« Prev Next »

Ectotherms, animals whose body temperature closely tracks ambient temperature, occur in virtually every ecological niche on Earth. By virtue of some remarkable adaptations, they thrive even at high latitudes and altitudes in habitats characterized by seasonal or continuous cold (Addo-Bediako et al. 2000). Because aquatic habitats tend to be relatively warm and thermally stable, even in the Polar Regions, this article focuses on ectotherms that occupy terrestrial, arboreal, or intertidal habitats where temperatures may fall appreciably below the freezing point (FP) of body fluids.
For any ectotherm, even brief exposure to subzero temperatures carries the risk of irreparable injury or death. Cold impairs cellular functions by rigidifying membranes, slowing ion pumps, inducing oxidative damage, denaturing proteins, and altering energy balance. Freezing of tissues also provokes myriad stresses that are injurious and potentially lethal to most species. Even for cold-hardy ectotherms, survival depends on cooling rate, exposure temperature, and the duration and frequency of subzero chilling episodes.
Not surprisingly, a species' capacity for cold hardiness is well matched to the thermal regimen to which it has adapted. It may exhibit a latitudinal or altitudinal cline such that populations inhabiting colder regions are adequately protected. Within a given population, survival limits, and even the cold hardiness mechanism (i.e., freeze avoidance or freeze tolerance), can change from year to year (Kukal & Duman 1989). For ectotherms with complex life cycles, such as holometabolous insects, cold hardiness commonly is most pronounced in the overwintering life stages (Salt 1961).
Thermal Environments of Cold-hardy Ectotherms
Avoidance often is an animal's primary means to protection from extreme temperatures. If migration to warmer climes is not an option, survival may depend on finding an overwintering site, or hibernaculum, that insulates from damaging cold. For example, some toads and terrestrial turtles, being proficient excavators, descend into the soil column and overwinter below the reach of frost. Various snakes and woodland salamanders evade frost by following abandoned rodent burrows or root channels to underground lairs. Nevertheless, many species encounter subzero cold, either because their winter refuge lacks adequate insulation or because they are behaviorally active during cold weather (Figure 1).

To survive in winter, even cold-hardy ectotherms must seek thermally buffered sites. The ideal hibernaculum also conceals its occupant from potential predators, permits gas exchange, and prevents excessive desiccation. Some species prefer relatively exposed sites from which they can readily detect environmental cues stimulating spring emergence. For others, such as plant gall-inhabiting insects (Baust et al. 1979) and the hatchlings of some turtles (Costanzo et al. 2008), there is no choice in the matter: winter is passed in the very place where one hatches.
The thermal regimens to which overwintering ectotherms are exposed vary geographically, with the intensity and frequency of subzero exposures increasing with altitude and latitude. Even locally, temperatures can range from mild to severe, depending on site characteristics and the physical features of individual hibernacula, and, being subject to the vagaries of the weather, can vary markedly from year to year (Figure 2). Within hibernacula, prevailing temperatures follow a pronounced seasonal rhythm, usually attaining the lowest values at high winter, and also oscillate on a diel cycle.

Freeze Avoidance
Many biologists would be surprised to learn that a solution or organism does not necessarily freeze at its FP, but under certain conditions can cool much further whilst remaining unfrozen (liquid), or "supercooled." Indeed, a small volume of pure water can be chilled to nearly -40°C before it spontaneously freezes at its so-called "supercooling point," or, more accurately, temperature of crystallization (Tc).
To exploit the purely physical phenomenon of supercooling in a freeze-avoidance strategy, an organism must remain free of potent ice-nucleating agents (INAs), any of various inorganic particulates, microorganisms, proteins, and organic residues that can organize water molecules into a crystalline arrangement. Ubiquitous in nature, INAs of various potencies occur in diverse habitats, including the overwintering sites of molluscs (Ansart et al. 2010), insects (Zachariassen & Kristiansen 2000), amphibians (Costanzo et al. 1999), and reptiles (Costanzo et al. 2000), and may enter the body through orifices or be inadvertently ingested with food. Many freeze-avoiding ectotherms prepare for dormancy by eliminating ingested INAs, and also by masking or inhibiting endogenous ice-nucleating proteins (Costanzo et al. 2003, Duman 2001). It is crucial that they avoid physical contact with ice, which potentially can invade the body and initiate freezing. Species that rely on supercooling for winter survival can reduce the risk of such "inoculative freezing" by selecting hibernacula that limit their exposure to environmental ice. Some harbor in their tissues antifreeze proteins (AFPs) that effectively inhibit inoculation (Duman 2001).
