Abstract
Corneal confocal microscopy (CCM) has been used to identify corneal nerve damage and increased Langerhans cell (LC) density in adults with Type 1 diabetes mellitus (T1DM). The purpose of this study was to evaluate whether corneal confocal microscopy can identify early corneal nerve damage and change in LC density in children and adolescents with T1DM. 64 participants with T1DM (age-14.6 ± 2.5 years, duration of diabetes-9.1 ± 2.7 years, HbA1c-75.66 ± 2.53 mmol/mol [9.1 ± 1.8%]) and 48 age-matched healthy control subjects underwent CCM. Sub-basal corneal nerve morphology and the density of mature and immature LCs was quantified. Corneal nerve fibre length and branch density were lower, whilst fibre density and tortuosity did not differ and both immature and mature LC density was significantly higher in T1DM compared to control subjects. There was no association between HbA1c and duration of diabetes with nerve fibre parameters or LC’s density. Children and adolescents with T1DM demonstrate early immune activation and nerve degeneration.
Similar content being viewed by others
Introduction
Advanced diabetic neuropathy is a cause of significant morbidity and mortality. It is therefore important to identify early nerve damage to limit the development and progression of this complication. We and others have demonstrated the utility of corneal confocal microscopy in identifying early corneal nerve loss in adults and small cohorts of young adolescents with Type 1 diabetes mellitus (T1DM) without neuropathy1,2,3,4,5 and was not associated with dry eye6. We have also demonstrated corneal nerve loss in adults and children with T1DM without retinopathy and microalbuminuria, suggesting that this may be the earliest microvascular complication7,8. Of prognostic relevance, a reduction in corneal nerve fibre length has been shown to predict the development of diabetic neuropathy in adults with T1DM9,10.
Corneal Langerhans cells (LC’s), first identified by Engelmann in 186711, are bone marrow derived antigen-presenting cells and a component of the corneal immune defence system12, located throughout the corneal epithelium13. LC’s in the central cornea are immature cells without dendritic structures while those in the periphery are mature and possess dendritic structures14. They reside primarily in the basal epithelium or sub-basal layer15 and several studies have used corneal confocal microscopy (CCM) to quantify LC’s in normal and pathologic human corneas11,15,16.
Studies have reported a link between LC’s and neuropathy with an increase in epidermal LC density being related to a reduction in the intra epidermal nerve fibre density (IENFD) in the footpad of Streptozotocin (STZ) diabetic rats17. Epidermal LC density has also been shown to be increased and related to reduced IENFD in patients with painful diabetic neuropathy18. Both epidermal and sub-epidermal LC’s are increased in the db/db mouse in the initial phase of mechanical allodynia and then decrease when mechanical allodynia diminishes19. We have previously demonstrated a three-fold increase in the density of LC’s in the central cornea of patients with diabetes with no or mild neuropathy20. However, in that study we were using a first-generation white light corneal confocal microscope, which cannot differentiate mature from immature LC’s. We have undertaken corneal confocal microscopy to quantify sub-basal corneal nerve pathology and LCs density in a large cohort of children and adolescents with T1DM. This may allow us to assess the utility of CCM in identifying early nerve fibre damage and insights into the mechanisms underlying early corneal nerve fibre loss in children with T1DM.
Research Design and Methods
Study subjects
This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary and Alberta Children’s Hospital. Children with a history of at least 5 years of T1DM aged 8 to 18 years and healthy age-matched control subjects were invited to participate. Subjects were recruited through the Alberta Children’s Hospital Diabetes Clinic and healthy controls were recruited from the patient’s family, relatives and through advertisement posters in general paediatric or paediatric ophthalmology clinics. Subjects with a known history of corneal abnormality, trauma or surgery, wearing contact lenses, any other cause of neuropathy, uncontrolled hypothyroidism and celiac disease were excluded from the study. This study adhered to the tenets of the Declaration of Helsinki and informed written consent was obtained from all participants aged 18 years old and from a parent and/or legal guardian of participants aged less than 18 years old.
