a reaction discovery approach
Remodelling of the natural product fumagillol employing a reaction discovery approach
The natural product fumagillol has been exploited as a stereochemically rich scaffold for the synthesis of a structurally unique, chemically diverse library with chemotypes distinctly different from the parent structure. Thus, fumagillol has been remodelled into a diverse array of isoindoles, isoquinolines, furans, mopholinones and benzoxazepines.
- Bradley R. Balthaser
- , Meghan C. Maloney
- & John K. Snyder
-
News & Views |
 Function first
Function first
The synthesis and biological investigation of a family of natural products and unnatural analogues illustrates the importance of considering both form and function in the planning of any synthesis.
- Andrew J. Phillips
-
Article |
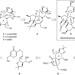 Gateway synthesis of daphnane congeners and their protein kinase C affinities and cell-growth activities
Gateway synthesis of daphnane congeners and their protein kinase C affinities and cell-growth activities
The daphnane diterpene orthoesters constitute a structurally fascinating family of natural products that exhibit remarkable and potent biological activities. A gateway strategy designed to provide general synthetic access to and biological evaluation of natural and non-natural daphnanes is described and used for yuanhuapin analogues.
- Paul A. Wender
- , Nicole Buschmann
- & Kate E. Longcore
-
News & Views |
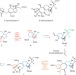 Making nematodes nervous
Making nematodes nervous
A highly inventive route for the synthesis of a key substance that stimulates potato cyst nematodes to hatch has been developed. This discovery has potential to impact food supplies, as treatment of crops with this compound could alleviate the devastating effect of these parasites.
- Scott A Snyder
-
Article |
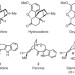 Synthesis of conolidine, a potent non-opioid analgesic for tonic and persistent pain
Synthesis of conolidine, a potent non-opioid analgesic for tonic and persistent pain
The first total synthesis of conolidine — representing the first asymmetric synthesis of a C5-nor-stemmadenine — is described. The chemical synthesis proceeds in just nine steps and gives an 18% overall yield from a commercially available pyridine — an achievement that led to the discovery that (+)-, (–)- and (±)-conolidine are potent non-opioid analgesics.
- Michael A. Tarselli
- , Kirsten M. Raehal
- & Glenn C. Micalizio
-
News & Views |
 C–H activation is a Reiske business
C–H activation is a Reiske business
Enzymes that selectively oxidize unactivated C–H bonds are capable of constructing complex molecules with high efficiency. A new member of this enzyme family is RedG, a Reiske-type oxygenase that catalyses chemically challenging cyclizations in the biosynthesis of prodiginine natural products.
- Steven D. Bruner
-
Article |
 Regio- and stereodivergent antibiotic oxidative carbocyclizations catalysed by Rieske oxygenase-like enzymes
Regio- and stereodivergent antibiotic oxidative carbocyclizations catalysed by Rieske oxygenase-like enzymes
Enzyme-mediated oxidative cyclizations in nature are a powerful demonstration of the utility of selective C–H activation. Here, Rieske oxygenase-like enzymes RedG and McpG are shown to mediate regio- and stereodivergent carbocyclization of undecylprodigiosin to streptorubin B and metacycloprodigiosin, respectively. Understanding these remarkably selective C–H activations could inspire the design of biomimetic catalysts with similar capabilities.
- Paulina K. Sydor
- , Sarah M. Barry
- & Gregory L. Challis
-
Research Highlights |
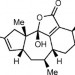 Conquering caribenol A
Conquering caribenol A
A delicate Diels–Alder reaction and a late-stage hydroxylation are the key steps in a total synthesis of a marine natural product.
- Stephen Davey
-
News & Views |
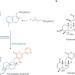 Not just passing through
Not just passing through
Identifying the genes responsible for each step of a natural product biosynthesis has allowed the synthesis to be 'hijacked' to make bioactive compounds, and reveals that some suspected transporter enzymes could have other important roles in fungal defence systems.
- Jason M. Crawford
- & Jon Clardy
-
News & Views |
 Back to basics
Back to basics
Taking inspiration from a proposed biosynthetic sequence, a cascade of simple reactions leads to an efficient synthesis of the alkaloid natural product (+)-fastigiatine.
- Brad M. Loertscher
- & Steven L. Castle
-
News & Views |
 Molecules under the microscope
Molecules under the microscope
A series of scanning probe microscopy experiments combined with density functional theory calculations have now been used to unambiguously determine the structure of a marine natural product. Can this method become generally useful for the determination of the structure of natural products?
- John W. Blunt
-
Article |
 Organic structure determination using atomic-resolution scanning probe microscopy
Organic structure determination using atomic-resolution scanning probe microscopy
The structure of many natural products can often only be confirmed by comparison with a synthetic sample. Here, scanning probe microscopy techniques allow the ultimate discrimination between structures suggested by the standard range of analytical techniques, proving the power of single-molecule imaging for molecular structure determination.
- Leo Gross
- , Fabian Mohn
- & Marcel Jaspars
-
Article |
 Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases
Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases
The structural diversity of natural products offers great potential for the identification of new drugs. Here, the gene cluster responsible for the production of a meroterpenoid, pyripyropene, has been identified, and reconstitution of the biosynthetic sequence involved allows the production of analogous synthetic structures with potentially new bioactivity.
- Takayuki Itoh
- , Kinya Tokunaga
- & Tetsuo Kushiro
-
News & Views |
 Towards artificial terpene cyclases
Towards artificial terpene cyclases
The plant-derived sesquiterpene englerin A is a potent inhibitor of several renal cancer cell lines. Two recent total syntheses have utilized cationic gold(I)-complexes to coax readily available open-chain precursors into englerin's challenging oxotricyclic core with enzyme-like precision.
- Matthieu Willot
- & Mathias Christmann
-
Research Highlights |
 Pseudosymmetry solved
Pseudosymmetry solved
Two different but successful approaches to the synthesis of (+)-complanadine A will help in investigations of its interesting biological activity.
- Stephen Davey
-
News & Views |
 Totally tubular peptide synthesis
Totally tubular peptide synthesis
The convergent total synthesis of the pore-forming polytheonamide B — a linear peptide natural product — pokes holes through perceived limitations in de novo peptide synthesis, and provides access to novel synthetic membrane channels.
- Craig J. Forsyth

 Anti-anthrax agents
Anti-anthrax agents Adjudazol assignment
Adjudazol assignment Flinderoles facilitated
Flinderoles facilitated