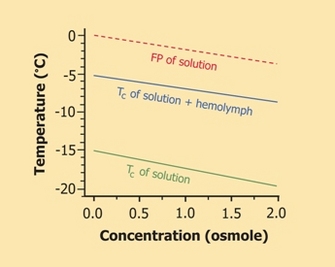
To avoid ice nucleation, many cold-hardy ectotherms accumulate one or more cryoprotectants in advance of winter (Zachariassen & Kristiansen 2000). Representing several classes of compounds, these solutes vary by species, but all are of low molecular mass and benign in high concentrations (Table 1). They not only colligatively depress the organism's FP, much as automotive antifreeze (e.g., ethylene glycol) prevents radiator fluid from freezing, but also can enhance supercooling (Figure 3). Supercooling capacity is further increased by partial dehydration of the body, which occurs preparatory to winter in many insects (Lee 2010).
| Class | Examples | Known From |
| Carbohydrates | polyhydric alcohols (glycerol, sorbitol, ethylene glycol); sugars (glucose, trehalose); cyclitols (myo-Inositol) | bacteria, marine and terrestrial invertebrates, amphibians, reptiles |
| Amino acids & derivatives | taurine, glycine, proline, alanine, asparagine, glutamic acid, lysine | bacteria, marine and terrestrial invertebrates |
| Methylamines | glycine betaine, glycerophosphorylcholine, trimethylamine oxide | bacteria, marine invertebrates, beetles |
| Urea | Terrestrial gastropods, amphibians, reptiles | |
| Table 1: Cryoprotectants used in animal freeze-avoidance and freeze-tolerance. | ||
Permutations of the freeze-avoidance strategy include vitrification, a process in which body fluids form a glass when cooled to extreme temperatures (Sformo et al. 2010), and cryoprotective dehydration, a survival adaptation of various invertebrates that overwinter in frozen substrata (Holmstrup et al. 2002, Sørensen & Holmstrup 2011). Because the vapor pressure of supercooled water exceeds that of ice, water tends to leave the unfrozen body until the internal vapor pressure reaches that of its frozen environment. Concomitantly, tissue FP drops as solutes become concentrated in a reduced water volume (and, commonly, as cryoprotectants are synthesized), thereby totally eliminating the risk of freezing. For this mechanism to be effective-and for ice inoculation to be avoided-dehydration must proceed quickly enough that the organism's FP consistently remains near the temperature of its cooling environment. Consequently, this strategy can be useful only to small ectotherms with a highly-permeable integument and profound desiccation tolerance.
Excessively deep or prolonged chilling can result in transient or permanent neuromuscular dysfunction or cold narcosis ("chill coma"), and even death (Salt 1961). Underlying mechanisms of chilling injury are not well understood, but probably include disturbance of ion homeostasis and metabolic functions, oxidative stress, and adverse phase changes in membranes (Kostal et al. 2006, MacMillan & Sinclair 2011).
Freeze Tolerance
Found in a small number of arthropods, molluscs, nematodes, annelids, amphibians, and reptiles, freeze tolerance is an adaptation for the survival of tissue freezing under ecologically-relevant thermal and temporal conditions. Freezing and thawing of the body, whilst potentially lethal, can be managed by mounting a diverse array of molecular and physiological responses that limit injury to cells and tissues.
For many species, freeze tolerance is expressed seasonally, usually developing in autumn in response to environmental cues and becoming most robust during the coldest months. In the laboratory, capacity for freezing survival can be enhanced by acclimation to high salinity and low environmental temperature, oxygen tension, and water potential (Lee 2010, Murphy 1983).
Control of the freezing process is key to survival in freeze-tolerant ectotherms (Lee & Costanzo 1998). In many invertebrates, which are prone to supercool owing to their small size, freezing is initiated by INAs in the hemolymph or other tissues. In other taxa, particularly species (e.g., amphibians) having a permeable integument, inoculation by external ice ensures that tissues freeze at relatively high temperatures (Costanzo & Lee 1996, Layne et al. 1990). Slow cooling enhances freezing survival, perhaps by allowing the organism adequate time to mount adaptive responses and by restricting ice to extracellular spaces; intracellular freezing is lethal for all but a few species (Lee 2010, Murphy 1983).
