Abstract
Endoscopic resection or esophagectomy has becoming the standard treatment for superficial esophageal squamous cell carcinomas (SESCC), but some patients may develop disease progression or second primary cancers after the therapies. Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), and platelet to lymphocyte ratio (PLR) reflect the balance between pro-cancer inflammatory and anti-cancer immune responses, however their roles in SESCC are still unknown. We consecutively enrolled patients with newly diagnosed SESCC (clinical stage Tis or T1N0M0) who were treated at our institute. Pre-treatment NLR, LMR and PLR were assessed and then correlated with clinical factors and long-term survival. A total of 156 patients were enrolled (152 males, 4 females; median age: 52.2 years), of whom 104 received endoscopic resection and 52 were treated with esophagectomy or chemoradiation.. During a mean follow-up period of 60.1 months, seventeen patients died of ESCCs, and 45 died of second primary cancers. The 5-year ESCC-specific survival and 5-year overall survival rate were 86% and 57%, respectively. LMR (P < 0.05) and NLR (P < 0.05), but not PLR were significantly correlated with overall survival. Receiver operating characteristic curve analysis showed optimal LMR and NLR cut-off values of 4 and 2.5, respectively, to predict a poor prognosis. Patients with a high NLR or low LMR tended to have longer tumor length, larger circumferential extension, and presence of second primary cancers. Multivariate Cox regression analysis showed that presence of second primary cancers (HR: 5.05, 95%CI: 2.75–9.28), low LMR (HR: 2.56, 95%CI: 1.09–6.03) were independent risk factors for poor survival. A low pre-treatment LMR may be a non-invasive pretreatment predictor of poor prognosis to guide the surveillance program, suggesting that anti-cancer immunity may play a role in the early events of esophageal squamous cancer.
Similar content being viewed by others
Introduction
Esophageal cancer is the eighth most common cancer and the sixth most common cause of cancer death worldwide1,2,3. The incidence is highest in Asia, with up to 95% being reported in China4. The prognosis of patients with esophageal neoplasia is still extremely poor, even if they receive aggressive surgery or chemoradiation therapy. Recently, image-enhanced endoscopy techniques such as Lugol chromoendoscopy and narrow-band imaging have been shown to improve the detection and diagnosis of superficial esophageal squamous cell carcinoma (SESCC)5,6. In addition, endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) and esophagectomy are the standard treatments for SESCC7. A recent meta-analysis revealed that TP53, CCND1, and MDM2 are the most prevalent gene mutations, which can highlight their role in the carcinogenesis of ESCC8. Nevertheless, some cases may develop recurrence, metastasis or second primary cancers (e.g. Head and neck cancers) after the standard treatment9,10. Previous studies showed the multiple small lugol-voiding lesions in the esophageal background mucosa, presence of poor histological features (e.g. deep submucosal invasion, lymphovascular invasion) in the resected specimens are associated with a worse outcome7,11,12,13,14,15. However, a pre-treatment non-invasive biomarker to predict the long-term outcome of SESCC has yet to be identified.
Several recent studies have demonstrated that systemic inflammation and anti-tumor immunity play crucial roles in carcinogenesis, treatment effect, and long-term outcomes16. Increasing evidence has demonstrated that neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), and platelet to lymphocyte ratio (PLR) reflect the balance between pro-cancer inflammatory and anti-cancer immune responses, and they have been associated with the prognosis of gastroesophageal tract cancers17,18,19,20,21.
Most previous studies have reported the roles of NLR/LMR/PLR in patients with advanced-stage esophageal squamous cell carcinoma (ESCC) or in those who received chemoradiation therapy21,22,23,24, however studies focusing on superficial esophageal cancer or endoscopic resection are still lacking. Therefore, the aims of this study were to evaluate the associations between NLR, LMR, PLR and SESCC, and identify a non-invasive convenient biomarker for the long-term prognosis of patients with SESCC. The realization of the prognostic significance of these systemic inflammatory response markers in patients with SESCC may not only guide the surveillance program after treatment, but also provide the potential targets for prevention of esophageal cancers.
