« Prev Next »
Introduction
Plant growth and development largely depend on the combination and concentration of mineral nutrients available in the soil. Plants often face significant challenges in obtaining an adequate supply of these nutrients to meet the demands of basic cellular processes due to their relative immobility. A deficiency of any one of them may result in decreased plant productivity and/or fertility. Symptoms of nutrient deficiency may include stunted growth, death of plant tissue, or yellowing of the leaves caused by a reduced production of chlorophyll, a pigment needed for photosynthesis. Nutrient deficiency can have a significant impact on agriculture, resulting in reduced crop yield or reduced plant quality. Nutrient deficiency can also lead to reduced overall biodiversity since plants serve as the producers that support most food webs.
Changes in the climate and atmosphere can have serious effects on plants, including changes in the availability of certain nutrients. In a world of continual global climate change, it is important to understand the strategies that plants have evolved to allow them to cope with some of these obstacles.
Two classes of nutrients are considered essential for plants: macronutrients and micronutrients. Macronutrients are the building blocks of crucial cellular components like proteins and nucleic acids; as the name suggests, they are required in large quantities. Nitrogen, phosphorus, magnesium, and potassium are some of the most important macronutrients. Carbon, hydrogen, and oxygen are also considered macronutrients as they are required in large quantities to build the larger organic molecules of the cell; however, they represent the non-mineral class of macronutrients. Micronutrients, including iron, zinc, manganese, and copper, are required in very small amounts. Micronutrients are often required as cofactors for enzyme activity.
Mineral nutrients are usually obtained from the soil through plant roots, but many factors can affect the efficiency of nutrient acquisition. First, the chemistry and composition of certain soils can make it harder for plants to absorb nutrients. The nutrients may not be available in certain soils, or may be present in forms that the plants cannot use. Soil properties like water content, pH, and compaction may exacerbate these problems.
Second, some plants possess mechanisms or structural features that provide advantages when growing in certain types of nutrient limited soils. In fact, most plants have evolved nutrient uptake mechanisms that are adapted to their native soils and are initiated in an attempt to overcome nutrient limitations. One of the most universal adaptations to nutrient-limited soils is a change in root structure that may increase the overall surface area of the root to increase nutrient acquisition or may increase elongation of the root system to access new nutrient sources. These changes can lead to an increase in the allocation of resources to overall root growth, thus resulting in greater root to shoot ratios in nutrient-limited plants (Lopez-Bucio et al., 2003).
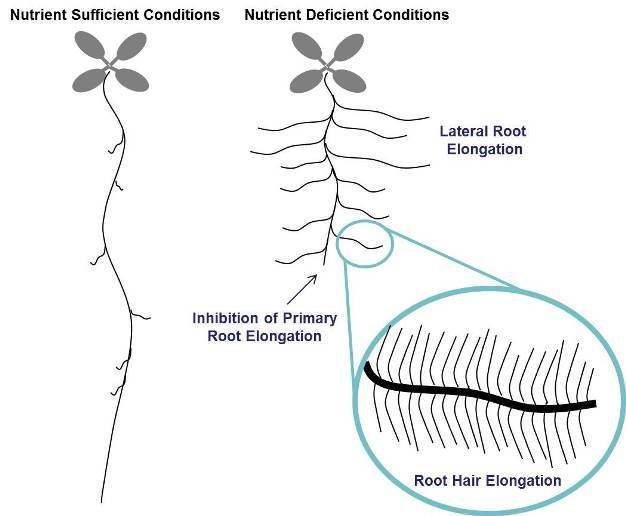
Plants are known to show different responses to different specific nutrient deficiencies and the responses can vary between species. As shown in Figure 1, the most common changes are inhibition of primary root growth (often associated with P deficiency), increase in lateral root growth and density (often associated with N, P, Fe, and S deficiency) and increase in root hair growth and density (often associated with P and Fe deficiency).
While nutrient deficiencies can pose serious threats to plant productivity, nutrients can become toxic in excess, which is also problematic. When some micronutrients accumulate to very high levels in plants, they contribute to the generation of reactive oxygen species (ROS), which can cause extensive cellular damage. Some highly toxic elements like lead and cadmium cannot be distinguished from essential nutrients by the nutrient uptake systems in the plant root, which means that in contaminated soils, toxic elements may enter the food web via these nutrient uptake systems, causing reduced uptake of the essential nutrient and significantly reduced plant growth and quality.
