« Prev Next »

Iron is one of the most abundant elements on Earth and is an essential nutrient for all organisms. It has two valence states: oxidized ferric iron, Fe(III) or Fe3+, and reduced ferrous iron, Fe(II) or Fe2+. In aerobic environments, when oxygen is present, iron exists in the ferric state:
2Fe2+ + ½O2 + 2H+ → 2Fe3+ + H2O
However, in anaerobic environments, such as subsurface habitats where oxygen is absent, iron exists in the Fe(II) state:
Fe3+ + reducing reagent + H2O → Fe2+ + oxidized product + H+
Here an element or compound acts as a reducing reagent and donates an electron to Fe3+. In doing so, the reducing reagent becomes oxidized and Fe3+ is reduced (i.e., gains an electron) to Fe2+. On Earth, reducing reagents can be abiotic, such as sulfur (S0) or hydrogen sulfide (H2S), or biotic, such as a protein or water-soluble organic compound.
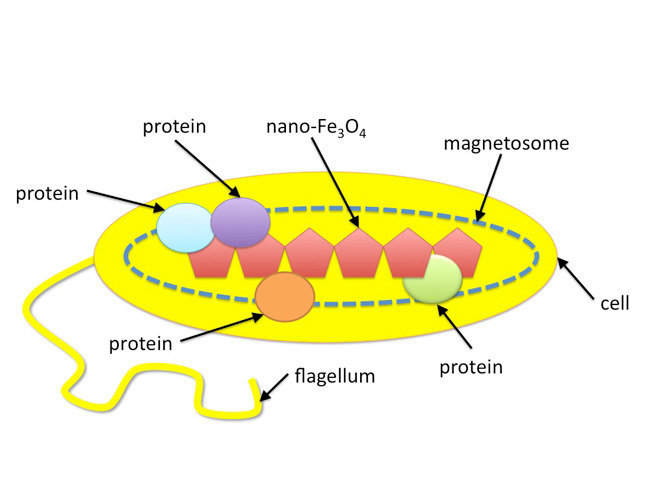
Earth's iron cycle is a very complex biogeochemical cycle that includes both biotic and abiotic components. In this article, we will illustrate the role that a group of single-celled microorganisms, called magnetotactic bacteria, play in Earth's iron cycle. Iron is particularly important in magnetotactic bacteria not only because iron functions as a protein cofactor for numerous metabolic pathways but also because these microorganisms incorporate large amounts of iron in the form of nanometer-sized iron mineral crystals within their cells (Bazylinski et al. 1995; Figure 1).
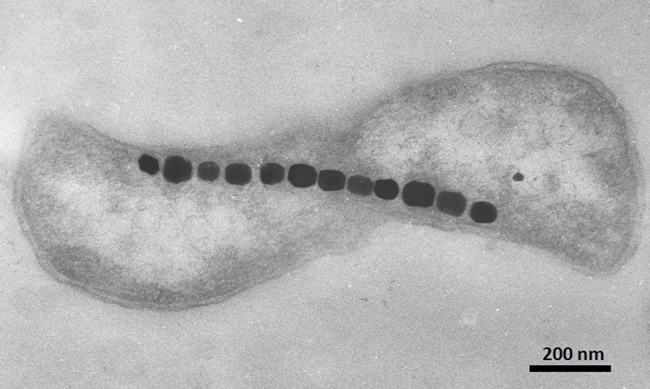
Blakemore (1975) first documented magnetotactic bacteria and found that the microorganisms synthesize chains of nano-sized magnetic particles that function as a compass needle. This "nano-compass" allows the microbe to passively orient itself in Earth's geomagnetic field (Figure 2). These magnetic nanoparticles are synthesized by a specific set of proteins that are contained within membrane-bound organelles called magnetosomes. Each bacterium contains 10–20 magnetosomes, each of which contains a magnetic nanoparticle. The magnetosomes are aligned in a chain-like fashion, which impart a magnetic dipole to the bacterial cell and allows the cells to "sense" Earth's geomagnetic field (Gorby et al. 1988). In addition to magnetosomes and magnetic nanoparticles, magnetotactic bacteria also have a flagellum, which they use for mobility.