Ectotherms vary markedly in their limits of freeze tolerance. Known from laboratory studies, freeze endurance extends from perhaps a few days to several months of continuous freezing, although multiple freeze-thaw episodes are commonly experienced in nature (Layne et al. 1999). Depending on the taxon, the minimum survivable temperature ranges from a degree or two below 0°C to about -80°C (Table 2). Despite this variation, apparently no ectotherm can withstand the freezing of more than 50-80% of their body water.
| Taxon | Examples | Lower Lethal Temperature (°C) | Reference |
| Marine invertebrates (intertidal) | barnacles, bivalves, gastropods | -20 | Loomis 1991, Murphy 1983 |
| Terrestrial annelids | earthworms, enchytraeids | -20 | Holmstrup 2007, Slotsbo et al. 2008 |
| Terrestrial molluscs | slugs, snails | -5 | Ansart and Vernon 2003 |
| Other terrestrial invertebrates | free-living nematodes, centipedes, flies, beetles, butterflies and moths, wasps | -80 | Lee 1991, Wharton 2002 |
| Amphibians | salamander, frogs | -40 (frogs, -6) | Storey and Storey 1992 |
| Reptiles | turtles, lizard, snake | -4 | Storey and Storey 1992 |
| Table 2: Thermal limits of freeze tolerance in animals | |||
Molecular and physiological adaptations to freezing and thawing help prevent cell death from dehydration, mechanical distortion, damage to macromolecules and membranes, metabolic perturbation, and ischemia/reoxygenation (Storey & Storey 1992). Dehydration is a particularly onerous problem. As ice accumulates in extracellular spaces, water is lost from the supercooled cells to the interstitium where solutes, rejected by the forming ice crystals, become increasingly concentrated in the as yet unfrozen solution. The rising osmotic potential of this solution withdraws water from inside the cells, progressively shrinking them and concentrating cytosolic solutes to potentially harmful levels (Mazur 1984). Oxidative damage, precipitated by the return of oxygen to thawed tissues, also contributes to cryoinjury (Storey & Storey 2010, Swartz 1972).
Freezing/thawing stress can be ameliorated by invoking mechanisms of anoxia tolerance, depressing metabolism, and accumulating one or more cryoprotectants, either in anticipation of or during freezing. Cryoprotectants used in freezing adaptation include a host of sugars and sugar alcohols, amino acids, and even the "waste product," urea (Table 1). By colligatively depressing the FP of body fluids, these solutes permit the cytoplasm to remain supercooled whilst the extracellular fluids freeze, and also limit ice formation (Storey 1997). Many play supportive roles in antioxidation, energy supply, macromolecular stabilization, and counteraction of perturbing solutes (Yancey 2005).
Freeze Avoidance or Freeze Tolerance?
Freeze avoidance is an effective survival strategy in small ectotherms that either use dry hibernacula or possess an impermeable integument. Although exposure to extreme cold or prolonged chilling can be harmful, cryoinjury can be minimized if freezing is avoided. Accordingly, freeze-avoiding species generally tolerate a relatively broad range of subzero temperatures. On the other hand, overwintering in the supercooled state is a precarious proposition, as death is the likely consequence of an inadvertent nucleation event.
Freeze-tolerant ectotherms may be of any size and utilize myriad hibernacula, including ones that harbor ice. For those overwintering in an icy environment, tissue freezing eliminates the vapor pressure gradient across the integument and thereby prevents the organism from desiccating. On the downside, freezing/thawing stresses can be highly deleterious and, therefore, survival is possible over a relatively limited range of temperatures and exposure durations.
Although most species rely on freeze avoidance or freeze tolerance, a very few are uniquely adapted to employ either strategy, where the mode used during any particular chilling episode depends on prevailing physiological and/or environmental conditions (Costanzo et al. 2008, Sformo et al. 2009, Sinclair et al. 2004). Intensive study of these species may provide new insights into how the various selective pressures that shape life history traits drive the evolutionary development of cold-hardiness strategies (Sinclair et al. 2003).
Acknowledgements
The support of the National Science Foundation (Grant Number IOS-1022788) is gratefully acknowledged. The manuscript was improved by comments by M. C. do Amaral, R. E. Lee, T. J. Muir, and two anonymous reviewers. Graphics are a courtesy of M. F. Wright.