Corneal confocal microscopy
All the participants underwent CCM using the Heidelberg Retinal Tomograph III Rostock Cornea Module (Heidelberg Engineering GmbH, Heidelberg, Germany) according to our previously published procedures21. HRT III is a class 1-laser system with 400 × 400 μm field of view and 10 μm/pixel optical resolution with a 2D digital image size of 384 × 384 pixels. Images were captured from the centre of the cornea using the section mode. Patients were assessed and CCM was performed at the Alberta Children’s hospital by three well-trained examiners and we have previously demonstrated the feasibility and reproducibility of this technique in children22,23. All participants were examined by an ophthalmologist after the CCM was performed to assess for any corneal alterations.
Demographic data included; age, gender, duration of diabetes, self-assessment pubertal stage (tanner stage I–V)24 and HbA1c. Six CCM images (3 per eye) of the corneal sub-basal nerve plexus were selected from 10–18 images and were analysed by a single examiner in a masked manner25. All the images were analysed manually using purpose-designed software (CCMetrics, MA Dabbah; Imaging Science and Biomedical Engineering, University of Manchester, Manchester, UK)26. Corneal nerves were traced using a digital pen and a wide screen tablet (Trust international B.V., China). Corneal nerve fibre morphological parameters included corneal nerve fibre density (CNFD); a measure of the total number of main corneal nerves/mm2, corneal nerve branch density (CNBD); the number of junctions between branches and main nerves/mm2, corneal nerve fibre length (CNFL); the total length of all corneal nerve fibres (mm/mm2) and corneal nerve fibre tortuosity (CNFT), as expressed by the tortuosity coefficient (TC).
LC’s were defined as bright dendritic structures in the images used to analyse the sub-basal corneal nerves11. LC’s less than 50 µm in length without dendritic structures were considered immature cells and those with a length greater than 50 µm and dendritic structures were considered as mature cells. The LC’s density (no./mm2) was derived by counting the total number of cells in the area of the cornea using the NBD feature and the length of each cell was calculated using the NFL feature of CCMetrics.
Statistical analysis
IBM SPSS v22 (Chicago, IL, USA) for Windows was used to analyse the results. Data are expressed as Mean ± SD and were tested for normality. Two independent sample t tests (for parametric variables) and Mann-Whitney U test (for non-parametric variable) were used to compare means between the two groups. Pearson correlation coefficient (Spearman rank correlation coefficient for non-parametric data) was used to assess the correlation between corneal LCs’ density and other clinical data. To assess categorical data, Pearson’s Chi-square (χ2) test of independence and a Fisher’s Exact test were used.
Results
Sixty-four subjects with T1DM (age: 14.6 ± 2.5 years, duration diabetes: 9.1 ± 2.7 years and HbA1c: 75.66 ± 2.53 mmol/mol [9.1 ± 1.8%]) and 55 aged-matched healthy controls (age: 13.6 ± 3.1 years) were studied. The demographic and CCM results of these subjects are summarized in Table 1.
Corneal nerve morphology
CNFL (mm/mm2) (22.8 ± 4.9 vs 24.8 ± 5.9, P = 0.03) and CNBD (no./mm2) (72.3 ± 29.4 vs 85.7 ± 36.8, P = 0.04) were significantly lower, with no difference in CNFD (no./mm2) (31.4 ± 7.6 vs 31.5 ± 6.8, P = 0.9) and CNFT (TC) (13.8 ± 5.2 vs 12.9 ± 2.9, P = 0.2) between individuals with T1DM and healthy controls (Fig. 1). No significant correlation was found between corneal nerve parameters with age, HbA1c and duration of diabetes.
Corneal nerve fibre parameters in children with Type 1 diabetes mellitus and healthy controls (bars indicate one standard error). (A) Corneal nerve fibre density (no./mm2). (B) Corneal nerve fibre branch density (no/mm2). (C) Corneal nerve fibre length (mm/mm2). (D) Corneal nerve fibre tortuosity (TC).
Langerhans cells
Mature LC density (no./mm2) (2.7 ± 4.3 vs 1.3 ± 2.09, P = 0.04), immature LC density (no./mm2) (48.9 ± 65.5 vs 16.6 ± 21.6, P = 0.005) and the total density of LC’s (no./mm2) (51.6 ± 68.6 vs 17.9 ± 22.6, P = 0.005) were significantly higher in individuals with T1DM compared to healthy controls (Fig. 2). The proportion of individuals with immature (85.9% vs 69.01%, P = 0.04) and mature (60% vs 38.2%, P = 0.04) LC’s was significantly greater in patients with T1DM compared to control subjects.
