Key Points
-
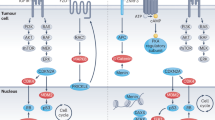
The available genomic data on adrenocortical tumours come from transcriptome (mRNA expression), miRNome (microRNA expression) and methylome (DNA methylation) analyses, as well as from comparative genomic hybridization and single nucleotide polymorphisms arrays
-
Genomic studies performed to date indicate that malignant and benign adrenocortical tumours can be discriminated using molecular tools, including profiling of mRNA expression, microRNA expression or of chromosomal alterations
-
Using transcriptome or methylome data, classification algorithms can identify subtypes of adrenocortical tumours that are associated with different outcomes; thus, these molecular features can help to determine prognosis
-
Large-scale clinical studies are needed to determine the part that molecular analyses should play in clinical practice; international collaborative research networks should enable recruitment of adequate cohorts in the near future
-
To improve personalization of prognostication and therapy, a detailed molecular classification of adrenocortical tumours should be developed through integration of data from wide-ranging and complementary 'omics' studies, particularly exome and whole-genome sequencing
Abstract
Pan-genomic analyses of genetic and epigenetic alterations and gene expression profiles are providing important new insights into the pathogenesis and molecular classification of cancers. The technologies and methods used for these studies are rapidly diversifying and improving. The use of such methodologies for the analysis of adrenocortical tumours has revealed clear transcriptomic (mRNA and microRNA expression profiles), epigenomic (DNA methylation profiles) and genomic (DNA mutations and chromosomal alterations) differences between benign and malignant tumours. Interestingly, genomic studies of adrenal cancers have also identified subtypes of malignant tumours, which demonstrate distinct patterns of molecular alterations and are associated with different clinical outcomes. These discoveries have created the opportunity for classifying adrenocortical tumours on the basis of molecular analyses. Following these genomic studies, efforts to develop new molecular tools that improve diagnosis and prognostication of patients with adrenocortical tumours have also been made. This Review describes the progress that has been made towards classification of adrenocortical tumours to date based on key genomic approaches. In addition, the potential for the development and use of various molecular tools to personalize the management of patients with adrenocortical tumours is discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Giordano, T. J. et al. Distinct transcriptional profiles of adrenocortical tumours uncovered by DNA microarray analysis. Am. J. Pathol. 162, 521–531 (2003).
de Fraipont, F. et al. Gene expression profiling of human adrenocortical tumours using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J. Clin. Endocrinol. Metab. 90, 1819–1829 (2005).
Velázquez-Fernández, D. et al. Expression profiling of adrenocortical neoplasms suggests a molecular signature of malignancy. Surgery 138, 1087–1094 (2005).
Slater, E. P. et al. Analysis by cDNA microarrays of gene expression patterns of human adrenocortical tumours. Eur. J. Endocrinol. 154, 587–598 (2006).
Lombardi, C. P. et al. Gene expression profiling of adrenal cortical tumours by cDNA macroarray analysis. Results of a preliminary study. Biomed. Pharmacother. 60, 186–190 (2006).
Fernandez-Ranvier, G. G. et al. Candidate diagnostic markers and tumour suppressor genes for adrenocortical carcinoma by expression profile of genes on chromosome 11q13. World J. Surg. 32, 873–881 (2008).
Fernandez-Ranvier, G. G. et al. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch. Surg. 143, 841–846 (2008).
Tömböl, Z. et al. Integrative molecular bioinformatics study of human adrenocortical tumours: microRNA, tissue-specific target prediction, and pathway analysis. Endocr. Relat. Cancer 16, 895–906 (2009).
Soon, P. S. et al. Microarray gene expression and immunohistochemistry analyses of adrenocortical tumours identify IGF2 and Ki-67 as useful in differentiating carcinomas from adenomas. Endocr. Relat. Cancer 16, 573–583 (2009).
Giordano, T. J. et al. Molecular classification and prognostication of adrenocortical tumours by transcriptome profiling. Clin. Cancer Res. 15, 668–676 (2009).