Glossary
antifreeze proteins: Class of specialized polypeptides or glycopolypeptides, also known as ice-structuring proteins (ISPs), that bind to minute ice crystals to inhibit the growth and recrystallization of ice in a solution.
antioxidation: Mounting defenses against the accumulation of free radicals and development of oxidative stress.
chill coma: Physiological state of being immobilized by the absence of neuromuscular function from the effects of low temperature on cell and tissue function.
cryoprotectants: A substance that acts through colligative or other properties to limit damage from exposure to low temperature and/or the freezing of biological tissue.
cryoinjury: A general term referring to perturbation or damage associated directly or indirectly from exposure to low temperature and/or the freezing of biological tissue.
hibernaculum: The protective shelter of a hibernating animal.
holometabolous: Adjective referring to an insect that undergoes complete metamorphosis, which includes four life stages: egg or embryo, larva, pupa, and imago or adult.
ice-nucleating proteins: Specialized protein with an ability to initiate the freezing of a solution, apparently by orienting water molecules into a crystalline lattice.
oxidative stress: A process in which free radicals accumulate in cells with deleterious consequences. Caused by an imbalance between the production of reactive oxygen species and the ability to detoxify the reactive intermediates or repair the resulting damage.
supercooling point: Supercooling, sometimes referred to as undercooling, is the tendency for a solution to remain in the liquid state when cooled below its freezing point. The supercooling point is taken as the lowest temperature achieved before the solution begins to freeze.
temperature of crystallization: Temperature at which a substance, supercooled or not, begins to freeze.
vapor pressure: The pressure exerted by a vapor in thermodynamic equilibrium with its solid or liquid phase. Relates to the propensity of particles to escape by evaporation (liquids) or sublimation (solids).
vitrification: The process by which a substance is converted at its glass transition temperature into an amorphous solid that is devoid of crystalline structure.
References and Recommended Reading
Ansart, A. & Vernon, P. Cold hardiness in molluscs. Acta Oecologia 24, 95-102 (2003).
Ansart, A., Nicolai, A., Vernon, P. & Madec, L. Do ice nucleating agents limit the supercooling ability of the land snail Cornu aspersum? Cryo-Letters 31, 329-340 (2010).
Baker, P. J. et al. Winter severity and phenology of spring emergence from the nest in freshwater turtles. Naturwissenschaften 97, 607-615 (2010).
Baust, J. G. et al. The diversity of overwintering strategies utilized by separate populations of gall insects. Physiological Zoology 52, 572-580 (1979).
Costanzo, J. P., Bayuk, J. M. & Lee, R. E. Inoculation by environmental ice nuclei in the freeze-tolerant wood frog, Rana sylvatica. Journal of Experimental Zoology 284, 7-14 (1999).
Costanzo, J. P., Claussen, D. L. & Lee, R. E. Natural freeze tolerance in a reptile. Cryo-Letters 9, 380-385 (1988).
Costanzo, J. P. & Lee, R. E. Mini review: Ice nucleation in freeze-tolerant vertebrates. Cryo-Letters 17, 111-118 (1996).
Costanzo, J. P., Lee, R. E. & Ultsch, G. R. Physiological ecology of overwintering in hatchling turtles. Journal of Experimental Zoology 309A, 297-379 (2008).
Costanzo, J. P. et al. Ice nuclei in soil compromise cold hardiness of hatchling painted turtles, Chrysemys picta. Ecology 81, 346-360 (2000).
Costanzo, J. P. et al. Endogenous and exogenous ice-nucleating agents constrain supercooling in the hatchling painted turtle. Journal of Experimental Biology 206, 477-485 (2003).
Costanzo, J. P. et al. Physiological ecology of overwintering in the hatchling painted turtle: multiple-scale variation in response to environmental stress. Physiological and Biochemical Zoology 77, 74-99 (2004).
Duman, J. G. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annual Review of Physiology 63, 327-357 (2001).
Holmstrup, M. E. Freeze tolerance in Aporrectodea caliginosa and other earthworms from Finland. Cryobiology 55, 80-86 (2007).
Holmstrup, M., Bayley, M. & Ramlov, H. Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable Arctic invertebrates. Proceedings of the National Academy of Sciences of the United States of America 99, 5716-5720 (2002).
Koštál, V., Yanagimoto, M. & Bastl, J. Chilling-injury and disturbance of ion homeostasis in the coxal muscle of the tropical cockroach (Nauphoeta cinerea). Comparative Biochemistry and Physiology B 143, 171-179 (2006).
