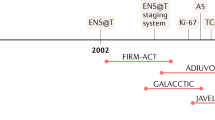
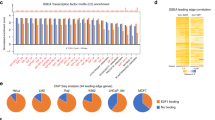
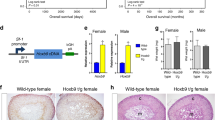
Abstract
Adrenocortical carcinoma is a rare malignancy with an annual worldwide incidence of 1–2 cases per 1 million and a 5-year survival rate of <60%. Although adrenocortical carcinoma is rare, such rare cancers account for approximately one third of patients diagnosed with cancer annually. In the past decade, there have been considerable advances in understanding the molecular basis of adrenocortical carcinoma. The genetic events associated with adrenocortical carcinoma in adults are distinct from those of paediatric cases, which are often associated with germline or somatic TP53 mutations and have a better prognosis. In adult primary adrenocortical carcinoma, the main somatic genetic alterations occur in genes that encode proteins involved in the WNT–β-catenin pathway, cell cycle and p53 apoptosis pathway, chromatin remodelling and telomere maintenance pathway, cAMP–protein kinase A (PKA) pathway or DNA transcription and RNA translation pathways. Recently, integrated molecular studies of adrenocortical carcinomas, which have characterized somatic mutations and the methylome as well as gene and microRNA expression profiles, have led to a molecular classification of these tumours that can predict prognosis and have helped to identify new therapeutic targets. In this Review, we summarize these recent translational research advances in adrenocortical carcinoma, which it is hoped could lead to improved patient diagnosis, treatment and outcome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bilimoria, K. Y. et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer 113, 3130–3136 (2008).
Else, T. et al. Adrenocortical carcinoma. Endocr. Rev. 35, 282–326 (2014).
Kebebew, E., Reiff, E., Duh, Q. Y., Clark, O. H. & McMillan, A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J. Surg. 30, 872–878 (2006).
Tella, S. H., Kommalapati, A., Yaturu, S. & Kebebew, E. Predictors of survival in adrenocortical carcinoma: an analysis from the national cancer database. J. Clin. Endocrinol. Metab. 103, 3566–3573 (2018).
Tierney, J. F. et al. National treatment practice for adrenocortical carcinoma: have they changed and have we made any progress? J. Clin. Endocrinol. Metab. 104, 5948–5956 (2019).
Kerkhofs, T. M. et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur. J. Cancer 49, 2579–2586 (2013).
McAteer, J. P., Huaco, J. A. & Gow, K. W. Predictors of survival in pediatric adrenocortical carcinoma: a Surveillance, Epidemiology, and End Results (SEER) program study. J. Pediatr. Surg. 48, 1025–1031 (2013).
Fassnacht, M. et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer 115, 243–250 (2009).
Elhassan, Y. S. et al. S-GRAS score for prognostic classification of adrenocortical carcinoma: an international, multicenter ENSAT study. Eur. J. Endocrinol. 186, 25–36 (2021).
Beuschlein, F. et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J. Clin. Endocrinol. Metab. 100, 841–849 (2015).
Michalkiewicz, E. et al. Clinical and outcome characteristics of children with adrenocortical tumors: a report from the International Pediatric Adrenocortical Tumor Registry. J. Clin. Oncol. 22, 838–845 (2004).
Loncar, Z. et al. Survival and prognostic factors for adrenocortical carcinoma: a single institution experience. BMC Urol. 15, 43 (2015).
Li, P., Su, X., Zhang, X., Sun, L. & Zhang, G. Prognostic factors of adrenocortical carcinoma: experience from a Regional Medical Center in Eastern China. Int. J. Gen. Med. 16, 453–465 (2023).
Lughezzani, G. et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the International Union Against Cancer-staging system: a North American validation. Eur. J. Cancer 46, 713–719 (2010).
Terzolo, M. et al. Results of the ADIUVO study, the first randomized trial on adjuvant mitotane in adrenocortical carcinoma patients. J. Endocr. Soc. 5, A166–A167 (2021).
Fassnacht, M. et al. Combination chemotherapy in advanced adrenocortical carcinoma. N. Engl. J. Med. 366, 2189–2197 (2012).
Fassnacht, M. et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 179, G1–G46 (2018).
Challis, B. G. et al. Familial adrenocortical carcinoma in association with Lynch syndrome. J. Clin. Endocrinol. Metab. 101, 2269–2272 (2016).
Gatta-Cherifi, B. et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’Etude des Tumeurs Endocrines database. Eur. J. Endocrinol. 166, 269–279 (2012).
Hampel, H. et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N. Engl. J. Med. 352, 1851–1860 (2005).
Herrmann, L. J. et al. TP53 germline mutations in adult patients with adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 97, E476–E485 (2012).
MacFarland, S. P. et al. Management of adrenal masses in patients with Beckwith–Wiedemann syndrome. Pediatr. Blood Cancer 64, 10.1002/pbc.26432 (2017).
Pinto, E. M. et al. Identification of clinical and biologic correlates associated with outcome in children with adrenocortical tumors without germline TP53 mutations: a st jude adrenocortical tumor registry and children’s oncology group study. J. Clin. Oncol. 35, 3956–3963 (2017).
Raymond, V. M. et al. Prevalence of germline TP53 mutations in a prospective series of unselected patients with adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 98, E119–E125 (2013).
Raymond, V. M. et al. Adrenocortical carcinoma is a Lynch syndrome-associated cancer. J. Clin. Oncol. 31, 3012–3018 (2013).
Seki, M. et al. Loss of normal allele of the APC gene in an adrenocortical carcinoma from a patient with familial adenomatous polyposis. Hum. Genet. 89, 298–300 (1992).