De Reyniès, A. et al. Gene expression profiling reveals a new classification of adrenocortical tumours and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 27, 1108–1115 (2009).
Laurell, C. et al. Transcriptional profiling enables molecular classification of adrenocortical tumours. Eur. J. Endocrinol. 161, 141–152 (2009).
Zhao, J. et al. Combined comparative genomic hybridization and genomic microarray for detection of gene amplifications in pulmonary artery intimal sarcomas and adrenocortical tumours. Genes Chromosomes Cancer 34, 48–57 (2002).
Stephan, E. A. et al. Adrenocortical carcinoma survival rates correlated to genomic copy number variants. Mol. Cancer Ther. 7, 425–431 (2008).
Szabó, P. M. et al. Meta-analysis of adrenocortical tumour genomics data: novel pathogenic pathways revealed. Oncogene 29, 3163–3172 (2010).
Barreau, O. et al. Clinical and pathophysiological implications of chromosomal alterations in adrenocortical tumours: an integrated genomic approach. J. Clin. Endocrinol. Metab. 97, E301–E311 (2012).
Rechache, N. S. et al. DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumours. J. Clin. Endocrinol. Metab. 97, E1004–E1013 (2012).
Fonseca, A. L. et al. Comprehensive DNA methylation analysis of benign and malignant adrenocortical tumours. Genes Chromosomes Cancer 51, 949–960 (2012).
Barreau, O. et al. Identification of a CpG island methylator phenotype in adrenocortical carcinomas. J. Clin. Endocrinol. Metab. 98, E174–E184 (2013).
Soon, P. S. et al. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin. Cancer Res. 15, 7684–7692 (2009).
Patterson, E. E., Holloway, A. K., Weng, J., Fojo, T. & Kebebew, E. MicroRNA profiling of adrenocortical tumours reveals miR-483 as a marker of malignancy. Cancer 117, 1630–1639 (2011).
Schmitz, K. J. et al. Differential expression of microRNA-675, microRNA-139-3p and microRNA-335 in benign and malignant adrenocortical tumours. J. Clin. Pathol. 64, 529–535 (2011).
Özata, D. M. et al. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr. Relat. Cancer 18, 643–655 (2011).
Chabre, O. et al. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr. Relat. Cancer 20, 579–594 (2013).
Ronchi, C. L. et al. Single nucleotide polymorphism array profiling of adrenocortical tumours—evidence for an adenoma carcinoma sequence? PLoS ONE 8, e73959 (2013).
De Martino, M. C. et al. Molecular screening for a personalized treatment approach in advanced adrenocortical cancer. J. Clin. Endocrinol. Metab. 98, 4080–4088 (2013).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Van 't Veer, L. J. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
Bhattacharjee, A. et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl Acad. Sci. USA 98, 13790–13795 (2001).
Garber, M. E. et al. Diversity of gene expression in adenocarcinoma of the lung. Proc. Natl Acad. Sci. USA 98, 13784–13789 (2001).
Beer, D. G. et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat. Med. 8, 816–824 (2002).
Bittner, M. et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406, 536–540 (2000).
Singh, D. et al. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell 1, 203–209 (2002).
Alizadeh, A. A. et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000).
Shipp, M. A. et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat. Med. 8, 68–74 (2002).
Golub, T. R. et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286, 531–537 (1999).
Gruvberger, S. et al. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 61, 5979–5984 (2001).
Gelmon, K. et al. Targeting triple-negative breast cancer: optimising therapeutic outcomes. Ann. Oncol. 23, 2223–2234 (2012).
McDermott, U., Downing, J. R. & Stratton, M. R. Genomics and the continuum of cancer care. N. Engl. J. Med. 364, 340–350 (2011).
Garraway, L. A. Genomics-driven oncology: framework for an emerging paradigm. J. Clin. Oncol. 31, 1806–1814 (2013).