Kukal, O. & Duman, J. G. Switch in the overwintering strategy of two insect species and latitudinal differences in cold hardiness. Canadian Journal of Zoology 67, 825-827 (1989).
Layne, J. R., Edgar, C. L. & Medwith, R. E. Cold hardiness in the woolly bear caterpillar (Pyrrharctia isabella Lepidoptera: Arctiidae). American Midland Naturalist 141, 293-304 (1999).
Layne, J. R., Lee, R. E. & Huang, J. L. Inoculation triggers freezing at high subzero temperatures in a freeze-tolerant frog (Rana sylvatica) and insect (Eurosta solidaginis). Canadian Journal of Zoology 68, 506-510 (1990).
Lee, R. E. "A primer on insect cold-tolerance," in Low Temperature Biology of Insects. eds. D. L. Denlinger & R. E. Lee (New York, NY: Cambridge University Press, 2010), 3-24.
Lee, R. E. & Costanzo, J. P. Biological ice nucleation and ice distribution in cold-hardy ectothermic animals. Annual Review of Physiology 60, 55-72 (1998).
Lee, R. E., Zachariassen, K. E. & Baust, J. G. Effect of cryoprotectants on the activity of hemolymph nucleating agents in physical solutions. Cryobiology 18, 511-514 (1981).
Loomis, S. H. "Comparative invertebrate cold hardiness," in Insects at Low Temperature. eds. R. E. Lee & D. L. Denlinger (New York, NY: Chapman and Hall, 1991), 301-317.
MacMillan, H. A. & Sinclair, B. J. Mechanisms underlying insect chill-coma. Journal of Insect Physiology 51, 1173-1182 (2011).
Mazur, P. Freezing of living cells: mechanisms and implications. American Journal of Physiology 247, C125-C142 (1984).
Murphy, D. J. Freezing resistance in intertidal invertebrates. Annual Review of Physiology 45, 289-299 (1983).
Salt, R. W. Principles of insect cold-hardiness. Annual Review of Entomology 6, 55-74 (1961).
Sformo, T. et al. Simultaneous freeze tolerance and avoidance in individual fungus gnats, Exechia nugatoria. Journal of Comparative Physiology B 179, 897-902 (2009).
Sformo, T. et al. Deep supercooling, vitrification and limited survival to -100°C in the Alaskan beetle Cucujus clavipes puniceus (Coleoptera: Cucujidae) larvae. Journal of Experimental Biology 213, 502-509 (2010).
Sinclair, B. J., Addo-Bediako, A. & Chown, S. L. Climatic variability and the evolution of insect freeze tolerance. Biological Reviews 78, 181-195 (2003).
Sinclair, B. J. et al. Cold tolerance of Littorinidae from southern Africa: Intertidal snails are not constrained to freeze tolerance. Journal of Comparative Physiology B 174, 617-624 (2004).
Slotsbo, S. et al. Freeze tolerance and accumulation of cryoprotectants in the enchytraeid Enchytraeus albidus (Oligochaeta) from Greenland and Europe. Cryobiology 57, 286-291 (2008).
Sørensen, J. G. & Holmstrup, M. Cryoprotective dehydration is widespread in Arctic springtails. Journal of Insect Physiology In press, (2011).
Storey, K. B. Organic solutes in freezing tolerance. Comparative Biochemistry and Physiology A 117A, 319-326 (1997).
Storey, K. B. & Storey, J. M. Natural freeze tolerance in ectothermic vertebrates. Annual Reviews of Physiology 54, 619-637 (1992).
Storey, K. B. & Storey, J. M. "Oxygen: Stress and adaptation in cold-hardy insects," in Low Temperature Biology of Insects. eds. D. L. Denlinger & R. E. Lee, Jr. 141-165 (Cambridge University Press, 2010).
Swartz, H. M. Effect of oxygen on freezing damage: II. Physical-chemical effects. Cryobiology 8, 255-264 (1972).
Wharton, D. A. Life at the Limits. Cambridge, UK: Cambridge University Press, 2002.
Yancey, P. H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. Journal of Experimental Biology 208, 2819-2830 (2005).
Zachariassen, K. E. & Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 41, 257-279 (2000).






