Shiroky, J. S., Lerner-Ellis, J. P., Govindarajan, A., Urbach, D. R. & Devon, K. M. Characteristics of adrenal masses in familial adenomatous polyposis. Dis. Colon Rectum 61, 679–685 (2018).
Wakatsuki, S. et al. Adrenocortical tumor in a patient with familial adenomatous polyposis: a case associated with a complete inactivating mutation of the APC gene and unusual histological features. Hum. Pathol. 29, 302–306 (1998).
Wasserman, J. D. et al. Prevalence and functional consequence of TP53 mutations in pediatric adrenocortical carcinoma: a Children’s Oncology Group study. J. Clin. Oncol. 33, 602–609 (2015).
Juhlin, C. C. et al. What did we learn from the molecular biology of adrenal cortical neoplasia? From histopathology to translational genomics. Endocr. Pathol. 32, 102–133 (2021).
Grisanti, S., Cosentini, D., Sigala, S. & Berruti, A. Molecular genotyping of adrenocortical carcinoma: a systematic analysis of published literature 2019–2021. Curr. Opin. Oncol. 34, 19–28 (2022).
Else, T. et al. Adrenocortical carcinoma and succinate dehydrogenase gene mutations: an observational case series. Eur. J. Endocrinol. 177, 439–444 (2017).
Grisanti, S. et al. 29MO Germline variants NGS characterization in patients with non-syndromic adrenocortical carcinoma. ESMO Open 8, 101050 (2023).
Custodio, G. et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J. Clin. Oncol. 31, 2619–2626 (2013).
Latronico, A. C. et al. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 86, 4970–4973 (2001).
Pinto, E. M. et al. Founder effect for the highly prevalent R337H mutation of tumor suppressor p53 in Brazilian patients with adrenocortical tumors. Arq. Bras. Endocrinol. Metab. 48, 647–650 (2004).
Ribeiro, R. C. et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc. Natl Acad. Sci. USA 98, 9330–9335 (2001).
Mete, O. et al. Overview of the 2022 who classification of adrenal cortical tumors. Endocr. Pathol. 33, 155–196 (2022).
Minner, S., Schreiner, J. & Saeger, W. Adrenal cancer: relevance of different grading systems and subtypes. Clin. Transl. Oncol. 23, 1350–1357 (2021).
Weiss, L. M. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am. J. Surg. Pathol. 8, 163–169 (1984).
Weiss, L. M., Medeiros, L. J. & Vickery, A. L. Jr Pathologic features of prognostic significance in adrenocortical carcinoma. Am. J. Surg. Pathol. 13, 202–206 (1989).
Pittaway, J. F. H. & Guasti, L. Pathobiology and genetics of adrenocortical carcinoma. J. Mol. Endocrinol. 62, R105–R119 (2019).
Assie, G. et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 46, 607–612 (2014). This study demonstrates that cataloguing the genomic changes in adrenocortical carcinoma reveals distinct molecular groups and that these are associated with prognosis.
Zheng, S. et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 29, 723–736 (2016). This study comprehensively identifies genomic alterations associated with adult adrenocortical carcinoma and their association with patient outcome.
Sbiera, I. et al. Role of FGF receptors and their pathways in adrenocortical tumors and possible therapeutic implications. Front. Endocrinol. 12, 795116 (2021).
Tamburello, M. et al. FGF/FGFR signaling in adrenocortical development and tumorigenesis: novel potential therapeutic targets in adrenocortical carcinoma. Endocrine 77, 411–418 (2022).
Pereira, S. S. et al. IGF2 role in adrenocortical carcinoma biology. Endocrine 66, 326–337 (2019).
Pinto, E. M. et al. Genomic landscape of paediatric adrenocortical tumours. Nat. Commun. 6, 6302 (2015). This study shows the genomic alterations that are prevalent in and unique to paediatric adrenocortical carcinomas.
Pozdeyev, N. et al. Targeted genomic analysis of 364 adrenocortical carcinomas. Endocr. Relat. Cancer 28, 671–681 (2021). This study is one of the largest mutational analyses performed using NGS and identifies novel genomic alterations that could be targeted for therapy.
Hoadley, K. A. et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173, 291–304.e6 (2018).
Knijnenburg, T. A. et al. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep. 23, 239–254.e6 (2018).
Sondka, Z. et al. The COSMIC cancer gene census: describing genetic dysfunction across all human cancers. Nat. Rev. Cancer 18, 696–705 (2018).
Kurtz, A. et al. Somatic mitochondrial DNA mutations in neurofibromatosis type 1-associated tumors. Mol. Cancer Res. 2, 433–441 (2004).
Wang, Q. et al. Neurofibromatosis type 1 gene as a mutational target in a mismatch repair-deficient cell type. Hum. Genet. 112, 117–123 (2003).
Santos, M. A. et al. DNA-damage-induced differentiation of leukaemic cells as an anti-cancer barrier. Nature 514, 107–111 (2014).
Pemov, A., Park, C., Reilly, K. M. & Stewart, D. R. Evidence of perturbations of cell cycle and DNA repair pathways as a consequence of human and murine NF1-haploinsufficiency. BMC Genomics 11, 194 (2010).
Bielski, C. M. et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat. Genet. 50, 1189–1195 (2018).
Letouze, E. et al. SNP array profiling of childhood adrenocortical tumors reveals distinct pathways of tumorigenesis and highlights candidate driver genes. J. Clin. Endocrinol. Metab. 97, E1284–E1293 (2012).
Zack, T. I. et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013).
Juhlin, C. C. et al. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 100, E493–E502 (2015).
Gicquel, C. et al. Rearrangements at the 11p15 locus and overexpression of insulin-like growth factor-II gene in sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 78, 1444–1453 (1994).
Gicquel, C. et al. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: study on a series of 82 tumors. J. Clin. Endocrinol. Metab. 82, 2559–2565 (1997).
