« Prev Next »

Sunlight is the ultimate energy source for virtually all life on Earth. This energy is harnessed by primary producers, organisms capable of using solar energy in photosynthesis, to create energy-rich sugars and sugar-derived molecules as the basis of the Earth's supply of food, fuels, and materials like wood and cotton. Photosynthetic organisms are also the source of all free oxygen, a molecule essential to almost all life. Primary producers come in all forms and sizes, from photosynthetic microbes to giant algae and plants, and populate both oceans and land.
Harnessing solar energy, however, is hazardous, and presents a formidable challenge to photosynthetic organisms. In fact, un-utilized light energy (any energy not converted to stable products like sugars) has the potential to destroy the light-collecting system itself. Moreover, such un-utilized energy can also destroy other major cell components and swiftly kill the whole cell. How? In photosynthesis, a light-absorbing pigment (typically chlorophyll) becomes energized after absorbing a photon of light (Figure 1). This energized state is what ultimately supplies the energy for the synthesis of energy-rich sugars. However, when more light is absorbed than can be utilized for sugar production, the (un-utilized, or excess) excitation energy can instead be passed off to oxygen. The resulting highly reactive oxygen has the potential to destroy the photosynthetic system and lead to cell death. Photosynthesis as we know it could therefore not exist (in an oxygen-containing atmosphere) without powerful photo-protective mechanisms, protecting against potential damage by un-utilized light energy.
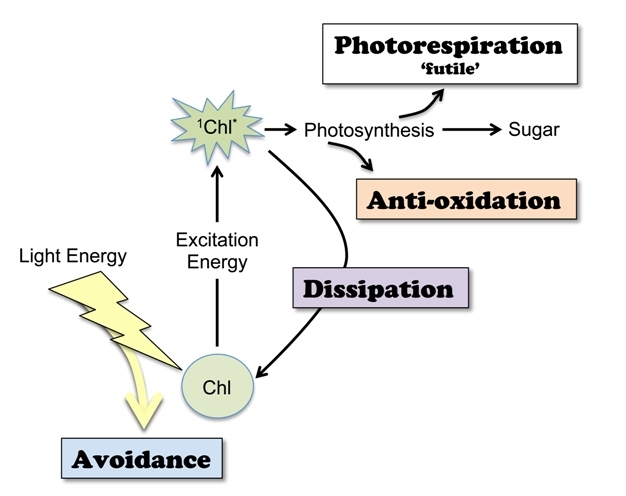
Alternative Utilization of Absorbed Light Energy
Even when the plant is not actively growing or storing products of photosynthesis, routes exist for the "utilization" of absorbed light energy through energy-consuming biochemical pathways that do not lead to sugar production. These paths are "futile" in that they do not generate energy-rich molecules for metabolism and growth, but they do fulfill a useful function in that they provide a pathway to get rid of potentially destructive excess excitation energy under mild stress. Under harsher conditions, different photo-protective mechanisms (see below), not based on biochemical reactions that may be inhibited by severe stress, are needed.