Materials and methods
Patients and design
We consecutively enrolled patients with newly diagnosed SESCC (clinical stage Tis or T1N0M0) at E-Da Hospital from January 2008 to October 2018. All of the included patients received treatment and follow-up at our institute, and pre-treatment complete blood cell count and differential count data were available. All patients underwent computed tomography (CT) to confirm that there was no lymph node involvement or distant metastasis. The baseline demographic and endoscopic characteristics, and data on alcohol drinking, betel nut chewing, and cigarette smoking were extracted from medical records. Treatment was performed based on the National Comprehensive Cancer Network (NCCN) clinical guidelines25. After treatment, the pathological stages, cancer invasion depth and the status of lymphovascular invasion were recorded based on the histopathological evaluation. The date of last follow-up or death was ascertained from medical records or by telephone contact. The data of cause of death and whether the patients presented with secondary primary cancer10,12, which indicated a second cancer develop on another site, such as head & neck or lung, were extracted from medical records. Informed consent was obtained from all of the patients. The study protocol was approved by the Institutional Review Board of E-Da Hospital (EMRP39101N) and conformed to the Declaration of Helsinki and Good Clinical Practice guidelines.
Measurement of pre-treatment NLR, LMR and PLR
The pretreatment hematological parameters, including neutrophil count, lymphocyte count, monocyte count and platelet count were collected within 2 weeks before the initial treatment. The NLR, PLR and LMR were calculated using the absolute values of the corresponding hematological parameters, and they were then correlated with clinical features and long-term survival.
Statistical analysis
All statistical analyses were performed using SPSS software (SPSS for Windows, version 22.0; SPSS Inc., Chicago, IL, USA). Comparisons between the different treatment groups and the clinical characteristics were performed using the χ2 test or the t-test as appropriate. The cumulative cancer-related survival rates were estimated using Kaplan–Meier curves and assessed using the log-rank test. Cox proportional hazard analysis was used to assess the factors associated with a worse outcome. A p value less than 0.05 was considered to indicate a statistically significant difference.
Results
Patient and endoscopic characteristics
A total of 156 patients (152 males and 4 females) with SESCC were included in this study, including 59 with high-grade intraepithelial neoplasia and 97 with T1 squamous cell carcinoma. The median age was 52.2 years, and more than 90% of the patients drank alcohol and/or smoked cigarettes. The mean tumor size was 3.6 cm. Of the enrolled patients, 104 were treated with endoscopic resection and 52 were treated with esophagectomy or chemoradiation. A total of 68 patients were found to have second primary cancers during the follow-up period, including 61 head & neck cancers, 3 gastric cancers, 2 lung cancers and 2 unknown primaries. The demographic and clinical data of the patients are shown in Table 1.
NLR, LMR and PLR and their correlations with clinical features
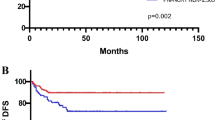
The mean absolute neutrophil, lymphocyte, monocyte and platelet counts were 4.8 ± 3.0 × 103/μl, 1.4 ± 0.8 × 103/μl, 0.5 ± 0.2 × 103/μl, and 221.4 ± 92.1 × 103/μl, respectively. The mean pre-treatment NLR, LMR and PLR values were 6.38, 3.45, and 14.6, respectively. During a mean follow-up period of 60.1 months (range 4–146 months), 17 patients died of ESCC progression, 45 patients died of second primary cancers (38 head & neck cancers; 3 gastric cancers; 2 lung cancers and 2 unknown primary cancers). The 5-year overall survival and ESCC-related survival rate were 57% and 86%, respectively (Fig. 1). Receiver operating characteristic (ROC) curve analysis to identify optimal cut-off values showed an NLR cut-off value of 2.5 (sensitivity: 0.77, specificity: 0.45; area under the curve [AUC]: 0.62), LMR cut-off value of 4 (sensitivity: 0.24, specificity: 0.6; AUC: 0.39), and PLR cut-off value of 5 (sensitivity: 0.92, specificity: 0.18; AUC: 0.62) to predict a poor overall survival of the patients (Fig. 2). Based on these cut-off values, subgroup analysis for survival was performed. Patients with a high NLR (> 2.5) or low LMR (< 4), but not PLR were significantly associated with a worse survival (P < 0.01, Fig. 3A-C). Furthermore, patients with concomitant high-NLR & low-LMR have the worst overall survival (Fig. 3D). A high NLR was correlated with longer tumor length, and larger tumor circumference extension (P < 0.05, Table 2) and tended to be associated with a higher risk of second primary cancer (P = 0.069). A low LMR tended to be associated with a larger tumor size (P = 0.06), larger circumferential extension (P = 0.09) and deeper cancer invasion depth (P = 0.09). Compared to the existing predicting system using the pathological features, including presence of deep submucosal invasion [HR: 0.74, 95% CI: 0.37–1.49, P = 0.402] or presence of lymphovascular invasion [HR: 1.25, 95% CI: 0.51–3.06, P = 0.627], the LMR < 4 [HR: 2.48, 95% CI: 1.11–5.53, P = 0.026] but not NLR > 2.5 [HR: 1.56, 95% CI: 0.70–3.45, P = 0.278] is a significant independent predictor for a worse long-term outcome after resection. Multivariate Cox regression analysis showed that presence of second primary cancers (HR: 5.05, 95% CI: 2.75–9.28, P < 0.001), low LMR (HR: 2.56, 95% CI: 1.09–6.03, P = 0.03), but not NLR (P = 0.75) were independent risk factors associated with a poor prognosis (Table 3).
Discussion
Esophageal cancers are a major global health issue with poor prognosis despite advances in treatment modalities in recent decades. Currently, the existing treatment guidelines suggest using the pathological features and staging in resected specimes to predict the long-term outcome of superficial ESCC14,15. There are no pre-treatment non-invasive biomarkers available to stratify the risk of a poor prognosis. In the present study, we assessed the roles of NLR, LMR and PLR in SESCC, and identified that a low LMR (< 4) was an effective predictor of poor survival. The predictive performance of LMR is better than the currently existing system, potentially because it may reflect the balance between pro-cancer inflammatory and anti-cancer immune responses, as well as the potential impacts on developing second primary cancers that may reduce the overall survival. In contrast, the pathological findings in resected specimens just reflect the local status. To the best of our knowledge, the present study is the first to assess the roles of these inflammatory markers in patients with early-stage esophageal cancers. The pre-treatment value of LMR is easily obtainable, and in the age of individualized patient care and precision medicine, it may represent a risk stratification tool for superficial ESCC patients.
Previous studies have shown that inflammation plays a key role in cancer development, treatment effect, and long-term surviva17,26. The proposed mechanism is that cancer-related inflammation can cause DNA damage, promote angiogenesis and cell proliferation, suppress antitumor immunity, and impact the response to anticancer therapies27,28. In addition, inflammatory responses may help to induce certain populations of tumor stem cells which are critical for resistance and metastasis of tumor tissue29. Tumor-infiltrating neutrophils may be associated with a poor prognosis through the promotion of angiogenesis, cell mobility, and migration17. Lymphocytes have been associated with tumor cell removal and improved tumor surveillance. Previous studies have demonstrated an association between a low peripheral lymphocyte count and poorer survival in different types of cancer30,31. Activated platelets can induce coagulation and interact with tumor cells through paracrine signaling or direct contact, thereby inducing tumor cell growth, angiogenesis and poor survival32,33. Monocytes, which differentiate into tissue macrophages and dendritic cells, can mediate tumor-associated monocyte infiltration in solid tumors, and induce various chemokines such as transforming growth factor (TGF)-α, tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 to promote tumorigenesis, angiogenesis and distant metastasis34,35. This may explain the association between a high monocyte count and poorer survival. NLR, LMR, and PLR have been reported to predict the potential suppression of host immune response and survival of cancer patients16,17. One study demonstrated that patients with ESCC and an NLR > 2.33 had a better pathological response after neoadjuvant chemoradiotherapy22. A recent study also found that high NLR (> 2.20) and PLR (> 110) were associated with larger tumor size, and that NLR was a prognostic factor for resectable ESCC23. Another retrospective study of 1587 ESCC patients who underwent esophagectomy found