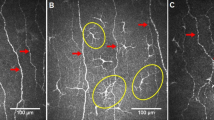
(A) Mature LC’s density (no./mm2) in children with Type 1 diabetes mellitus and healthy controls (bars indicate one standard error). (B) Immature LC’s density (no./mm2) in children with Type 1 diabetes mellitus and healthy controls (bars indicate one standard error). (C) A CCM image of the corneal sub-basal nerve plexus in a healthy control. (D) A CCM image in a child with Type 1 diabetes mellitus showing a reduction in corneal nerves and increased immature (arrows) and mature (circle) LC’s.
Correlations
CNFD (r = 0.2, P = 0.01), age (r = 0.2, P = 0.03) and pubertal stage (Tanner staging) (r = 0.34, P = 0.005) correlated significantly with the density of mature LC’s in patients with T1DM. Pubertal stage correlated significantly with the total LC density (r = 0.25, P = 0.04) and immature LC density (r = 0.2, P = 0.02) in patients with T1DM, but not in controls. The proportion of participants who had gone through puberty did not differ significantly between control subjects (44/55–80%) and participants with T1DM (59/64–90%). The duration of diabetes, HbA1c, CNBD, CNFL and CNFT did not correlate with LC density.
Discussion
Subclinical DN has been reported in 25% of newly diagnosed and 50% of children within 5 years of being diagnosed with T1DM27. However, DN is often asymptomatic in children and early diagnosis is difficult. Techniques used to detect early DN have limited utility in children because they are subjective (symptoms/signs, quantitative sensory testing), uncomfortable (nerve conduction studies) or invasive (skin biopsies)27. We have established CCM as an objective measure of DN in adults with T1DM, T2DM and subjects with impaired glucose tolerance2,28,29,30.
In a pilot study, we previously reported that CCM is an acceptable procedure, but we found no abnormality of corneal nerves in a small cohort of children with T1DM or T2DM23. However, we and others have recently shown that there is an early abnormality in corneal nerve fibres in larger cohorts of children and adolescents with T1DM4,8. Certainly, studies in adults with diabetes show a global reduction in CNFD, CNBD and CNFL and an increase in CNFT31. We now show a significant reduction in corneal nerve branch density and length with preserved nerve fibre density and tortuosity in a large cohort of children with T1DM. This reduction in CNBD and CNFL represents the earliest pathology to the most distal nerves, sparing the more proximal major nerves represented by CNFD. Indeed, an increase in CNBD is indicative of nerve fibre regeneration in individuals treated with CSII32 and after simultaneous pancreas and kidney transplantation29. An increase in corneal nerve fibre area has been shown to be the earliest measure of nerve fibre repair33. Indeed, we have also shown that more prominent nerve fibre loss at the inferior whorl reflects the earliest damage to the most distal nerve fibres, consistent with the dying back process in diabetic neuropathy34.
Previous ex vivo35 and in vivo20 studies have shown an increase in LC density in the diabetic cornea. We show a threefold increase in the density of both mature and immature LC’s in the diabetic cornea. Studies have suggested a possible interaction between LC’s and nerves in the pathogenesis of neuropathy36, indicating neuro-immune communication37. Nerves can also influence immune cell activity by releasing cytokines and neuropeptides38, with relevant neuropeptide receptors being expressed by resident immune cells39. DC’s express neurotrophic factors such as CNTF, which promotes re-innervation and corneal denervation has been associated with a reduction in DC density and altered morphology40. Leppin et al.35 demonstrated a significant increase in DC’s using CCM and related it to total corneal nerve fibre length in STZ-diabetic mice. We now confirm and extend our previous observations showing a significant increase in the density of LC’s in patients with diabetes but find no correlation with the severity of corneal nerve damage, HbA1c or duration of diabetes. There was a correlation between pubertal stage and the density of LC’s in T1DM, but not in controls even though the proportion of participants who had gone through puberty was comparable. This requires further study as there is a link between pubertal stage and progression of neuropathy in youth with diabetes41. Ideally this requires a longitudinal study to assess the temporal change in LC density and corneal nerve morphology during puberty to see if this differs between patients with T1DM and controls.
In conclusion, CCM has identified a small but significant reduction in corneal nerve branch density and length and an increase in LC’s in children and adolescents with T1DM. This early corneal nerve damage was not related to glycemic control, diabetes duration or LC density. The role of CCM as an early diagnostic marker of sub-clinical diabetic neuropathy in children and the potential role of LC’s in mediating this early pathology merits further study.