In order to maintain nutrient homeostasis, plants must regulate nutrient uptake and must respond to changes in the soil as well as within the plant. Thus, plant species utilize various strategies for mobilization and uptake of nutrients as well as chelation, transport between the various cells and organs of the plant and storage to achieve whole-plant nutrient homeostasis. Here, we briefly describe a few examples of strategies used by plants to acquire nutrients from the soil.
Plant Acquisition of Nutrients: Direct Uptake from the Soil
Potassium. Potassium (K) is considered a macronutrient for plants and is the most abundant cation within plant cells. Potassium has a number of important functions within plants, including balancing the charges of cellular anions, enzyme activation, control of stomatal opening/closing and serving as an osmoticum for cellular growth.
Potassium deficiency occurs frequently in plants grown on sandy soils resulting in a number of symptoms including browning of leaves, curling of leaf tips and yellowing (chlorosis) of leaves, as well as reduced growth and fertility.
Potassium uptake processes have been the subject of intense study for several decades. Early studies indicated that plants utilize both high and low affinity transport systems to directly acquire potassium from the soil. Low affinity transport systems generally function when potassium levels in the soil are adequate for plant growth and development. This process is mediated by ion channels in the plasma membrane of root cells, allowing passive transport of K+ from areas of relatively high external concentration into the plant cells where the concentration of K+ is lower. The expression of these low affinity transporters does not appear to be significantly affected by potassium availability.
Under conditions of potassium limitation, in contrast, plants usually induce high affinity K+ transport systems. There are likely many proteins involved in high affinity potassium transport, but in Arabidopsis, two proteins have been identified as the most important transporters in this process. Interestingly, one of these transporters, AtHAK5, is a carrier protein and thought to mediate active transport of potassium into plant roots, whereas the other protein, AKT1, is a channel protein and likely mediates a passive transport mechanism with an increased affinity for K+ under conditions of potassium limitation (Pyo et al., 2010). More recent work shows that plants contain a number of different transport systems to acquire potassium from the soil and distribute it within the plants. Although much remains to be learned about potassium uptake and translocation in plants, it is clear that the mechanisms involved are complex and tightly controlled to allow the plant to acquire sufficient amounts of potassium from the soil under varying conditions.
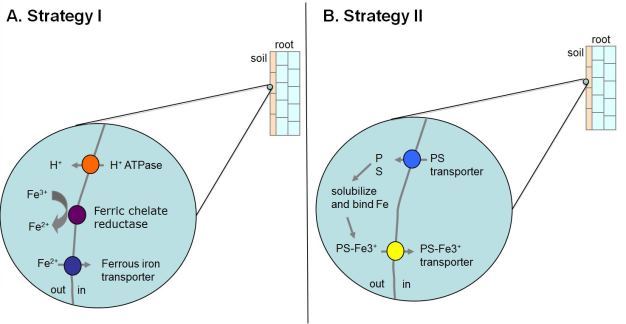
Iron. Iron is essential for plant growth and development and is required as a cofactor for proteins that are involved in a number of important metabolic processes including photosynthesis and respiration. Despite the fact that iron is the fourth most abundant element in the earth's crust, it is often limiting for plants due to the fact that it tends to form insoluble complexes in aerobic soils of neutral to basic pH (Guerinot & Yi, 1994). It is thought that iron limitation is a problem for plants on as much as 30% of soils worldwide. Iron-deficient plants often display interveinal chlorosis, in which the veins of the leaf remain green while the areas between the veins are yellow (Figure 2). Due to the limited solubility of iron in many soils, plants often must first mobilize iron in the rhizosphere (a region of the soil that surrounds, and is influenced by, the roots) before transporting it into the plant. Two distinct mechanisms have evolved that are utilized by plants to acquire iron from the soil, termed the Strategy I and Strategy II responses (Connolly & Walker, 2008).

In contrast, the grasses utilize strategy II to acquire iron under conditions of iron limitation (Figure 3B). Following the imposition of iron limitation, strategy II species begin to synthesize special molecules called phytosiderophores (PSs) that display high affinity for ferric iron. PSs are secreted into the rhizosphere where they bind tightly to ferric iron. Finally, the PS-ferric iron complexes are transported into root cells by PS-Fe(III) transporters. Interestingly, while both strategies are relatively effective at allowing plants to acquire iron from the soil, the strategy II response is thought to be more efficient because grass species tend to grow better in calcareous soils (which have a high pH and thus have limited iron available for uptake by plants).