Cutting Down on Light Absorption
An obvious option for avoiding damage by excess light is to decrease the actual absorption of sunlight. This can be accomplished by a wide variety of approaches. In plants, light absorption can be lowered at the leaf level, either by growth of the leaf at a steep angle (Figure 2b) or by rapid leaf movements displayed by certain plant species. The latter movements expose the leaf to the sun when light energy can be fully utilized in photosynthesis, and turn the leaf away from the sun when light absorption exceeds light utilization. Chlorophyll-containing structures (chloroplasts) can also be spread out along the tops of cells to increase light absorption, or instead be pressed against the sides of cells to minimize absorption when there is too much light (Figure 2c). Another option is to reflect sunlight striking the leaf by virtue of light-reflecting leaf hairs, wax, or even a layer of white salt. Moreover, at the level of molecules, the plant can synthesize more or less of the photosynthetic pigments themselves as well as of the proteins that bind and display these (Figure 3). An extension of this conceptual approach is employed by long-lived plants that drop their leaves altogether for an entire harsh season — a cold winter or a scorching hot, dry summer. However, these absorption-lowering strategies are not universally employed by plants because safety (no leaves, no damage) must be balanced against the need for photosynthesis and growth. Most plants do maintain light-harvesting structures that periodically absorb more light than photosynthesis can utilize, and simply remove the excess energy (see below). As an extreme example, slowly growing plants in subalpine regions and in deserts are often evergreen (maintain green leaves!) and rely heavily on the harmless removal of much of the absorbed energy.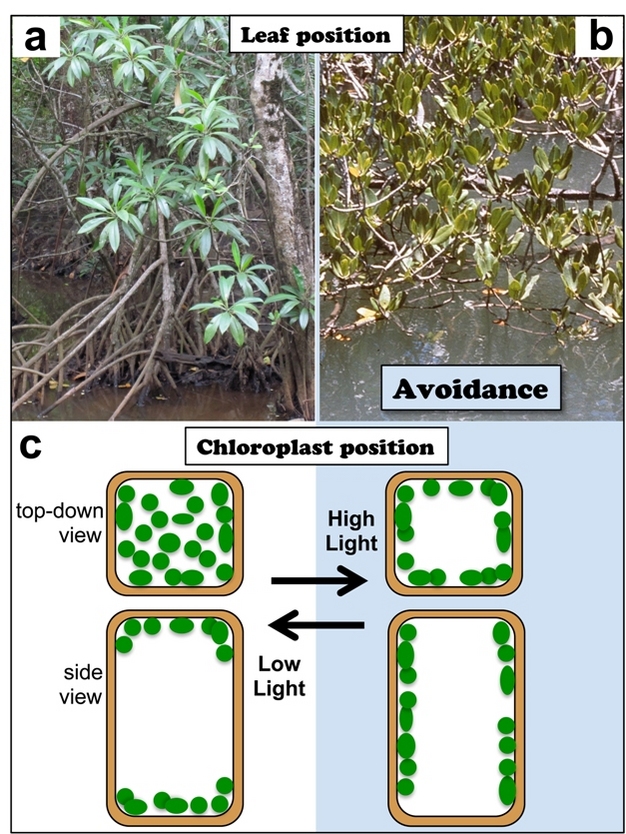

Harmless Dissipation of Excess Absorbed Light Directly in the Chloroplast
Absorption of a photon of sunlight propels photosynthetic pigments into their excited state, from which un-utilized energy is readily transferred to oxygen. Such a transfer to oxygen can be prevented by swift de-excitation of the excited pigment, whereby the excitation energy is harmlessly removed as thermal (heat) energy. All plants and photosynthetic algae regularly employ photo-protective thermal dissipation of excess absorbed light. In sunny environments-without any additional stress-plants smoothly and elegantly reconfigure their solar-collector pigment complexes every day, from a state favoring efficient light utilization in the low-light morning hours to ever-increasing levels of efficient thermal dissipation of un-utilized energy towards peak light intensities at noon (Figure 4). Even the fastest growing, most rapidly photosynthesizing crops and weeds dissipate about half of the sunlight they absorb at peak intensity. For more slowly growing evergreen plants, the fraction of dissipated light is even higher, often as much as 90–95% at peak intensity. Under the influence of environmental stresses, thermal dissipation of absorbed, but un-utilized, light changes from this latter pattern, of only being turned on for a few hours a day, to staying at high levels all day long (Figure 4). The greenness of over-wintering needles of