Data Availability
The dataset generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alam, U. et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PloS one 12, e0180175, https://doi.org/10.1371/journal.pone.0180175 (2017).
Ahmed, A. et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes care 35, 821–828, https://doi.org/10.2337/dc11-1396 (2012).
Sivaskandarajah, G. A. et al. Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes care 36, 2748–2755, https://doi.org/10.2337/dc12-2075 (2013).
Szalai, E. et al. Early Corneal Cellular and Nerve Fiber Pathology in Young Patients With Type 1 Diabetes Mellitus Identified Using Corneal Confocal Microscopy. Investigative ophthalmology & visual science 57, 853–858, https://doi.org/10.1167/iovs.15-18735 (2016).
Perkins, B. A. et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia 61, 1856–1861, https://doi.org/10.1007/s00125-018-4653-8 (2018).
Ferdousi, M. et al. No Relation Between the Severity of Corneal Nerve, Epithelial, and Keratocyte Cell Morphology With Measures of Dry Eye Disease in Type 1 Diabetes. Investigative ophthalmology & visual science 59, 5525–5530, https://doi.org/10.1167/iovs.18-25321 (2018).
Petropoulos, I. N. et al. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PloS one 10, e0123517, https://doi.org/10.1371/journal.pone.0123517 (2015).
Gotze, A. et al. The corneal subbasal nerve plexus and thickness of the retinal layers in pediatric type 1 diabetes and matched controls. Scientific reports 8, 14, https://doi.org/10.1038/s41598-017-18284-z (2018).
Pritchard, N. et al. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes care 38, 671–675, https://doi.org/10.2337/dc14-2114 (2015).
Lewis, E. J. et al. Using in vivo corneal confocal microscopy to identify diabetic sensorimotor polyneuropathy risk profiles in patients with type 1 diabetes. BMJ open diabetes research & care 5, e000251, https://doi.org/10.1136/bmjdrc-2016-000251 (2017).
Zhivov, A., Stave, J., Vollmar, B. & Guthoff, R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 243, 1056–1061, https://doi.org/10.1007/s00417-004-1075-8 (2005).
Knickelbein, J. E., Watkins, S. C., McMenamin, P. G. & Hendricks, R. L. Stratification of Antigen-presenting Cells within the Normal Cornea. Ophthalmology and eye diseases 1, 45–54 (2009).
Hamrah, P., Zhang, Q., Liu, Y. & Dana, M. R. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Investigative ophthalmology & visual science 43, 639–646 (2002).
Hamrah, P., Huq, S. O., Liu, Y., Zhang, Q. & Dana, M. R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. Journal of leukocyte biology 74, 172–178 (2003).
Rosenberg, M. E., Tervo, T. M., Muller, L. J., Moilanen, J. A. & Vesaluoma, M. H. In vivo confocal microscopy after herpes keratitis. Cornea 21, 265–269 (2002).
Mastropasqua, L. et al. Epithelial dendritic cell distribution in normal and inflamed human cornea: in vivo confocal microscopy study. American journal of ophthalmology 142, 736–744, https://doi.org/10.1016/j.ajo.2006.06.057 (2006).
Lauria, G. et al. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. Journal of the peripheral nervous system: JPNS 10, 202–208, https://doi.org/10.1111/j.1085-9489.2005.0010210.x (2005).
Casanova-Molla, J. et al. Epidermal Langerhans cells in small fiber neuropathies. Pain 153, 982–989, https://doi.org/10.1016/j.pain.2012.01.021 (2012).
Dauch, J. R. et al. Neurogenic factor-induced Langerhans cell activation in diabetic mice with mechanical allodynia. Journal of neuroinflammation 10, 64, https://doi.org/10.1186/1742-2094-10-64 (2013).
Tavakoli, M., Boulton, A. J., Efron, N. & Malik, R. A. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Contact lens & anterior eye: the journal of the British Contact Lens Association 34, 7–11, https://doi.org/10.1016/j.clae.2010.08.007 (2011).
Chen, X. et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes care 38, 1138–1144, https://doi.org/10.2337/dc14-2422 (2015).