Plant Acquisition of Nutrients: Symbioses with Soil-based Microorganisms
Nitrogen Fixation. Despite the fact that nitrogen is the most abundant gaseous element in the atmosphere, plants are unable to utilize the element in this form (N2) and may experience nitrogen deficiency in some soils that have low nitrogen content. Since nitrogen is a primary component of both proteins and nucleic acids, nitrogen deficiency imposes significant limitations to plant productivity. In an agricultural setting, nitrogen deficiency can be combated by the addition of nitrogen-rich fertilizers to increase the availability of nutrients and thereby increase crop yield. However, this can be a dangerous practice since excess nutrients generally end up in ground water, leading to eutrophication and subsequent oxygen deprivation of connected aquatic ecosystems.
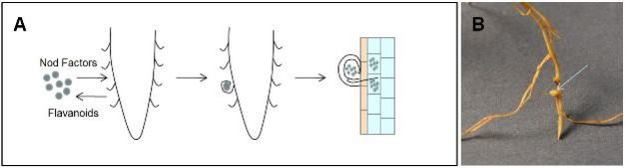
Plants are able to directly acquire nitrate and ammonium from the soil. However, when these nitrogen sources are not available, certain species of plants from the family Fabaceae (legumes) initiate symbiotic relationships with a group of nitrogen fixing bacteria called Rhizobia. These interactions are relatively specific and require that the host plant and the microbe recognize each other using chemical signals. The interaction begins when the plant releases compounds called flavanoids into the soil that attract the bacteria to the root (Figure 4). In response, the bacteria release compounds called Nod Factors (NF) that cause local changes in the structure of the root and root hairs. Specifically, the root hair curls sharply to envelop the bacteria in a small pocket. The plant cell wall is broken down and the plant cell membrane invaginates and forms a tunnel called an infection thread that grows to the cells of the root cortex. The bacteria become wrapped in a plant derived membrane as they differentiate into structures called bacteroids. These structures are allowed to enter the cytoplasm of cortical cells where they convert atmospheric nitrogen to ammonia, a form that can be used by the plants. In return, the bacteroids receive photosynthetically derived carbohydrates to use for energy production (reviewed by Limpens & Bisseling, 2003; Ferguson et al. 2010).
Mycorrhizal interactions with plants.
The most common endomycorrhizal interaction occurs between arbuscular mycorrhizal fungi (AMF; also called Vesicular-Arbuscular Mycorrhiza or VAM) and a variety of species of grasses, herbs, trees and shrubs. When phosphate is available in the soil, plants are able to acquire it directly via root phosphate transporters. However, under low phosphate conditions, plants become reliant on interactions with mycorrhizal fungi for phosphorus acquisition. Mycorrhizal spores present in the soil are germinated by compounds released from the plant. Hyphae extend from the germinating spore and penetrate the epidermis of the plant root. Inside the root, the hyphae branch and penetrate cortical cells, where highly branched structures called arbuscules develop (Figure 5). Externally, hyphae extend into the soil beyond the area accessible to the root. This kind of symbiosis facilitates plant phosphorus uptake from the soil by increasing the root's absorptive surface area. Since plants take up phosphorus at a much higher rate than phosphorus diffuses into the soil surrounding the root, a phosphorus depletion zone is quickly established, limiting uptake of phosphorus by the plant. However, AMF hyphae form a bridge between the internal root environment and the area beyond this depletion zone enabling the plant to acquire significantly more phosphorus through its symbiotic partner than it could on its own (reviewed by Karandashov & Bucher 2005).