conifers (Figure 5) betrays the fact that, in harsh environments with continuously frozen soils, 100% of the absorbed light is dissipated via photo-protective thermal dissipation throughout the winter. None of the absorbed light is utilized for photosynthesis in these over-wintering trees that stop growing and photosynthesizing altogether. The reward for the strategy of keeping green leaves is the almost instant re-activation of photosynthesis (and discontinuation of thermal dissipation) in these evergreens when soils thaw in the spring, and when deciduous trees labor for a month to re-grow their leaves.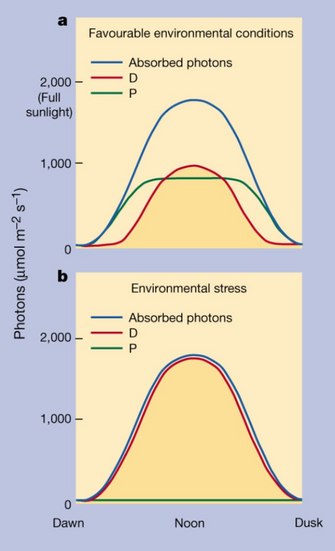

A Peek at Dissipation Mechanisms
The key mechanism for thermal dissipation involves a group of yellow (xanthophyll) pigments, specific ones of which facilitate the harmless dissipation of excess energy from photosynthesis' light-collecting pigments (Figure 6). In sunny, low-stress environments, leaves contain a non-dissipating xanthophyll in the morning and gradually convert this to a closely related xanthophyll, the main dissipater zeaxanthin, by midday. The plant species (those with the lowest photosynthesis rates) featuring the highest zeaxanthin levels dissipate the most light. On the other hand, evergreen plants subjected to environmental stresses (that lower photosynthesis rates and increase excess light levels) arrest their xanthophyll conversions and keep high levels of zeaxanthin (and thermal dissipation) in place all day long. Thermal dissipation is not activated by the mere presence of zeaxanthin, but requires an additional trigger for the actual engagement of dissipation. In over-wintering conifers in frozen soils, zeaxanthin levels and dissipation engagement remain high all winter. In contrast, leaves under the canopy of a rainforest-intermittently pierced by intense shafts of sunlight-keep zeaxanthin around all day, instantly engage thermal dissipation during each sunfleck, and then bounce back to efficient utilization of the low background level of light for photosynthesis.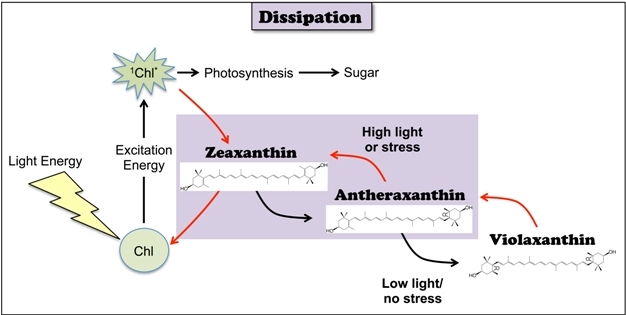
Zeaxanthin-facilitated thermal dissipation of excess energy appears to require the least investment of metabolic energy to put and keep in place (and never more so than when long-term retention of high zeaxanthin levels is involved) compared to other photo-protective mechanisms, and this may be the reason why the former is so ubiquitously employed. At the same time, the de-excitation of the very same state of chlorophyll otherwise used to drive photosynthesis potentially cuts into the efficiency of photosynthetic light utilization, thus warranting the multi-level fine-tuning and control of its engagement in harmless dissipation. Remarkably, zeaxanthin also plays a key role in the protection of the human/animal eye against damage by intense light. In contrast to plants, which synthesize as much zeaxanthin as they need, animals do not possess the ability to synthesize zeaxanthin, and instead depend on their diet for supplying this critical protector. In addition to serving as a photo-protector, zeaxanthin also functions as a multi-purpose antioxidant that can detoxify reactive oxygen and its derivates in both plants and animals. In humans, zeaxanthin likely plays a role in protection against a number of chronic diseases.