Peixoto Lira, R. C. et al. IGF2 and IGF1R in pediatric adrenocortical tumors: roles in metastasis and steroidogenesis. Endocr. Relat. Cancer 23, 481–493 (2016).
Guillaud-Bataille, M. et al. IGF2 promotes growth of adrenocortical carcinoma cells, but its overexpression does not modify phenotypic and molecular features of adrenocortical carcinoma. PLoS ONE 9, e103744 (2014).
Nielsen, H. M. et al. Copy number variations alter methylation and parallel IGF2 overexpression in adrenal tumors. Endocr. Relat. Cancer 22, 953–967 (2015).
Rosati, R. et al. High frequency of loss of heterozygosity at 11p15 and IGF2 overexpression are not related to clinical outcome in childhood adrenocortical tumors positive for the R337H TP53 mutation. Cancer Genet. Cytogenet. 186, 19–24 (2008).
Szabo, P. E., Tang, S. H., Silva, F. J., Tsark, W. M. & Mann, J. R. Role of CTCF binding sites in the Igf2/H19 imprinting control region. Mol. Cell Biol. 24, 4791–4800 (2004).
Barlaskar, F. M. et al. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 94, 204–212 (2009).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT00831844 (2015).
Fassnacht, M. et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 16, 426–35 (2015).
Sasano, H., Satoh, F. & Nakamura, Y. Roles of the pathologist in evaluating surrogate markers for medical therapy in adrenocortical carcinoma. Endocr. Pathol. 25, 366–370 (2014).
Adam, P. et al. Epidermal growth factor receptor in adrenocortical tumors: analysis of gene sequence, protein expression and correlation with clinical outcome. Mod. Pathol. 23, 1596–1604 (2010).
Quinkler, M. et al. Treatment of advanced adrenocortical carcinoma with erlotinib plus gemcitabine. J. Clin. Endocrinol. Metab. 93, 2057–2062 (2008).
Ardolino, L., Hansen, A., Ackland, S. & Joshua, A. Advanced adrenocortical carcinoma (ACC): a review with focus on second-line therapies. Horm. Cancer 11, 155–169 (2020).
Boumahdi, S. & de Sauvage, F. J. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 19, 39–56 (2020).
Quintanal-Villalonga, Á. et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 17, 360–371 (2020).
Gara, S. K. et al. Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors. Nat. Commun. 9, 4172 (2018). This study demonstrates that genomic alterations between primary adrenocortical carcinoma and recurrent and metastatic adrenocortical carcinoma are different with the latter exhibiting more tumour heterogeneity.
Heaton, J. H. et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am. J. Pathol. 181, 1017–1033 (2012).
Berthon, A. et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum. Mol. Genet. 19, 1561–1576 (2010).
Lin, S. & Gregory, R. I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 15, 321–333 (2015).
Huarte, M. The emerging role of lncRNAs in cancer. Nat. Med. 21, 1253–1261 (2015).
Liu, J., Kahri, A. I., Heikkila, P., Ilvesmaki, V. & Voutilainen, R. H19 and insulin-like growth factor-II gene expression in adrenal tumors and cultured adrenal cells. J. Clin. Endocrinol. Metab. 80, 492–496 (1995).
Hatada, I. & Mukai, T. Genomic imprinting of p57KIP2, a cyclin-dependent kinase inhibitor, in mouse. Nat. Genet. 11, 204–206 (1995).
Liu, J., Kahri, A. I., Heikkila, P. & Voutilainen, R. Ribonucleic acid expression of the clustered imprinted genes, p57KIP2, insulin-like growth factor II, and H19, in adrenal tumors and cultured adrenal cells. J. Clin. Endocrinol. Metab. 82, 1766–1771 (1997).
Soon, P. S. et al. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin. Cancer Res. 15, 7684–7692 (2009).
Feinmesser, M. et al. Specific microRNAs differentiate adrenocortical adenomas from carcinomas and correlate with Weiss histopathologic system. Appl. Immunohistochem. Mol. Morphol. 23, 522–531 (2015).
Koperski, Ł. et al. Next-generation sequencing reveals microRNA markers of adrenocortical tumors malignancy. Oncotarget 8, 49191–49200 (2017).
Chehade, M., Bullock, M., Glover, A., Hutvagner, G. & Sidhu, S. Key microRNA’s and their targetome in adrenocortical cancer. Cancers 12, 2198 (2020).
Mitchell, P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl Acad. Sci. USA 105, 10513–10518 (2008).
Chabre, O. et al. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr. Relat. Cancer 20, 579–594 (2013).
Szabó, D. R. et al. Analysis of circulating microRNAs in adrenocortical tumors. Lab. Invest. 94, 331–339 (2014).
Patel, D. et al. MiR-34a and miR-483-5p are candidate serum biomarkers for adrenocortical tumors. Surgery 154, 1224–1228 (2013).
Wu, Y. et al. MicroRNA-205 suppresses the growth of adrenocortical carcinoma SW-13 cells via targeting Bcl-2. Oncol. Rep. 34, 3104–3110 (2015).
Jain, M. et al. ZNF367 inhibits cancer progression and is targeted by miR-195. PLoS ONE 9, e101423 (2014).
Kwok, G. T. Y. et al. microRNA-431 as a chemosensitizer and potentiator of drug activity in adrenocortical carcinoma. Oncologist 24, e241–e250 (2019).
Hassan, N., Zhao, J. T., Glover, A., Robinson, B. G. & Sidhu, S. B. Reciprocal interplay of miR-497 and MALAT1 promotes tumourigenesis of adrenocortical cancer. Endocr. Relat. Cancer 26, 677–688 (2019).
Kalinowski, F. C. et al. microRNA-7: a tumor suppressor miRNA with therapeutic potential. Int. J. Biochem. Cell Biol. 54, 312–317 (2014).
