Abstract
Celiac disease (CeD) is a common small bowel enteropathy characterized by an altered adaptive immune system and increased mucosal antigen presenting cells. This study aims to establish if quantification of corneal Langerhans cells (LCs) using corneal confocal microscopy (CCM) could act as a surrogate marker for antigen presenting cell status and hence disease activity in children with CeD. Twenty children with stable CeD and 20 age-matched controls underwent CCM and quantification of central corneal total, mature and immature LC density. There was no difference in age (11.78 ± 1.7 vs. 12.83 ± 1.91; P = 0.077) or height (1.38 ± 0.14 vs. 1.44 ± 0.13; P = 0.125). BMI (18.81 ± 3.90 vs. 22.26 ± 5.47; P = 0.031) and 25 OHD levels (43.50 ± 13.36 vs. 59.77 ± 22.45; P = 0.014) were significantly lower in children with CeD compared to controls. The total (33.33(16.67–59.37) vs. 51.56(30.21–85.42); P = 0.343), immature (33.33(16.67–52.08) vs. 44.79(29.17–82.29); P = 0.752) and mature (1.56(0–5) vs. 1.56(1.04–8.33); P = 0.752) LC density did not differ between the CeD and control groups. However, immature (r = 0.535, P = 0.015), mature (r = 0.464, P = 0.039), and total (r = 0.548, P = 0.012) LC density correlated with age. Immature (r = 0.602, P = 0.038) and total (r = 0.637, P = 0.026) LC density also correlated with tissue transglutaminase antibody (Anti-TtG) levels assessed in 12/20 subjects with CeD. There was no difference in corneal LC density between children with CeD and controls. However, the correlation between corneal LC density and anti-TtG levels suggests a relationship with disease activity in CeD and requires further study.
Similar content being viewed by others
Introduction
Celiac disease (CeD) affects ~ 0.7% of the world population1, but may be more prevalent in the Middle East2,3, especially in Qatar4. It is characterized by varying degrees of intestinal malabsorption, caused by an inappropriate immune response to ingested wheat gluten containing gliadin. Histopathological studies demonstrate villous atrophy with defective transepithelial5,6 and paracellular uptake of gliadin by the intestinal mucosa of patients with active celiac disease.
Circulating dendritic cells are recruited to the inflamed mucosa in those with active CeD, and indeed , there is a significant increase in the number of dendritic cells (DC) in the lamina propria of patients with active celiac disease, which reverts to normal with a gluten-free diet7,8. DCs isolated from patients with active celiac disease behave as APCs and transcribe IFN-gamma9, a key cytokine in the pathogenesis of CeD. The level of auto-antibodies to the enzyme transglutaminase 2 (TG2) and gliadin in gluten-consuming subjects are used as a diagnostic adjunct and marker of disease activity. Intriguingly, TG2 is expressed on most cell surfaces including monocytes and APCs, suggesting that it may facilitate the uptake of gluten10.
Corneal Langerhans cells (LC’s) are APCs which modulate the immune response in the cornea11,12. We have used corneal confocal microscopy (CCM) a rapid, non-invasive and well-tolerated ophthalmic imaging technique to quantify the number of mature and immature corneal LC’s11,12,13,14,15. Moreover, we and others have shown increased LC’s in patients with type 1 diabetes11,16, latent autoimmune diabetes of adults (LADA)16, multiple sclerosis (MS)12, long-COVID17, dry eye disease18, systemic lupus erythematosus (SLE)19, fibromyalgia20, thyroid-associated ophthalmopathy21, and chronic inflammatory demyelinating polyneuropathy (CIDP)22. These studies suggest that corneal immune cells are associated with a number of immune and inflammatory diseases. In the present study we have quantified mature, immature and total numbers of LCs and related them to clinical parameters and anti-TtG level in children with CeD.
Results
There was no significant difference in age, height, or tissue transglutaminase antibody between children with CeD and the control group. BMI and vitamin D were significantly lower in children with CeD compared to the control group (Table 1).
Langerhans cells
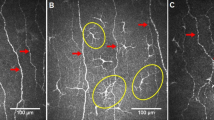
The total, immature and mature LC density in the central cornea did not differ between children with CeD and controls (Fig. 1a,b) (Table 2).
Correlation between Langerhans cell densities and clinical parameters.
Using spearman correlation, immature (r = 0.535, P = 0.015), mature (r = 0.464, P = 0.039), and total (r = 0.548, P = 0.012) LC density correlated with age. In 12/20 children with CeD who had recently undergone assessment of anti-TtG levels, there was a significant correlation with immature LC density (r = 0.602, P = 0.038) and total LC density (r = 0.637, P = 0.026) (Fig. 2a–c).