Gordon, G. J. et al. Identification of novel candidate oncogenes and tumour suppressors in malignant pleural mesothelioma using large-scale transcriptional profiling. Am. J. Pathol. 166, 1827–1840 (2005).
Phillips, H. S. et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157–173 (2006).
Remke, M. et al. Adult medulloblastoma comprises three major molecular variants. J. Clin. Oncol. 29, 2717–2723 (2011).
Burnichon, N. et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 20, 3974–3985 (2011).
Ribeiro, R. C. et al. The International Pediatric Adrenocortical Tumour Registry initiative: contributions to clinical, biological, and treatment advances in pediatric adrenocortical tumours. Mol. Cell. Endocrinol. 351, 37–43 (2012).
Bertherat, J., Mosnier-Pudar, H. & Bertagna, X. Adrenal incidentalomas. Curr. Opin. Oncol. 14, 58–63 (2002).
Grumbach, M. M. et al. Management of the clinically inapparent adrenal mass ('incidentaloma'). Ann. Intern. Med. 138, 424–429 (2003).
Libè, R., Fratticci, A. & Bertherat, J. Adrenocortical cancer: pathophysiology and clinical management. Endocr. Relat. Cancer 14, 13–28 (2007).
Abiven, G. et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumours in a series of 202 consecutive patients. J. Clin. Endocrinol. Metab. 91, 2650–2655 (2006).
Fassnacht, M. et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 115, 243–250 (2009).
Assié, G. et al. Prognostic parameters of metastatic adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 92, 148–154 (2007).
Weiss, L. M. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumours. Am. J. Surg. Pathol. 8, 163–169 (1984).
Lau, S. K. & Weiss, L. M. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum. Pathol. 40, 757–768 (2009).
Tissier, F. et al. Adrenocortical tumours: improving the practice of the Weiss system through virtual microscopy: a National Program of the French Network INCa-COMETE. Am. J. Surg. Pathol. 36, 1194–1201 (2012).
Stojadinovic, A. et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J. Clin. Oncol. 20, 941–950 (2002).
Beuschlein, F. et al. Prognostic value of histological markers in localized adrenocortical carcinoma after complete resection. Presented at the 95th Annual Meeting of the Endocrine Society (San Francisco, 2013).
Sbiera, S. et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumours. J. Clin. Endocrinol. Metab. 95, E161–E171 (2010).
Gaujoux, S. et al. β-catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin. Cancer Res. 17, 328–336 (2011).
Gicquel, C. et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumours. Cancer Res. 61, 6762–6767 (2001).
Reincke, M. et al. p53 mutations in human adrenocortical neoplasms: immunohistochemical and molecular studies. J. Clin. Endocrinol. Metab. 78, 790–794 (1994).
Libè, R. et al. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clin. Cancer Res. 13, 844–850 (2007).
Suh, I., Guerrero, M. A. & Kebebew, E. Gene-expression profiling of adrenocortical carcinoma. Expert Rev. Mol. Diagn. 9, 343–351 (2009).
Bussey, K. J. & Demeure, M. J. Genomic and expression profiling of adrenocortical carcinoma: application to diagnosis, prognosis and treatment. Future Oncol. 5, 641–655 (2009).
Soon, P. S. & Sidhu, S. B. Molecular basis of adrenocortical carcinomas. Minerva Endocrinol. 34, 137–147 (2009).
Assié, G. et al. The pathophysiology, diagnosis and prognosis of adrenocortical tumours revisited by transcriptome analyses. Trends Endocrinol. Metab. 21, 325–334 (2010).
Giordano, T. J. Adrenocortical tumours: an integrated clinical, pathologic, and molecular approach at the University of Michigan. Arch. Pathol. Lab. Med. 134, 1440–1443 (2010).
Ragazzon, B., Assié, G. & Bertherat, J. Transcriptome analysis of adrenocortical cancers: from molecular classification to the identification of new treatments. Endocr. Relat. Cancer 18, R15–R27 (2011).