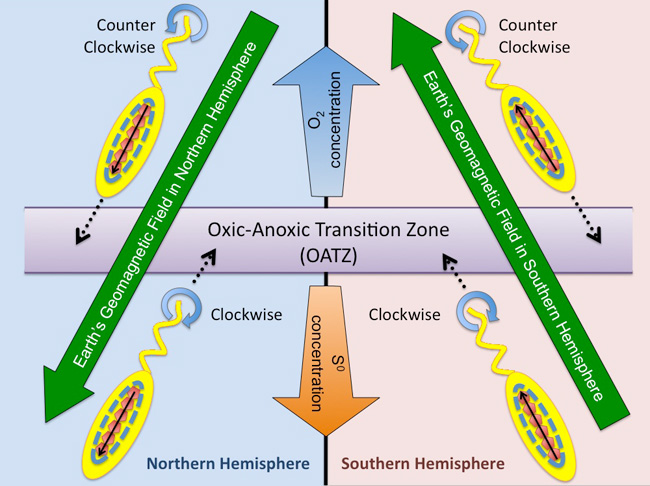
Magnetotactic bacteria are prokaryotic microorganisms ubiquitous to freshwater and marine habitats. They are all microaerophiles or anaerobes and prefer environments that contain little to no oxygen (Bazylinski et al. 1995, Blakemore 1975). Because these organisms thrive in environments that lack oxygen, it is believed that these microbes may represent some of Earth's earliest inhabitants. Magnetotaxis is the process by which magnetotactic bacteria orientate themselves within Earth's geomagnetic field and then use their flagellum to swim to oxygen-poor sediments where the oxygen and redox conditions preferred by these organisms are found (Spormann & Wolfe 1984; Figure 3).
These microbes prefer the oxic-anoxic transition zone (OATZ) where oxygenated water (or sediment) meets oxygen-deficient water (or sediment). The bacteria internally synthesize a chain of magnetic nanoparticles (shown as red pentagons in Figure 3), which act as a nano-sized magnet that allows the bacteria to passively align with Earth's geomagnetic field lines in both the Northern and Southern Hemispheres. Bacteria located in environments with higher than optimal oxygen concentration (top of the diagram) swim forward to the OATZ by rotating their flagella counter clockwise. Conversely, bacteria located in environments having lower than optimal oxygen concentration (bottom of diagram) rotate their flagella clockwise and swim backward to the OATZ (Figure. 3).
Magnetotactic bacteria are typically divided into three groups according to the type of magnetic mineral nanocrystal, which they synthesize: 1. magnetite (Fe3O4), 2. greigite (Fe3S4) or a combination of greigite and pyrite (FeS2), 3. a combination of magnetite and greigite (Bazylinski et al. 1995, Frankel et al. 1979, Mann et al. 1990). At lease twenty species of magnetite-producing magnetotactic bacteria have been cultivated in pure culture whereas greigite-producing magnetotactic bacteria have not yet been isolated in pure culture. This difficulty is likely due to a number of factors, not the least of which is their preference for marine habitats having little to no oxygen and relatively high concentrations of hydrogen sulfide (H2S). This makes research on this class of magnetotactic bacteria much more difficult. However, magnetotactic bacteria that synthesize iron sulfide minerals can sequester a large amount of sulfur and may therefore exert significant effects on the biogeochemical cycling of sulfur, in addition to iron, on Earth (Faivre & Schüler 2008).
Whereas most magnetite-producing magnetotactic bacteria occur in a water column's oxic-anoxic transition zone, most iron-sulfide-producing magnetotactic bacteria occur in the anaerobic zone just below the oxic-anoxic interface where H2S becomes detectable (Bazylinski & Frankel 2004; Figure 3). It has also been reported that magnetotactic bacteria from an oligotrophic lake (i.e., a lake having little nutrient content) sediment were phylogenetically and morphologically much more diverse than those in habitats with high content of organic carbon (Schüler 1999). So magnetotactic bacteria can vary according to which hemisphere they are found as well as the type of ecosystem in which they live.
The process by which Fe3O4 (or Fe3S4) is synthesized by magnetotactic bacteria is still not clear. Researchers believe several distinct steps are involved in this biomineralization process. These steps include iron uptake by the bacterial cell, magnetosome vesicle formation within the bacteria, iron transport into the magnetosome vesicle, and protein-mediated Fe3O4 or Fe3S4 biomineralization within the magnetosomes (Bazylinski & Frankel 2004, Komeili et al. 2004, Schüler 1999).