Gara, S. K. et al. Integrated genome-wide analysis of genomic changes and gene regulation in human adrenocortical tissue samples. Nucleic Acids Res. 43, 9327–9339 (2015).
Glover, A. R. et al. Long noncoding RNA profiles of adrenocortical cancer can be used to predict recurrence. Endocr. Relat. Cancer 22, 99–109 (2015). One of the first studies to show that the lncRNA expression profile of adrenocortical carcinoma is different from that of benign adrenocortical carcinoma and normal adrenal cortex.
Buishand, F. O. et al. Adrenocortical tumors have a distinct, long, non-coding RNA expression profile and LINC00271 is downregulated in malignancy. Surgery 167, 224–232 (2020).
Long, B. et al. Long noncoding RNA ASB16-AS1 inhibits adrenocortical carcinoma cell growth by promoting ubiquitination of RNA-binding protein HuR. Cell Death Dis. 11, 995 (2020).
Guo, N., Sun, Q., Fu, D. & Zhang, Y. Long non-coding RNA UCA1 promoted the growth of adrenocortical cancer cells via modulating the miR-298-CDK6 axis. Gene 703, 26–34 (2019).
Tombol, Z. et al. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocr. Relat. Cancer 16, 895–906 (2009).
Duregon, E. et al. MicroRNA expression patterns in adrenocortical carcinoma variants and clinical pathologic correlations. Hum. Pathol. 45, 1555–1562 (2014).
Ozata, D. M. et al. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr. Relat. Cancer 18, 643–655 (2011).
Patterson, E. E., Holloway, A. K., Weng, J., Fojo, T. & Kebebew, E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 117, 1630–1639 (2011).
Ettaieb, M., Kerkhofs, T., van Engeland, M. & Haak, H. Past, present and future of epigenetics in adrenocortical carcinoma. Cancers 12, 1218 (2020).
Rechache, N. S. et al. DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumors. J. Clin. Endocrinol. Metab. 97, E1004–E1013 (2012).
Creemers, S. G. et al. Methylation of IGF2 regulatory regions to diagnose adrenocortical carcinomas. Endocr. Relat. Cancer 23, 727–737 (2016).
Barreau, O. et al. Identification of a CpG island methylator phenotype in adrenocortical carcinomas. J. Clin. Endocrinol. Metab. 98, E174–E184 (2013).
Jouinot, A. et al. DNA methylation is an independent prognostic marker of survival in adrenocortical cancer. J. Clin. Endocrinol. Metab. 102, 923–932 (2017).
Lippert, J. et al. Prognostic role of targeted methylation analysis in paraffin-embedded samples of adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 107, 2892–2899 (2022).
Mohan, D. R. et al. Targeted assessment of G0S2 methylation identifies a rapidly recurrent, routinely fatal molecular subtype of adrenocortical carcinoma. Clin. Cancer Res. 25, 3276–3288 (2019).
Clay, M. R. et al. DNA methylation profiling reveals prognostically significant groups in pediatric adrenocortical tumors: a report from the International Pediatric Adrenocortical Tumor Registry. JCO Precis. Oncol. 3, PO.19.00163 (2019).
Drelon, C. et al. EZH2 is overexpressed in adrenocortical carcinoma and is associated with disease progression. Hum. Mol. Genet. 25, 2789–2800 (2016).
Tabbal, H. et al. EZH2 cooperates with E2F1 to stimulate expression of genes involved in adrenocortical carcinoma aggressiveness. Br. J. Cancer 121, 384–394 (2019).
Kebebew, E. Adrenal incidentaloma. N. Engl. J. Med. 384, 1542–1551 (2021).
Rege, J., Turcu, A. F., Else, T., Auchus, R. J. & Rainey, W. E. Steroid biomarkers in human adrenal disease. J. Steroid Biochem. Mol. Biol. 190, 273–280 (2019).
Taylor, D. R. et al. A 13-steroid serum panel based on LC-MS/MS: use in detection of adrenocortical carcinoma. Clin. Chem. 63, 1836–1846 (2017). Along with Rege et al. (2019), this study shows the utility of measuring levels of steroids and their metabolities as biomarkers of adrenocortical carcinoma.
Chortis, V. et al. Urine steroid metabolomics as a novel tool for detection of recurrent adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 105, e307–e318 (2020).
Suzuki, S. et al. Steroid metabolites for diagnosing and predicting clinicopathological features in cortisol-producing adrenocortical carcinoma. BMC Endocr. Disord. 20, 173 (2020).
Bancos, I. et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 8, 773–781 (2020).
Patel, D. et al. Unique and novel urinary metabolomic features in malignant versus benign adrenal neoplasms. Clin. Cancer Res. 23, 5302–5310 (2017). This study shows that patients with adrenocortical carcinoma have a unique urinary metabolome, which derives from the tumour tissue.
Mathe, E. A. et al. Noninvasive urinary metabolomic profiling identifies diagnostic and prognostic markers in lung cancer. Cancer Res. 74, 3259–3270 (2014).
Haznadar, M. et al. Urinary metabolites diagnostic and prognostic of intrahepatic cholangiocarcinoma. Cancer Epidemiol. Biomark. Prev. 28, 1704–1711 (2019).
Foster, P. A. & Mueller, J. W. SULFATION PATHWAYS: insights into steroid sulfation and desulfation pathways. J. Mol. Endocrinol. 61, T271–T283 (2018).
Sun, N. et al. Prognostic relevance of steroid sulfation in adrenocortical carcinoma revealed by molecular phenotyping using high-resolution mass spectrometry imaging. Clin. Chem. 65, 1276–1286 (2019).
Sigala, S. et al. A comprehensive investigation of steroidogenic signaling in classical and new experimental cell models of adrenocortical carcinoma. Cells 11, 1439 (2022).