Pacaud, D. et al. The Reliability and Reproducibility of Corneal Confocal Microscopy in Children. Investigative ophthalmology & visual science 56, 5636–5640, https://doi.org/10.1167/iovs.15-16995 (2015).
Sellers, E. A. et al. The acceptability and feasibility of corneal confocal microscopy to detect early diabetic neuropathy in children: a pilot study. Diabetic medicine: a journal of the British Diabetic Association 30, 630–631, https://doi.org/10.1111/dme.12125 (2013).
Duke, P. M., Litt, I. F. & Gross, R. T. Adolescents’ self-assessment of sexual maturation. Pediatrics 66, 918–920 (1980).
Kalteniece, A. et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PloS one 12, e0183040, https://doi.org/10.1371/journal.pone.0183040 (2017).
Dabbah, M. A., Graham, J., Petropoulos, I. N., Tavakoli, M. & Malik, R. A. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Medical image analysis 15, 738–747, https://doi.org/10.1016/j.media.2011.05.016 (2011).
Nelson, D. et al. Comparison of conventional and non-invasive techniques for the early identification of diabetic neuropathy in children and adolescents with type 1 diabetes. Pediatric diabetes 7, 305–310, https://doi.org/10.1111/j.1399-5448.2006.00208.x (2006).
Tavakoli, M. et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes care 33, 1792–1797, https://doi.org/10.2337/dc10-0253 (2010).
Tavakoli, M. et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 62, 254–260, https://doi.org/10.2337/db12-0574 (2013).
Rosenberg, M. E. et al. Corneal structure and sensitivity in type 1 diabetes mellitus. Investigative ophthalmology & visual science 41, 2915–2921 (2000).
Petropoulos, I. N. et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes care 36, 3646–3651, https://doi.org/10.2337/dc13-0193 (2013).
Azmi, S. et al. Corneal Confocal Microscopy Identifies Small-Fiber Neuropathy in Subjects With Impaired Glucose Tolerance Who Develop Type 2 Diabetes. Diabetes care 38, 1502–1508, https://doi.org/10.2337/dc14-2733 (2015).
Brines, M. et al. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Scientific reports 8, 4734, https://doi.org/10.1038/s41598-018-23107-w (2018).
Kalteniece, A. et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Scientific reports 8, 3283, https://doi.org/10.1038/s41598-018-21643-z (2018).
Leppin, K. et al. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Investigative ophthalmology & visual science 55, 3603–3615, https://doi.org/10.1167/iovs.14-14307 (2014).
Cruzat, A. et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Investigative ophthalmology & visual science 52, 5136–5143, https://doi.org/10.1167/iovs.10-7048 (2011).
Seyed-Razavi, Y., Chinnery, H. R. & McMenamin, P. G. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Investigative ophthalmology & visual science 55, 1313–1320, https://doi.org/10.1167/iovs.13-12995 (2014).
Lambrecht, B. N. Immunologists getting nervous: neuropeptides, dendritic cells and T cell activation. Respiratory research 2, 133–138 (2001).
Peters, E. M. et al. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. The Journal of investigative dermatology 126, 1937–1947, https://doi.org/10.1038/sj.jid.5700429 (2006).
Yu, F. X., Gao, N. & Sun, H. Hyperglycemia targets sensory nerve-dendritic cell interactions, resulting in diabetic corneal neuropathy. Investigative ophthalmology & visual science 55, 4706–4706 (2014).
Riihimaa, P. H. et al. Peripheral nerve function is increasingly impaired during puberty in adolescents with type 1 diabetes. Diabetes care 24, 1087–1092 (2001).
Acknowledgements
Alberta Children’s Hospital Research Institute facilitated this research. This research was funded by awards from Juvenile Diabetes Research Foundation International (Grant: 17-2008-1032).
Author information
Authors and Affiliations
Contributions
M.F. researched data, performed analysis and wrote the manuscript, K.R. researched data, J.K.M. researched data, H.V. researched data, C.M. researched data, R.A.M. designed the study, reviewed and revised the manuscript and D.P. designed the study, reviewed and revised the manuscript. R.A.M. is the guarantor of this work and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferdousi, M., Romanchuk, K., Mah, J.K. et al. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci Rep 9, 8758 (2019). https://doi.org/10.1038/s41598-019-45116-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45116-z
This article is cited by
-
Corneal Langerhans cells in children with celiac disease
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.