Iron uptake systems in magnetotactic bacteria vary from one species to another. Fe2+ is very soluble at neutral pH, and Fe2+ is quickly oxidized to Fe3+ under the aerobic (oxygenated) conditions. As a result, iron on Earth's surface exists primarily as Fe(III)-containing minerals, which are insoluble at neutral pH. In order to utilize solid-phase Fe3+, some magnetotactic bacteria are believed to synthesize iron-binding biomolecules called a siderophores (Bazylinski & Frankel 2004, Paoletti & Blakemore 1986). Siderophores are ferric ion (Fe3+) chelators produced by bacteria and fungi growing under low iron stress (Neilands 1995). The siderophores have a high affinity for Fe3+ and bind, or chelate, the iron. Next, it is believed that the Fe3+-sideophore complex enters the cell and then Fe3+ is cleaved from the siderophore. Once inside the cell, proteins reduce (i.e., donate electrons to) the Fe(III), converting the iron to Fe2+ which is then taken up by the magnetosomes. The magnetotactic bacteria M. magneticum AMB-1 uses a protein called MagA to transport Fe2+ in an energy dependent manner into magnetosomes, which leads to an accumulation of Fe2+ within the subcellular vesicle (Nakamura et al. 1995). Two additional proteins have been identified within the magnetosome membrane of M. gryphiswaldense, MamB and MamM. Both proteins have also been shown to function in the transport of iron into the magnetosomes (Schübbe et al. 2003).
Once inside the magnetosome, proteins called Mms-proteins direct the biomineralization of magnetite (or greigite) nanocrystals (Figure 4). The Mms-proteins are a unique set of transmembrane or soluble proteins that control the nucleation and directed growth of magnetite or greigite within the magnetosome vesicles. Four Mms-proteins in particular were found to be tightly bound to magnetic magnetite particles in M. magneticum AMB-1 (Arakaki et al. 2003). These proteins are: Mms5, Mms6, Mms7 (homolog MamD in M. gryphiswaldense), and Mms13 (homolog MamC in M. gryphiswaldense) are believed to control the nucleation and growth of the magnetic mineral nanoparticles (Arakaki et al. 2003, Schübbe et al. 2003).
Mms5, Mms6, Mms7, and Mms13 share similarities in their primary amino acid sequences. All four proteins contain a hydrophobic N-terminus that is a putative transmembrane region that allows the four proteins to become integrated within the lipid bilayer membrane of the magnetosomes. The proteins also contain a hydrophilic C-terminus containing dense carboxyl and hydroxyl groups, which have strong affinity for metal ions and are believed to function as bridging ligands. Only one protein, Mms6, has been studied in detail. Arakaki et al. (2003) suggested that the magnetite crystals formed in vitro in the presence of Mms6 are similar to those produced by intact cells while the magnetite crystals produced without Mms6 in vitro showed no homogeneity in shape and size. Arakaki and colleagues also point out that the hydroxyl group at the C-terminus of the Mms6 protein might function as a template for Fe3O4 crystal formation, and control the shape of the crystals (Arakaki et al. 2003).
It has been observed that biomineralization in magnetotactic bacteria provides highly uniform magnetite crystals with narrow size distributions (an average diameter of 50–100 nm; Mann et al. 1990; Figure 2). Furthermore, magnetite crystals in magnetosomes are of high chemical purity (Arakaki et al. 2003, Bazylinski et al. 1995, Bazylinski & Frankel 2003). The crystals are composed of iron and oxygen with the chemical formula Fe3O4 (magnetite) or iron and sulfur with the chemical formula Fe3S4 (greigite). The size, type, and morphology of magnetic crystals vary from species to species but are highly conserved within the same bacterial species or genus (Bazylinski & Frankel 2004). The three most common magnetic crystal morphologies are elongated prismatic, roughly cuboidal, and tooth-shaped (Bazylinski et al. 1995, Blakemore 1975, Mann et al. 1990). In contrast, magnetite crystals produced by abiotic mineralization have low crystallinity and broad size distributions.
Magnetotactic bacteria consist of up to 3% iron by dry weight (10-13 to 10-15 grams of iron per cell) (Bazylinski & Frankel 2003, Bazylinski et al. 2007, Mann et al. 1984). After magnetotactic bacteria die, magnetosome chains deposit in the sediment, and become magnetofossils, which can carry useful paleomagnetic information about past environments on Earth. Faivre & Schüler (2008) estimated that, in some locations on Earth, up to 10% of the iron contained within sediment may be contributed by magnetotactic bacteria. Recently, Haltia-Hovi and colleagues suggested that fossil magnetosome concentrations could be used as a climate indicator (Haltia-Hovi et al. 2010). Magnetosome production was influenced by changes in the bacteria's natural habitat (e.g., organic matter), which is directly controlled by climate. However, this type of analysis can be difficult. Faivre & Schüler (2008) pointed out that magnetofossils often mix with soils in the sediment and may be reduced or oxidized in different environmental conditions, which often challenge a researcher's ability to discern the biogenic information contained within the magnetofossils.