Assié, G., Giordano, T. J. & Bertherat, J. Gene expression profiling in adrenocortical neoplasia. Mol. Cell. Endocrinol. 351, 111–117 (2012).
Jain, M., Rechache, N. & Kebebew, E. Molecular markers of adrenocortical tumours. J. Surg. Oncol. 106, 549–556 (2012).
Lehmann, T. & Wrzesinski, T. The molecular basis of adrenocortical cancer. Cancer Genet. 205, 131–137 (2012).
Zsippai, A. et al. mRNA and microRNA expression patterns in adrenocortical cancer. Am. J. Cancer Res. 1, 618–628 (2011).
Fassnacht, M. et al. Update in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 98, 4551–4564 (2013).
Giordano, T. J. The argument for mitotic rate-based grading for the prognostication of adrenocortical carcinoma. Am. J. Surg. Pathol. 35, 471–473 (2011).
Fragoso, M. C. et al. Combined expression of BUB1B, DLGAP5, and PINK1 as predictors of poor outcome in adrenocortical tumours: validation in a Brazilian cohort of adult and pediatric patients. Eur. J. Endocrinol. 166, 61–67 (2012).
Lazar, C. et al. Batch effect removal methods for microarray gene expression data integration: a survey. Brief. Bioinform. 14, 469–490 (2013).
Feng, H. et al. Opportunities and methods for studying alternative splicing in cancer with RNA-Seq. Cancer Lett. 340, 179–191 (2013).
Kjellman, M. et al. Genetic aberrations in adrenocortical tumours detected using comparative genomic hybridization correlate with tumour size and malignancy. Cancer Res. 56, 4219–4223 (1996).
Zhao, J. et al. Analysis of genomic alterations in sporadic adrenocortical lesions. Gain of chromosome 17 is an early event in adrenocortical tumourigenesis. Am. J. Pathol. 155, 1039–1045 (1999).
Dohna, M. et al. Adrenocortical carcinoma is characterized by a high frequency of chromosomal gains and high-level amplifications. Genes Chromosomes Cancer 28, 145–152 (2000).
Sidhu, S. et al. Comparative genomic hybridization analysis of adrenocortical tumours. J. Clin. Endocrinol. Metab. 87, 3467–3474 (2002).
Gruschwitz, T., Breza, J., Wunderlich, H. & Junker, K. Improvement of histopathological classification of adrenal gland tumours by genetic differentiation. World J. Urol. 28, 329–334 (2010).
Chang, H. et al. Exome sequencing reveals comprehensive genomic alterations across eight cancer cell lines. PLoS ONE 6, e21097 (2011).
MacConaill, L. E. Existing and emerging technologies for tumour genomic profiling. J. Clin. Oncol. 31, 1815–1824 (2013).
Kulis, M. & Esteller, M. DNA methylation and cancer. Adv. Genet. 70, 27–56 (2010).
Mukamel, Z. & Tanay, A. Hypomethylation marks enhancers within transposable elements. Nat. Genet. 45, 717–718 (2013).
Gicquel, C. et al. Rearrangements at the 11p15 locus and overexpression of insulin-like growth factor-II gene in sporadic adrenocortical tumours. J. Clin. Endocrinol. Metab. 78, 1444–1453 (1994).
Gicquel, C. et al. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumours: study on a series of 82 tumours. J. Clin. Endocrinol. Metab. 82, 2559–2565 (1997).
Gao, Z. H. et al. Association of H19 promoter methylation with the expression of H19 and IGF-II genes in adrenocortical tumours. J. Clin. Endocrinol. Metab. 87, 1170–1176 (2002).
Simons, C. C. et al. A novel classification of colorectal tumours based on microsatellite instability, the CpG island methylator phenotype and chromosomal instability: implications for prognosis. Ann. Oncol. 24, 2048–2056 (2013).
Liu, J., Li, X. D., Vaheri, A. & Voutilainen, R. DNA methylation affects cell proliferation, cortisol secretion and steroidogenic gene expression in human adrenocortical NCI-H295R cells. J. Mol. Endocrinol. 33, 651–662 (2004).