Discussion
Gluten-induced activation of the adaptive immune response and immune mediated tissue inflammation occurs in celiac disease. We show normal anti-TtG levels and LC density in children with stable CeD. Previous studies have shown increased LC’s in a variety of autoimmune conditions including diabetes11,16, latent autoimmune diabetes of adults (LADA)16, multiple sclerosis (MS)12, long-COVID17, dry eye disease18, systemic lupus erythematous (SLE)19 and fibromyalgia20. Our group was the the first to show increased LC’s in patients with chronic inflammatory demyelinating polyneuropathy (CIDP), suggesting that corneal LC’s may be a surrogate marker of antigen presenting cell status in peripheral nerves22. Indeed, recent studies in patients with CIDP have shown that increased LC’s may predict increased rates of disease progression and neurological disability23,24. Furthermore, treatment of a 27-year-old patient with subacute anti-neurofascin-155 neuropathy with rituximab was associated with an improvement in clinical and neurophysiological parameters and a reduction in antibody titers and LC density25.
Interestingly, despite overall normal anti-TtG level, immature and total LC densities correlated with anti-TtG levels. Anti-TtG levels are increased in CeD, but also other inflammatory conditions such as Crohn’s disease, ulcerative colitis, primary biliary cirrhosis26, rheumatoid arthritis27, and chronic liver disease28 which has been attributed to an overexpression of TtG, and its involvement in apoptosis, tissue repair, and fibrosis29.
We also found a lower level of 25OHD in patients with CeD, consistent with a higher prevalence of vitamin D deficiency in children with newly diagnosed celiac disease30 and a non-linear relationship between vitamin D deficiency and the development of CeD in at-risk children31. In a recent study of 200 Saudi adolescent girls with marked vitamin D deficiency a strong inverse correlation was observed between TtG and 25 OHD levels32 . Vitamin D is a key mediator of innate immune function and signals directly to alter macrophage and dendritic cell function33. We found no correlation between 25OHD levels and LC density, although a previous study demonstrated an inverse correlation between vitamin D levels and dendritic cells in patients with dry eye34.
In conclusion, children with stable CeD have normal corneal LC numbers. However, even though, anti-TtG levels were within the normal range there was a correlation with LC density, suggesting there may be sub-clinical immune activation in children with stable CeD. This is an exploratory study with a cross-sectional design and small sample size. Nevertheless, the association between anti-TtG levels and LC’s merits further study to assess if corneal LCs could be used to monitor disease activity and adherence to a gluten-free diet in patients with CeD.
Methods
Children with a confirmed diagnosis of celiac disease and a positive serology test for anti-TtG antibodies (available in 12/20) with a disease duration of 4.49 ± 4.02 years and 20 healthy controls were recruited. Inclusion criteria were age between 8 and 17 years and a diagnosis of CeD. Exclusion criteria were any history of a cause of neuropathy, malignancy, vitamin B12 or folate deficiency, liver, or renal dysfunction. Participants were also excluded in they had corneal pathology, allergy to the eye-drops or previous ocular trauma or surgery in the past six months. Participants with a history of or current contact lens use were excluded. The study was approved by the Ethics Committee of Weill Cornell Medicine-Qatar (IRB 1700032) and Sidra Medicine (IRB 1500758–3) and was undertaken according to the principles of the Helsinki Declaration. Written informed consent and assent were obtained from all participants and their parents.
Corneal confocal microscopy procedure
Corneal confocal microscopy was undertaken using the Heidelberg Retina Tomograph Cornea Module (Heidelberg Engineering, Germany). Anesthetic drops Bausch & Lomb Minims ® (Oxybuprocaine hydrochloride 0.4% w/v) were used to numb both eyes to limit irritation and discomfort during the examination. A drop of hypotears gel (Carbomer 0.2% eye gel) was placed on the tip of the objective lens and a sterile disposable TomoCap was placed over the lens, allowing optical coupling of the objective lens to the cornea. Images were captured from the central cornea to quantify corneal Langerhans cells in the sub-basal layer. The investigator (HG) was blind to the study group when performing CCM and analyzing CCM images.
Image selection and quantification
Six images were selected from the central cornea, excluding those with pressure lines and out of focus images. Langerhans cells were identified as white, bright structures13. Total, mature (with dendrites) and immature (without dendrites and a total end-to-end length less than 25 µm)35 LC’s were counted manually using CCMetrics software11. The investigator was blind to disease and control group during selection and quantification of the CCM images using anonymized codes for each participant.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics software Version 27 and P < 0.05 was considered statistically significant. Normality of the data was assessed using the Shapiro–Wilk test, histograms and normal Q-Q plot. Data are expressed as mean ± SD for the normally distributed variables and as median(range) for the skewed variables. Inferential analyses were conducted for the corneal nerve parameters and clinical demographics using both parametric (T-test) and non-parametric (Mann–Whitney U) tests, with post-hoc adjustment. To investigate the association between corneal nerve metrics and clinical variables, Pearson and Spearman correlation were performed as appropriate. Graphpad prism version 9 was used to build dot plots.