It has even been suggested that organisms similar to Earth's magnetotactic bacteria may have inhabited other planets in our solar system. Several years ago researchers showed that magnetite crystals contained within the Martian meteorite ALH84001 exhibited characteristics similar to crystals synthesized by magnetotactic bacteria on Earth (McKay 1996, Thomas-Keprta 2002). As we begin to understand Earth's magnetotactic bacteria and the mechanism by which these organisms synthesize iron oxides and sulfides, we may also be able to answer whether the magnetite observed in the Martian meteorite ALH84001 (or others) may be biotic in origin as well. Such insight might help us understand the origin of life on Earth and elsewhere in the universe.
Acknowledgements
The support of US National Science Foundation (Grant Number EAR-0920299 and EAR-0920718) is gratefully acknowledged.
References and Recommended Reading
Arakaki, A., Webb J., & Matsunaga, T. A novel protein tightly bound to bacterial magnetic particles in Magnetospirillum magneticum strain AMB-1. Journal of Biological Chemistry 278, 8745–8750 (2003).
Bazylinski, D. A. et al.. Controlled biomineralization of magnetite (Fe3O4) and greigite (Fe3S4) in a magnetotactic bacterium. Applied and Environmental Microbiology 61, 3232–3239 (1995).
Bazylinski, D. A. & Frankel R. B. Biologically controlled mineralization in prokaryotes. Reviews in Mineralogy and Geochemistry 54, 217–247 (2003).
Bazylinski, D. A. & Frankel R. B. Magnetosome formation in prokaryotes. Nature Reviews Microbiology 2, 217–230 (2004).
Bazylinski, D. A., Frankel R. B., & Konhauser, K. O. Modes of biomineralization of magnetite by microbes. Geomicrobiology Journal 24, 456–475 (2007).
Blakemore, R. Magnetotactic bacteria. Science 24, 377–379 (1975).
Faivre, D. & Schüler D. Magnetotactic bacteria and magnetosomes. Chemistry Reviews 108, 4875–4898 (2008).
Frankel, R. B., Blakemore, R. P. & Wolfe R. S. Magnetite in freshwater magnetotactic bacteria. Science 203, 1355–1356 (1979).
Gorby, Y. A., Beveridge T. J., & Blakemore R. P. Characterization of the bacterial magnetosome membrane. Journal of Bacteriology 170, 834–841 (1988).
Haltia-Hovi, E. et al. Magnetic properties and environmental changes recorded in Lake Lehmilampi (Finland) during the Holocene. Journal of Paleolimnology 43, 1–13 (2010).
Komeili, A., Vali H., Beveridge T. J. & Newman D. K. Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proceedings of the National Academy of Sciences USA 101, 3839–3844 (2004).
Mann, S., Frankel, R. B., & Blakemore R. P. Structure, morphology and crystal growth of bacterial magnetite. Nature 310, 405–407 (1984).
Mann, S. et al. Biomineralization of ferrimagnetic greigite (Fe3O4) and iron pyrite (FeS2) in a magnetotactic bacterium. Nature 343, 258–260 (1990).
McKay, C. P. Oxygen and the rapid evolution of life on Mars. In Chemical Evolution: Physics of the Origin and Evolution of Life, eds. Chela-Flores J. & François R. (New York, NNY: Springer, 1996: 177-18.
Nakamura, C., Kikuchi T., Burgess J. G. & Matsunaga T. An iron-regulated gene, magA, encoding an iron transport protein of Magnetospirillum sp. strain AMB-1. Journal of Biological Chemistry 270, 28392–28396 (1995).
Neilands, J. B. Siderophores: Structure and Function of Microbial Iron Transport Compounds. Journal of Biological Chemistry 270, 26723–26726 (1995).
Paoletti, L. C. & Blakemore, R. P. Hydroxamate production by Aquaspirillum magnetotacticum. Journal of Bacteriology 167, 73–76 (1986).
Schübbe, S. et al. Characterization of a spontaneous nonmagnetic mutant of Magnetospirillum gryphiswaldense reveals a large deletion comprising a putative magnetosome island. Journal of Bacteriology 185, 5779–5790 (2003).
Schüler, D. Formation of magnetosomes in magnetotactic bacteria. Journal of Molecular Microbiology and Biotechnology 1, 79–86 (1999).
Spormann A. M. & Wolfe R. S. Chemotactic, magnetotactic and tactile behavior in a magnetic spirillum. FEMS Microbiology Letters 22,171–177 (1984).
Thomas-Keprta K. L. et al. Magnetofossils from ancient mars: a robust biosignature in the martian meteorite ALH84001. Applied and Environmental Microbiology 68, 3663–3672 (2002).