Utriainen, P., Liu, J., Kuulasmaa, T. & Voutilainen, R. Inhibition of DNA methylation increases follistatin expression and secretion in the human adrenocortical cell line NCI-H295R. J. Endocrinol. 188, 305–310 (2006).
Suh, I. et al. Antineoplastic effects of decitabine, an inhibitor of DNA promoter methylation, in adrenocortical carcinoma cells. Arch. Surg. 145, 226–232 (2010).
Simi, L. et al. Seladin-1 expression is regulated by promoter methylation in adrenal cancer. BMC Cancer 10, 201 (2010).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Wang, Q. et al. Briefing in family characteristics of microRNAs and their applications in cancer research. Biochim. Biophys. Acta 1844, 191–197 (2014).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005).
Xi, Y. et al. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA 13, 1668–1674 (2007).
Laios, A. et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol. Cancer 7, 35 (2008).
Hui, A. B. et al. Robust global micro-RNA profiling with formalin-fixed paraffin-embedded breast cancer tissues. Lab. Invest. 89, 597–606 (2009).
Leite, K. R. et al. miRNA analysis of prostate cancer by quantitative real time PCR: comparison between formalin-fixed paraffin embedded and fresh-frozen tissue. Urol. Oncol. 29, 533–537 (2011).
De Preter, K. et al. miRNA expression profiling enables risk stratification in archived and fresh neuroblastoma tumour samples. Clin. Cancer Res. 17, 7684–7692 (2011).
Hall, J. S. et al. Enhanced stability of microRNA expression facilitates classification of FFPE tumour samples exhibiting near total mRNA degradation. Br. J. Cancer 107, 684–694 (2012).
Culpin, R. E., Sieniawski, M., Proctor, S. J., Menon, G. & Mainou-Fowler, T. MicroRNAs are suitable for assessment as biomarkers from formalin-fixed paraffin-embedded tissue, and miR-24 represents an appropriate reference microRNA for diffuse large B-cell lymphoma studies. J. Clin. Pathol. 66, 249–252 (2013).
Li, X., Lu, Y., Chen, Y., Lu, W. & Xie, X. MicroRNA profile of paclitaxel-resistant serous ovarian carcinoma based on formalin-fixed paraffin-embedded samples. BMC Cancer 13, 216 (2013).
Mwenifumbo, J. C. & Marra, M. A. Cancer genome-sequencing study design. Nat. Rev. Genet. 14, 321–332 (2013).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Acknowledgements
J. Bertherat's research on adrenal cancer is supported in part by the COMETE Network (Programme Hospitalier de Recherche Clinique; grant AOM95201), the INCa Recherche Translationelle (grant 2009-RT-02), and ENSAT-CANCER Health (FP7 program grant F2-2010-259735).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching the data and to writing the article. J. Bertherat and G. Assié discussed the content and reviewed and/or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Key genomic studies in adrenocortical carcinoma published to date (XLS 61 kb)
Rights and permissions
About this article
Cite this article
Assié, G., Jouinot, A. & Bertherat, J. The 'omics' of adrenocortical tumours for personalized medicine. Nat Rev Endocrinol 10, 215–228 (2014). https://doi.org/10.1038/nrendo.2013.272
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.272
This article is cited by
-
Adrenocortical carcinoma: the dawn of a new era of genomic and molecular biology analysis
Journal of Endocrinological Investigation (2018)
-
Wichtige Aspekte des Nebennierenrindenkarzinoms
best practice onkologie (2017)
-
Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours
Endocrine Pathology (2017)
-
Wichtige Aspekte des Nebennierenrindenkarzinoms
Der Onkologe (2016)
-
5th International ACC Symposium: Classification of Adrenocortical Cancers from Pathology to Integrated Genomics: Real Advances or Lost in Translation?
Hormones and Cancer (2016)