Data availability
Data is available upon reasonable request to the corresponding author.
References
Glissen Brown, J. R. & Singh, P. Coeliac disease. Paediatr. Int. Child Health 39, 23–31. https://doi.org/10.1080/20469047.2018.1504431 (2019).
Aljebreen, A. M., Almadi, M. A., Alhammad, A. & Al Faleh, F. Z. Seroprevalence of celiac disease among healthy adolescents in Saudi Arabia. World J. Gastroenterol. 19, 2374–2378. https://doi.org/10.3748/wjg.v19.i15.2374 (2013).
Abu-Zeid, Y. A., Jasem, W. S., Lebwohl, B., Green, P. H. & ElGhazali, G. Seroprevalence of celiac disease among United Arab Emirates healthy adult nationals: A gender disparity. World J. Gastroenterol. 20, 15830–15836. https://doi.org/10.3748/wjg.v20.i42.15830 (2014).
Haris, B. et al. The prevalence, immune profile, and clinical characteristics of children with celiac disease and type 1 diabetes mellitus in the state of Qatar. J. Pediatr. Endocrinol. Metab. 34, 1457–1461. https://doi.org/10.1515/jpem-2021-0452 (2021).
Schulzke, J. D., Bentzel, C. J., Schulzke, I., Riecken, E. O. & Fromm, M. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr. Res. 43, 435–441. https://doi.org/10.1203/00006450-199804000-00001 (1998).
Schumann, M. et al. Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut 57, 747–754. https://doi.org/10.1136/gut.2007.136366 (2008).
Raki, M. et al. A unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells. Gastroenterology 131, 428–438. https://doi.org/10.1053/j.gastro.2006.06.002 (2006).
Di Sabatino, A. et al. Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology 133, 1175–1187. https://doi.org/10.1053/j.gastro.2007.08.018 (2007).
Kim, S. Y., Jeong, E. J. & Steinert, P. M. IFN-gamma induces transglutaminase 2 expression in rat small intestinal cells. J. Interferon Cytokine Res. 22, 677–682. https://doi.org/10.1089/10799900260100169 (2002).
Akimov, S. S. & Belkin, A. M. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood 98, 1567–1576. https://doi.org/10.1182/blood.v98.5.1567 (2001).
Ferdousi, M. et al. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci. Rep. 9, 8758. https://doi.org/10.1038/s41598-019-45116-z (2019).
Khan, A. et al. Corneal immune cells are increased in patients with multiple sclerosis. Transl. Vis. Sci. Technol. 10, 19. https://doi.org/10.1167/tvst.10.4.19 (2021).
Zhivov, A., Stave, J., Vollmar, B. & Guthoff, R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch. Clin. Exp. Ophthalmol. 243, 1056–1061. https://doi.org/10.1007/s00417-004-1075-8 (2005).
Rosenberg, M. E., Tervo, T. M., Muller, L. J., Moilanen, J. A. & Vesaluoma, M. H. In vivo confocal microscopy after herpes keratitis. Cornea 21, 265–269. https://doi.org/10.1097/00003226-200204000-00006 (2002).
Mastropasqua, L. et al. Epithelial dendritic cell distribution in normal and inflamed human cornea: In vivo confocal microscopy study. Am. J. Ophthalmol. 142, 736–744. https://doi.org/10.1016/j.ajo.2006.06.057 (2006).
D’Onofrio, L. et al. Small nerve fiber damage and langerhans cells in type 1 and type 2 diabetes and LADA measured by corneal confocal microscopy. Invest. Ophthalmol. Vis. Sci. 62, 5. https://doi.org/10.1167/iovs.62.6.5 (2021).
Bitirgen, G. et al. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br. J. Ophthalmol. https://doi.org/10.1136/bjophthalmol-2021-319450 (2021).
Machetta, F. et al. In vivo confocal microscopic evaluation of corneal langerhans cells in dry eye patients. Open Ophthalmol. J. 8, 51–59. https://doi.org/10.2174/1874364101408010051 (2014).
Resch, M. D. et al. Dry eye and corneal langerhans cells in systemic lupus erythematosus. J. Ophthalmol. 2015, 543835. https://doi.org/10.1155/2015/543835 (2015).