Fujisawa, Y. et al. Combined steroidogenic characters of fetal adrenal and Leydig cells in childhood adrenocortical carcinoma. J. Steroid Biochem. Mol. Biol. 159, 86–93 (2016).
Marti, N. et al. Androgen production in pediatric adrenocortical tumors may occur via both the classic and/or the alternative backdoor pathway. Mol. Cell Endocrinol. 452, 64–73 (2017).
Fenske, W. et al. Glucose transporter GLUT1 expression is an stage-independent predictor of clinical outcome in adrenocortical carcinoma. Endocr. Relat. Cancer 16, 919–928 (2009).
Satoh, K., Patel, D., Dieckmann, W., Nilubol, N. & Kebebew, E. Whole body metabolic tumor volume and total lesion glycolysis predict survival in patients with adrenocortical carcinoma. Ann. Surg. Oncol. 22 (Suppl. 3), S714–S720 (2015).
Wrenn, S. M. et al. Higher SUVmax on FDG-PET is associated with shorter survival in adrenocortical carcinoma. Am. J. Surg. 225, 309–314 (2023).
Pinheiro, C. et al. GLUT1 expression in pediatric adrenocortical tumors: a promising candidate to predict clinical behavior. Oncotarget 8, 63835–63845 (2017).
Assié, G. et al. Value of molecular classification for prognostic assessment of adrenocortical carcinoma. JAMA Oncol. 5, 1440–1447 (2019).
de Reyniès, A. et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 27, 1108–1115 (2009).
Marquardt, A. et al. Identifying new potential biomarkers in adrenocortical tumors based on mrna expression data using machine learning. Cancers 13, 4671 (2021).
Yi, X. et al. Identification of four novel prognostic biomarkers and construction of two nomograms in adrenocortical carcinoma: a multi-omics data study via bioinformatics and machine learning methods. Front. Mol. Biosci. 9, 878073 (2022).
Paré, L. et al. Association between PD1 mRNA and response to anti-PD1 monotherapy across multiple cancer types. Ann. Oncol. 29, 2121–2128 (2018).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e14 (2018).
Mohan, D. R., Lerario, A. M. & Hammer, G. D. Therapeutic targets for adrenocortical carcinoma in the genomics era. J. Endocr. Soc. 2, 1259–1274 (2018).
Tian, X. et al. Identification of tumor-infiltrating immune cells and prognostic validation of tumor-infiltrating mast cells in adrenocortical carcinoma: results from bioinformatics and real-world data. Oncoimmunology 9, 1784529 (2020).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015).
Landwehr, L. S. et al. Interplay between glucocorticoids and tumor-infiltrating lymphocytes on the prognosis of adrenocortical carcinoma. J. Immunother. Cancer 8, e000469 (2020).
Coutinho, A. E. & Chapman, K. E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 335, 2–13 (2011).
Marx, C., Wolkersdorfer, G. W., Brown, J. W., Scherbaum, W. A. & Bornstein, S. R. MHC class II expression—a new tool to assess dignity in adrenocortical tumours. J. Clin. Endocrinol. Metab. 81, 4488–4491 (1996).
Ozdemirli, M. et al. Fas (CD95)/Fas ligand interactions regulate antigen-specific, major histocompatibility complex-restricted T/B cell proliferative responses. Eur. J. Immunol. 26, 415–419 (1996).
Brunner, T. et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373, 441–444 (1995).
Wolkersdörfer, G. W. et al. Prevalence of HLA-DRB1 genotype and altered Fas/Fas ligand expression in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 90, 1768–1774 (2005).
Hahne, M. et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science 274, 1363–1366 (1996).
Xiao, W. et al. Loss of fas expression and function is coupled with colon cancer resistance to immune checkpoint inhibitor immunotherapy. Mol. Cancer Res. 17, 420–430 (2019).
Pinto, E. M. et al. Prognostic significance of major histocompatibility complex class ii expression in pediatric adrenocortical tumors: a St. Jude and Children’s Oncology Group Study. Clin. Cancer Res. 22, 6247–6255 (2016).
Kanczkowski, W. et al. Abrogation of TLR4 and CD14 expression and signaling in human adrenocortical tumors. J. Clin. Endocrinol. Metab. 95, E421–E429 (2010).
Huang, B. et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 65, 5009–5014 (2005).
Szajnik, M. et al. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene 28, 4353–4363 (2009).
Liang, J. et al. Clinicopathological and prognostic characteristics of CD276 (B7-H3) expression in adrenocortical carcinoma. Dis. Markers 2020, 5354825 (2020).
Hofmeyer, K. A., Ray, A. & Zang, X. The contrasting role of B7-H3. Proc. Natl Acad. Sci. USA 105, 10277–10278 (2008).
Liao, H., Ding, M., Zhou, N., Yang, Y. & Chen, L. B7-H3 promotes the epithelial-mesenchymal transition of NSCLC by targeting SIRT1 through the PI3K/AKT pathway. Mol. Med. Rep. 25, 79 (2022).
Raj, N. et al. PD-1 blockade in advanced adrenocortical carcinoma. J. Clin. Oncol. 38, 71–80 (2020).
Habra, M. A. et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J. Immunother. Cancer 7, 253 (2019).
Head, L. et al. Response to immunotherapy in combination with mitotane in patients with metastatic adrenocortical cancer. J. Endocr. Soc. 3, 2295–2304 (2019).
Bedrose, S. et al. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J. Immunother. Cancer 8, e001009 (2020).
Carneiro, B. A. et al. Nivolumab in metastatic adrenocortical carcinoma: results of a phase 2 trial. J. Clin. Endocrinol. Metab. 104, 6193–6200 (2019).
McGregor, B. A. et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer 127, 840–849 (2021).