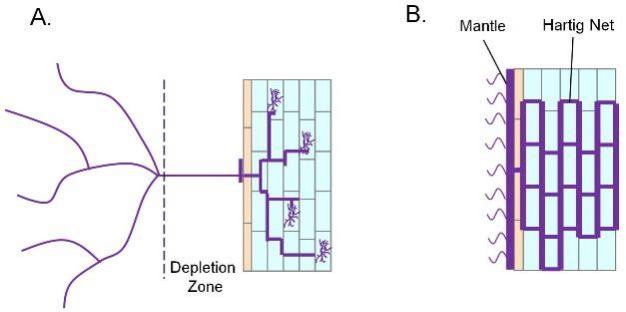
Ectomycorrhizal fungi (EcM) form associations with many tree species (birch, oak, spruce, pine, fir), making these microorganisms an important component of the biodiversity found in forest ecosystems, particularly temperate and boreal forests in the northern hemisphere. Although the molecular mechanisms underlying initiation of EcM-plant interactions are not well defined, it is clear that signaling molecules are released by both symbiotic partners, resulting in fungal hyphae growth towards compatible plant roots. The EcM forms an extensive hyphal sheath, or mantle, around the root cap and a hartig net of hyphae that extends into the root itself and surrounds the cells within the root cortex. Hyphae from the mantle extend into the surrounding soil, providing availability and storage of nutrients like phosphorus and nitrogen for the plant. This is particularly important for trees growing in forest soils because a large portion of the nutrients available are located in the litter layer and are not accessible to the roots of these trees. The EcM produce enzymes that can digest this organic material and also have the ability to mobilize the nutrients to the hartig net, making them available to the plant. In this way, the interaction between trees and EcM is not only beneficial, but in many cases, is essential (reviewed by Nehls et al., 2008).
As is the case with bacterial symbioses, the benefit to the fungal partners in these relationships involves transfer of photosynthetic carbohydrates from the plant to the microorganism for metabolism and energy production. However, the nutritional advantage for plants of such symbiotic relationships far outweighs the energetic cost imposed by hosting microorganisms like rhizobacteria, AMF, and EcM.
Summary
References and Recommended Reading
Beyer P. Golden Rice and "Golden" crops for human nutrition. New Biotechnology 27, 478-481 (2010).
Britto, D. T. & H. J. Kronzucker. Cellular mechanisms of potassium transport in plants. Physiologia Plantarum 133, 637-650 (2008).
Connolly, E. L. & E. L. Walker. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Current Opinion in Plant Biology 11, 530-535 (2008).
Ferguson B. J. et al. Molecular Analysis of Legume Nodule Development and Autoregulation. Journal of Integrative Plant Biology 52, 61-76 (2010).
Graham L., Graham J. & L. Wilcox. Plant Biology. Upper Saddle River, NJ: Pearson Prentice Hall, 2006.
Guerinot M. L. & Y. Yi. Iron: Nutritious, Noxious and Not Readily Available. Plant Physiology 104, 815-820 (1994).
Hell R. & H. Hillebrand. Plant concepts for mineral acquisition and allocation. Current Opinion in Biotechnology 12, 161-168 (2001).
Jones B. & K. Ljung. Subterranean space exploration: the development of root system architecture. Current Opinion in Plant Biology 15, 97-102 (2012).
Karandashov V. & M. Bucher. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends in Plant Science 10, 22-29 (2005).
Lopez-Bucio J. et al. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology 6, 280-287 (2003).
Limpens E. & T. Bisseling. Signaling in symbiosis. Current Opinion in Plant Biology 6, 343-350 (2003).
Nehls U. et al. Sugar for my honey: Carbohydrate partitioning in ectomycorrhizal symbiosis. Phytochemistry 68, 82-91 (2007).
Nehls U. Mastering ectomycorrhizal symbiosis: the impact of carbohydrates. Journal of Experimental Botany 59, 1097-1108 (2008).
Pyo Y. J. et al. High Affinity K+ Transport in Arabidopsis: AtHAK5 and AKT1 are Vital for Seedling Establishment and Postgermination Growth under Low-Potassium Conditions. Plant Physiology 153, 863-875 (2010).
Sprent J. 60Ma of legume nodulation. What's new? What's changing? Journal of Experimental Botany 59, 1081-1084 (2008).
Vance C. Symbiotic Nitrogen Fixation and Phosphorus Acquisition. Plant Nutrition in a World of Declining Renewable Resources. Plant Physiology 127, 390-397 (2001).
Very, A. & H. Sentenac. Molecular Mechanisms and Regulation of K+ Transport in Higher Plants. Annual Review Plant Biology 54, 575-603 (2003).