Detoxification of Reactive Oxygen Formed
While thermal dissipation of energy is instrumental in preventing the formation of reactive oxygen, additional photo-protective mechanisms exist to detoxify any reactive oxygen that is formed (Figure 7). In a series of steps, reactive oxygen is rendered non-reactive and ultimately converted to harmless H2O or O2. While some of these processes can proceed in metabolically inactive leaves, others depend on metabolic energy and on enzymes, whose availability and function may be diminished under severe stress. All photosynthetic organisms employ photo-protective detoxification, but the latter is not the main route for excess energy removal. This may be due in part to the energy cost of detoxification and in part to the fact that removal of already formed reactive oxygen is not as pro-active and "safe" as the removal of excess energy by thermal dissipation before reactive oxygen is formed.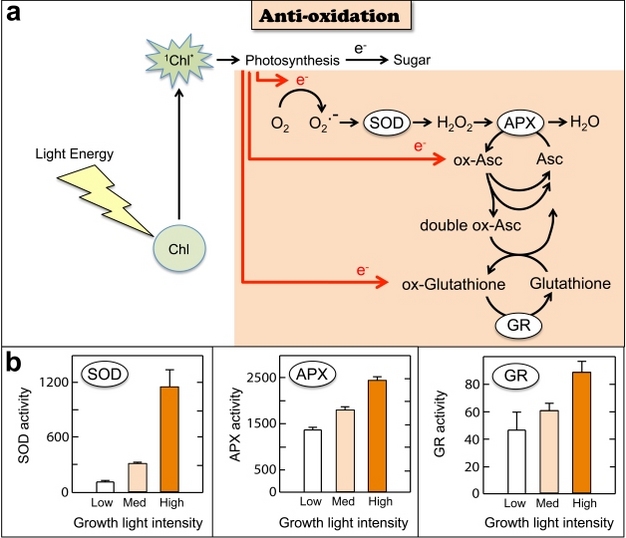
Each Environmental Challenge Begets its Own Specific Photo-Protective Solution
The existence of a multitude of alternative or concurrent photo-protective processes to make the absorption of sunlight for photosynthesis safe underlines the critical importance of photo-protection. However, this suite of multiple photo-protective mechanisms does not merely constitute redundant layers of safety nets, but occurs in nature in varied combinations that meet the distinct challenges presented by each specific environment as well as by the needs of plant species with contrasting life spans and growth rates.Not surprisingly, all photosynthetic organisms possess a suite of formidable photo-protective mechanisms that are used on a daily basis. However, among different plant species, the exact combination of photo-protective mechanisms employed varies tremendously depending on-for example-growth rate and growth pattern. For instance, a fast-growing, short-lived annual (with a high rate of photosynthesis) requires less photo-protection compared to a slow-growing, long-lived evergreen (with a low rate of photosynthesis). In a nutshell, the lower the photosynthesis rate of a plant, the greater its need for photo-protection. Moreover, the magnitude of photo-protection needed varies greatly depending on the environment. Favorable environmental conditions-for example, those with plenty of water and nutrients for plant growth-favor high photosynthesis rates and thereby maximize utilization of absorbed sunlight in photosynthesis. In contrast, drought, nutrient deficiency, excessive heat or cold, and many other unfavorable conditions or stresses, slow plant growth and lower the rate of photosynthesis, and thereby increase the fraction of absorbed light that remains un-utilized.
References and Recommended Reading
Adams, W. W. III. & Demmig-Adams, B. "Photoprotection (Plants)." McGraw-Hill Yearbook of Science & Technology 2012, 11 (13). (New York, NY: McGraw-Hill, 2012) 490-493.
Adams, W. W. III. et al. Photoprotective strategies of overwintering evergreens. BioScience 54, 41-49 (2004).
Apel, K. & Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Reviews of Plant Biology 55, 373-399 (2004).
Demmig-Adams, B. & Adams, W. W. III. Harvesting sunlight safely. Nature 403, 371-374 (2000).
———. Antioxidants in photosynthesis and human nutrition. Science 298, 2149-2153 (2002).
———. Photoprotection in an ecological context: The remarkable complexity of thermal dissipation. New Phytologist 172, 11-21 (2006).
Demmig-Adams, B., Adams, W. W. III. & Mattoo, A. K. eds. Photoprotection, Photoinhibition, Gene Regulation, and Environment. Advances in Photosynthesis and Respiration, 21. Dordrecht, Netherlands: Springer, 2006.
Demmig-Adams, B., Rixham, C. S. & Adams, W. W. III. "Carotenoids," in McGraw-Hill Yearbook of Science & Technology 2012 11 (3) (New York, NY: McGraw-Hill, 2012) 549-555.
Foyer, C. H. & Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxidants & Redox Signaling 11, 861-905 (2009).
Grace, S. C. & Logan, B. A. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiology 112, 1631-1640 (1996).
Kashahara, M. et al. Chloroplast avoidance movement reduces photodamage in plants. Nature 420, 829-832 (2002).
Niyogi, K. K. Photoprotection revisited: Genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology 50, 333-359 (1999).
Niyogi, K. K. Safety valves for photosynthesis. Current Opinion in Plant Biology 3, 455-460 (2000).
van Zanten, M. et al. On the relevance and control of leaf angle. Critical Reviews in Plant Sciences 29, 300-316 (2010).
Zhirong, L. et al. Sensing and responding to excess light. Annual Review of Plant Biology 60, 239-260 (2009).






