Grondal, S., Eriksson, B., Hagenas, L., Werner, S. & Curstedt, T. Steroid profile in urine: a useful tool in the diagnosis and follow up of adrenocortical carcinoma. Acta Endocrinol. 122, 656–663 (1990).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT04373265 (2023).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT04187404 (2023).
Armignacco, R. et al. The adipose stem cell as a novel metabolic actor in adrenocortical carcinoma progression: evidence from an in vitro tumor microenvironment crosstalk model. Cancers 11, 1931 (2019).
Jain, M. et al. TOP2A is overexpressed and is a therapeutic target for adrenocortical carcinoma. Endocr. Relat. Cancer 20, 361–370 (2013).
Satoh, K. et al. Identification of niclosamide as a novel anticancer agent for adrenocortical carcinoma. Clin. Cancer Res. 22, 3458–3466 (2016).
Jain, M. et al. Interleukin-13 receptor alpha2 is a novel therapeutic target for human adrenocortical carcinoma. Cancer 118, 5698–5708 (2012).
Barton, D. E., Foellmer, B. E., Wood, W. I. & Francke, U. Chromosome mapping of the growth hormone receptor gene in man and mouse. Cytogenet. Cell Genet. 50, 137–141 (1989).
Hantel, C. et al. Targeting heterogeneity of adrenocortical carcinoma: evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget 7, 79292–79304 (2016).
Cardoso, C. C., Bornstein, S. R. & Hornsby, P. J. Optimizing orthotopic cell transplantation in the mouse adrenal gland. Cell Transpl. 19, 565–572 (2010).
Ruggiero, C., Doghman-Bouguerra, M. & Lalli, E. How good are the current models of adrenocortical carcinoma for novel drug discovery? Expert Opin. Drug Discov. 17, 211–213 (2022).
Batisse-Lignier, M. et al. p53/Rb inhibition induces metastatic adrenocortical carcinomas in a preclinical transgenic model. Oncogene 36, 4445–4456 (2017). This study demonstrates that known genetic alterations in human adrenocortical carcinoma introduced into a mouse can cause adrenocortical carcinoma similar in phenotype and histology to those human tumours and that this mouse model can in turn be used to evaluate the efficacy of targeted therapies.
Borges, K. S. et al. Wnt/β-catenin activation cooperates with loss of p53 to cause adrenocortical carcinoma in mice. Oncogene 39, 5282–5291 (2020).
Warde, K. M. et al. Senescence-induced immune remodeling facilitates metastatic adrenal cancer in a sex-dimorphic manner. Nat. Aging 3, 846–865 (2023).
Wilmouth, J. J. Jr. et al. Sexually dimorphic activation of innate antitumor immunity prevents adrenocortical carcinoma development. Sci. Adv. 8, eadd0422 (2022).
Lyraki, R. et al. Crosstalk between androgen receptor and WNT/β-catenin signaling causes sex-specific adrenocortical hyperplasia in mice. Dis. Model. Mech. 16, dmm050053 (2023).
Kiseljak-Vassiliades, K. et al. Development of new preclinical models to advance adrenocortical carcinoma research. Endocr. Relat. Cancer 25, 437–451 (2018).
Lang, J. et al. Development of an adrenocortical cancer humanized mouse model to characterize anti-pd1 effects on tumor microenvironment. J. Clin. Endocrinol. Metab. 105, 26–42 (2020). This study develops a humanized adrenocortical carcinoma PDX mouse model to assess immunotherapy responses.
Pinto, E. M. et al. Establishment and characterization of the first pediatric adrenocortical carcinoma xenograft model identifies topotecan as a potential chemotherapeutic agent. Clin. Cancer Res. 19, 1740–1747 (2013).
Fiorentini, C. et al. Palbociclib inhibits proliferation of human adrenocortical tumor cells. Endocrine 59, 213–217 (2018).
Liang, R. et al. Targeted gene expression profile reveals CDK4 as therapeutic target for selected patients with adrenocortical carcinoma. Front. Endocrinol. 11, 219 (2020).
Hadjadj, D. et al. A hypothesis-driven approach identifies CDK4 and CDK6 inhibitors as candidate drugs for treatments of adrenocortical carcinomas. Aging 9, 2695–2716 (2017).
Nilubol, N. et al. Synergistic combination of flavopiridol and carfilzomib targets commonly dysregulated pathways in adrenocortical carcinoma and has biomarkers of response. Oncotarget 9, 33030–33042 (2018).
Doghman, M. et al. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res. 70, 4666–4675 (2010).
Doghman, M., Cazareth, J. & Lalli, E. The T cell factor/beta-catenin antagonist PKF115-584 inhibits proliferation of adrenocortical carcinoma cells. J. Clin. Endocrinol. Metab. 93, 3222–3225 (2008).
Gaujoux, S. et al. Silencing mutated β-catenin inhibits cell proliferation and stimulates apoptosis in the adrenocortical cancer cell line H295R. PLoS ONE 8, e55743 (2013).
Cerquetti, L. et al. Rosiglitazone induces autophagy in H295R and cell cycle deregulation in SW13 adrenocortical cancer cells. Exp. Cell Res. 317, 1397–1410 (2011).
Cantini, G. et al. Rosiglitazone inhibits adrenocortical cancer cell proliferation by interfering with the IGF-IR intracellular signaling. PPAR Res. 2008, 904041 (2008).
Ferruzzi, P. et al. Thiazolidinediones inhibit growth and invasiveness of the human adrenocortical cancer cell line H295R. J. Clin. Endocrinol. Metab. 90, 1332–1339 (2005).
Sirianni, R. et al. Targeting estrogen receptor-α reduces adrenocortical cancer (ACC) cell growth in vitro and in vivo: potential therapeutic role of selective estrogen receptor modulators (SERMs) for ACC treatment. J. Clin. Endocrinol. Metab. 97, E2238–E2250 (2012).