Klitsch, A. et al. Reduced association between dendritic cells and corneal sub-basal nerve fibers in patients with fibromyalgia syndrome. J. Peripher. Nerv. Syst. 25, 9–18. https://doi.org/10.1111/jns.12360 (2020).
Wu, L. Q. et al. Observation of corneal langerhans cells by in vivo confocal microscopy in thyroid-associated ophthalmopathy. Curr. Eye. Res. 41, 927–932. https://doi.org/10.3109/02713683.2015.1133833 (2016).
Stettner, M. et al. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann. Clin. Transl. Neurol. 3, 88–100. https://doi.org/10.1002/acn3.275 (2016).
Pitarokoili, K. et al. Neuroimaging markers of clinical progression in chronic inflammatory demyelinating polyradiculoneuropathy. Ther. Adv. Neurol. Disord. 12, 1756286419855485. https://doi.org/10.1177/1756286419855485 (2019).
Motte, J. et al. Corneal inflammatory cell infiltration predicts disease activity in chronic inflammatory demyelinating polyneuropathy. Sci. Rep. 11, 15150. https://doi.org/10.1038/s41598-021-94605-7 (2021).
Athanasopoulos, D. et al. Longitudinal study on nerve ultrasound and corneal confocal microscopy in NF155 paranodopathy. Ann. Clin. Transl. Neurol. 7, 1061–1068. https://doi.org/10.1002/acn3.51061 (2020).
Bizzaro, N. et al. IgA and IgG tissue transglutaminase antibody prevalence and clinical significance in connective tissue diseases, inflammatory bowel disease, and primary biliary cirrhosis. Dig. Dis. Sci. 48, 2360–2365. https://doi.org/10.1023/b:ddas.0000007875.72256.e8 (2003).
Picarelli, A. et al. Anti-tissue transglutaminase antibodies in arthritic patients: A disease-specific finding?. Clin. Chem. 49, 2091–2094. https://doi.org/10.1373/clinchem.2003.023234 (2003).
Vecchi, M. et al. High rate of positive anti-tissue transglutaminase antibodies in chronic liver disease. Role of liver decompensation and of the antigen source. Scand. J. Gastroenterol. 38, 50–54 (2003).
Mirza, A. et al. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. Am. J. Physiol. 272, G281-288. https://doi.org/10.1152/ajpgi.1997.272.2.G281 (1997).
Seth, A., Kumar, P. & Jain, A. Prevalence and management of vitamin D deficiency in children with newly diagnosed coeliac disease: cohort study. Paediatr. Int. Child Health 41, 247–252. https://doi.org/10.1080/20469047.2021.1996089 (2021).
Andren Aronsson, C. et al. 25(OH)D levels in infancy is associated with celiac disease autoimmunity in at-risk children: A case-control study. Front. Nutr. 8, 720041. https://doi.org/10.3389/fnut.2021.720041 (2021).
Sulimani, R. A. Celiac disease and severe vitamin D deficiency: The case for anti-tissue transglutaminase antibody screening. Arch. Osteoporos. 14, 30. https://doi.org/10.1007/s11657-018-0554-1 (2019).
Arnold, E. P. et al. Urodynamics of female incontinence: Factors influencing the results of surgery. Am. J. Obstet. Gynecol. 117, 805–813. https://doi.org/10.1016/0002-9378(73)90496-1 (1973).
Shetty, R. et al. Corneal dendritic cell density is associated with subbasal nerve plexus features, ocular surface disease index, and serum vitamin D in evaporative dry eye disease. Biomed. Res. Int. 2016, 4369750. https://doi.org/10.1155/2016/4369750 (2016).
Lagali, N. S. et al. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Sci. Rep. 8, 14248. https://doi.org/10.1038/s41598-018-32410-5 (2018).
Funding
This study was funded by the Sidra Internal Research Fund (SIRF 5011029001) and a Biomedical Research Program [BMRP- 5726113101] Grant from the Qatar National Research Fund (QNRF).
Author information
Authors and Affiliations
Contributions
R.A.M. and A.K.A. were the guarantors and designed the study; H.G., I.M., I.N.P., G.P., A.K., S.S., B.A., M.E., W.A., H.A., P.S., S.A. H.A., K.H., M.A.H., F.A., and K.A. participated in the data acquisition; H.G. and M.F. undertook the image analysis. H.G. undertook data analysis, data interpretation and drafted the initial manuscript; R.A.M., A.K.A., and K.H. revised the article critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gad, H., Mohammed, I., Saraswathi, S. et al. Corneal Langerhans cells in children with celiac disease. Sci Rep 12, 18289 (2022). https://doi.org/10.1038/s41598-022-22376-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22376-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.