Chimento, A. et al. GPER agonist G-1 decreases adrenocortical carcinoma (ACC) cell growth in vitro and in vivo. Oncotarget 6, 19190–19203 (2015).
Casaburi, I. et al. Estrogen related receptor α (ERRα) a promising target for the therapy of adrenocortical carcinoma (ACC). Oncotarget 6, 25135–25148 (2015).
Tamburello, M. et al. Preclinical evidence of progesterone as a new pharmacological strategy in human adrenocortical carcinoma cell lines. Int. J. Mol. Sci. 24, 6829 (2023).
Doghman, M. et al. Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonists. J. Clin. Endocrinol. Metab. 94, 2178–2183 (2009).
Cheng, Y., Kerppola, R. E. & Kerppola, T. K. ATR-101 disrupts mitochondrial functions in adrenocortical carcinoma cells and in vivo. Endocr. Relat. Cancer 23, 1–19 (2016).
LaPensee, C. R. et al. ATR-101, a selective and potent inhibitor of acyl-CoA acyltransferase 1, induces apoptosis in h295r adrenocortical cells and in the adrenal cortex of dogs. Endocrinology 157, 1775–1788 (2016).
Subramanian, C. et al. Synthetic high-density lipoprotein nanoparticles: a novel therapeutic strategy for adrenocortical carcinomas. Surgery 159, 284–294 (2016).
Bussey, K. J. et al. Targeting polo-like kinase 1, a regulator of p53, in the treatment of adrenocortical carcinoma. Clin. Transl. Med. 5, 1 (2016).
Martarelli, D., Pompei, P., Baldi, C. & Mazzoni, G. Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemother. Pharmacol. 61, 809–817 (2008).
Poli, G. et al. Metformin as a new anti-cancer drug in adrenocortical carcinoma. Oncotarget 7, 49636–49648 (2016).
Glover, A. R. et al. MicroRNA-7 as a tumor suppressor and novel therapeutic for adrenocortical carcinoma. Oncotarget 6, 36675–36688 (2015).
Babińska, A., Pęksa, R., Wiśniewski, P., Świątkowska-Stodulska, R. & Sworczak, K. Diagnostic and prognostic role of SF1, IGF2, Ki67, p53, adiponectin, and leptin receptors in human adrenal cortical tumors. J. Surg. Oncol. 116, 427–433 (2017).
Liu, S., Ding, G., Zhou, Z. & Feng, C. β-Catenin-driven adrenocortical carcinoma is characterized with immune exclusion. Onco Targets Ther. 11, 2029–2036 (2018).
Pennanen, M. et al. C-myc expression in adrenocortical tumours. J. Clin. Pathol. 71, 129–134 (2018).
Fernandez-Ranvier, G. G. et al. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch. Surg. 143, 841–846 (2008).
Aporowicz, M. et al. Minichromosome maintenance proteins MCM-3, MCM-5, MCM-7, and Ki-67 as proliferative markers in adrenocortical tumors. Anticancer Res. 39, 1151–1159 (2019).
Cheng, Y., Kou, W., Zhu, D., Yu, X. & Zhu, Y. Future directions in diagnosis, prognosis and disease monitoring of adrenocortical carcinoma: novel non-invasive biomarkers. Front. Endocrinol. 12, 811293 (2021).
Mytareli, C. et al. The diagnostic, prognostic and therapeutic role of mirnas in adrenocortical carcinoma: a systematic review. Biomedicines 9, 1501 (2021).
Cantini, G. et al. Prognostic and monitoring value of circulating tumor cells in adrenocortical carcinoma: a preliminary monocentric study. Cancers 12, 3176 (2020).
Creemers, S. G. et al. Identification of mutations in cell-free circulating tumor DNA in adrenocortical carcinoma: a case series. J. Clin. Endocrinol. Metab. 102, 3611–3615 (2017).
Zhang, F. et al. Prognostic role of Ki-67 in adrenocortical carcinoma after primary resection: a retrospective mono-institutional study. Adv. Ther. 36, 2756–2768 (2019).
Xu, W. H. et al. Screening and identification of potential prognostic biomarkers in adrenocortical carcinoma. Front. Genet. 10, 821 (2019).
Fragoso, M. C. et al. Combined expression of BUB1B, DLGAP5, and PINK1 as predictors of poor outcome in adrenocortical tumors: validation in a Brazilian cohort of adult and pediatric patients. Eur. J. Endocrinol. 166, 61–67 (2012).
Sbiera, S. et al. Assessment of VAV2 expression refines prognostic prediction in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 102, 3491–3498 (2017).
Faria, A. M. & Almeida, M. Q. Differences in the molecular mechanisms of adrenocortical tumorigenesis between children and adults. Mol. Cell Endocrinol. 351, 52–57 (2012).
Sbiera, S. et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J. Clin. Endocrinol. Metab. 95, E161–E171 (2010).
Miller, B. S., Gauger, P. G., Hammer, G. D., Giordano, T. J. & Doherty, G. M. Proposal for modification of the ENSAT staging system for adrenocortical carcinoma using tumor grade. Langenbecks Arch. Surg. 395, 955–961 (2010).
Mermejo, L. M. et al. Altered expression of noncanonical Wnt pathway genes in paediatric and adult adrenocortical tumours. Clin. Endocrinol. 81, 503–510 (2014).
Lavoie, J. M. et al. Whole-genome and transcriptome analysis of advanced adrenocortical cancer highlights multiple alterations affecting epigenome and DNA repair pathways. Cold Spring Harb. Mol. Case Stud. 8, a006148 (2022).
Legendre, C. R. et al. pathway implications of aberrant global methylation in adrenocortical cancer. PLoS ONE 11, e0150629 (2016).
Fonseca, A. L. et al. Comprehensive DNA methylation analysis of benign and malignant adrenocortical tumors. Genes Chromosomes Cancer 51, 949–960 (2012).
Gao, Z. H. et al. Association of H19 promoter methylation with the expression of H19 and IGF-II genes in adrenocortical tumors. J. Clin. Endocrinol. Metab. 87, 1170–1176 (2002).
Arlt, W. et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 96, 3775–3784 (2011).
Acknowledgements
The authors thank G. Kalafatis for her help with organizing the manuscript draft and for management of the references for the manuscript. The authors apologize to colleagues whose work they may not have cited given the space constraint for the article.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Michael Fassnacht, Joakim Crona, who co-reviewed with Liang Zhang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Area under the receiver operating characteristic (ROC) curve
-
(AUC). A statistical analysis used to measure the diagnostic accuracy of a test. The y axis represents the sensitivity (true positive rate) and the x axis represents 1 − specificity (true negative rate) of the test when using different cut-off values of the value measured. The closer the curve is to the left upper corner of the graph the more accurate the test is. Thus, a test with an AUC = 1 is perfect and a test with an AUC = 0.5 is completely random.
- Classical subtype
-
The common histological subtype based on microscopic features of eosinophilic cytoplasm, often with thick fibrous bands and capsule, necrosis and mitotic figures.
- Cushing syndrome
-
A disorder that is due to overproduction of cortisol over a prolonged period; also called hypercortisolism.
- Decision curve analysis
-
A method of statistical analysis to evaluate prediction models, diagnostic tests and molecular markers.
- Genomic imprinting
-
A mechanism of silencing of a gene in which the repressed allele is methylated and the active allele is unmethylated.
- Helsinki scoring system
-
A system for diagnosis and prognosis based on evaluation of a combination of morphological (mitoses and necrosis) and immunohistochemical (Ki-67) parameters in patients.
- Leydig cells
-
Cells in the testis that are the primary source of testosterone.
- Lin–Weiss–Bisceglia system
-
A modified Weiss scoring system, which recommends that for oncocytic adrenocortical neoplasms, a malignancy can be indicated in the presence of one major criterion and indicated as uncertain in the presence of only minor criteria or considered as benign in the absence of both major and minor criteria.
- Lynch syndrome
-
An inherited cancer syndrome that often genetically predisposes the patient to different types of cancer, especially colorectal cancer. Hence, it is also referred to as hereditary non-polyposis colorectal cancer.
- Metabolic tumour volume
-
(MTV). A measurement of the metabolically active tumour volume based on tumour segmentation for the amount of 18F-fluorodeoxyglucose taken up on PET.
- Myxoid subtype
-
When observed by microscopy, frequent cords or trabeculae of tumour cells appear floating in the stroma with diffuse pools or a lack of extracellular mucin. The tumour mucin is positive for Alcian blue.
- Nomograms
-
Graphical calculating devices enabling an approximate graphical computation of a mathematical model predicting the relationship between variables and the probability of the outcome associated with those variables.
- Oncocytic subtype
-
When observed by microscopy, abundant granular eosinophilic cytoplasm, excessive number of mitochondria and high-grade nuclear features are present. Frequent atypical mitotic figures and intranuclear inclusions are also observed.
- Primary hyperaldosteronism
-
A disorder that is due to overproduction and release of aldosterone from the adrenal glands.
- Reticulin algorithm
-
Distinguishes malignancy through an altered reticulin framework (a type of fibre in connective tissue composed of type III collagen in which these reticular fibres crosslink to form a fine meshwork) associated with either necrosis, a high mitotic rate or vascular invasion.
- Sarcomatoid subtype
-
When observed by microscopy, frequent spindle tumour cells as well as giant cells are present. There is also prominent nuclear pleomorphism and atypical mitotic figures.
- Scoring systems of Weiss et al.
-
The reference scoring method to distinguish between benign and malignant adrenocortical tumours in adults based on positive scores for features related to, for example, architecture, nucleus and the presence of any type of invasion, with each feature given a score of 1. A total score of 3 or more indicates a malignant tumour.
- Standardized uptake value
-
(SUV). A semi-quantitative measure of the amount of 18F-fluorodeoxyglucose taken up by a tumour with PET. The value is determined by the ratio of activity per unit volume of a region of interest to the activity per unit whole body volume. SUVs are reported as the mean (the average over the region of interest) and the maximum (the highest in the region of interest).
- Steroid sulfation
-
The sulfation of endogenous steroids. In general, sulfated steroids are not able to bind and activate their target nuclear receptors and also require active transport into cells by an anion transporter as they are no longer lipophilic owing to the sulfation.
- SV40 large T antigen
-
A dominant-acting oncoprotein derived from the polyomavirus SV40 and capable of inducing malignant transformation of various cell types. The transforming activity of this oncoprotein is largely due to its dysregulation of RB and p53.
- Telomere maintenance pathway
-
The molecular pathway that regulates telemore length, which is essential for cancer cells to proliferate and not undergo senescence or apoptosis.
- Total lesion glycolysis
-
(TLG). The product of the standardized uptake value and metabolic tumour volume, which more accurately reflects the glycolytic phenotype of a tumour and is associated with prognosis in several types of cancer.
- Virilization
-
The acquisition of adult male physical features that develop in a female or young male, precociously, owing to excess androgen production.
- Weighted correlation network analysis
-
(WGCNA). A widely used data mining method used especially for biological networks based on pairwise correlations between variables.
- Wieneke criteria
-
A scoring system based on tumour size, local invasion and histological features, which distinguishes between benign and malignant tumours as well as predicts the prognosis of paediatric adrenocortical carcinomas.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, C., Hu, J. & Kebebew, E. Advances in translational research of the rare cancer type adrenocortical carcinoma. Nat Rev Cancer 23, 805–824 (2023). https://doi.org/10.1038/s41568-023-00623-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00623-0