that a high pre-operative NLR (> 3.29) and low LMR (< 2.95) could predict worse survival in patients with ESCC24. A recent meta-analysis also confirmed that a high NLR may predict a poor prognosis, including overall survival, cancer-specific survival, and progression-free survival of ESCC patients36. However, the roles of NLR, LMR, and PLR in patients with early-stage cancers have rarely been reported. In the present study, we found that the patients with a high pretreatment NLR (> 2.5) or low LMR (< 4) had worse overall survival, suggesting that these parameters could serve as non-invasive, easily obtainable markers in clinical practice. In addition, we found that the patients with a high NLR or low LMR tended to have a larger tumor longitudinal and circumferential extension, suggesting that a lower lymphocyte count and relatively weak anti-tumor immunity may predispose to a larger tumor size and poor long-term survival. Moreover, due to the field cancerization theory9, the risk of developing second primary cancers were very high (43.5%) in SESCC patients and these may lead to a poor prognosis. Thus, surveillance for second primary is becoming an important issue for patients with SESCC. Our study showed patients with high-NLR tended to have second primary cancers (P = 0.069). The presence of second primary cancer or low-LMR were the significant independent predictors for worse survival. These findings not only provide a potential biomarker to predict the prognosis of superficial ESCC, but also provide a clue for the future development of immunotherapy.
There were several limitations to the current study. First, we did not investigate the effect of sequential changes in LMR on recurrent events and treatment response. Second, this study was conducted at a single medical center, and further multicenter studies are required to validate the performance of LMR as a risk assessment marker for SESCC. In conclusion, the patients with SECC (clinical Tis or T1N0M0) and a low pre-treatment LMR in peripheral blood in this study had a poorer prognosis. LMR values can be easily obtained from routinely collected blood samples, and could assist clinicians when deciding the surveillance program for SESCC patients.
In conclusion, minimally invasive endoscopic resection or esophagectomy is the standard treatment for SESCC. However, some patients may occur disease progression or develop second primary cancers after the therapies. Our study identified a low pre-treatment LMR may be a non-invasive pre-treatment predictor of poor prognosis to guide the surveillance program of SESCC, suggesting that anti-cancer immunity may play a role in the early events of esophageal squamous cancer.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Barret, M. & Prat, F. Diagnosis and treatment of superficial esophageal cancer. Ann. Gastroenterol. 31, 256–265. https://doi.org/10.20524/aog.2018.0252 (2018).
Ferlay, J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. https://doi.org/10.1002/ijc.29210 (2015).
Arnold, M., Soerjomataram, I., Ferlay, J. & Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 64, 381–387. https://doi.org/10.1136/gutjnl-2014-308124 (2015).
Kim, J. A. & Shah, P. M. Screening and prevention strategies and endoscopic management of early esophageal cancer. Chin. Clin. Oncol. 6, 50. https://doi.org/10.21037/cco.2017.09.05 (2017).
Muto, M. et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: A multicenter randomized controlled trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 28, 1566–1572. https://doi.org/10.1200/jco.2009.25.4680 (2010).
Oyama, T. et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: Magnifying endoscopic classification of the Japan esophageal society. Esophagus Off. J. Japan Esophageal Soc. 14, 105–112. https://doi.org/10.1007/s10388-016-0527-7 (2017).
Yeh, J. H. et al. Long-term outcomes of endoscopic submucosal dissection and comparison to surgery for superficial esophageal squamous cancer: A systematic review and meta-analysis. Therap. Adv. Gastroenterol. 13, 1756284820964316 (2020).
Naseri, A. et al. Systematic review and meta-analysis of the most common genetic mutations in esophageal squamous cell carcinoma. J. Gastrointest. Cancer https://doi.org/10.1007/s12029-021-00721-y (2021).
Slaughter, D. P., Southwick, H. W. & Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6, 963–968 (1953).
Katada, C. et al. Risk of superficial squamous cell carcinoma developing in the head and neck region in patients with esophageal squamous cell carcinoma. Laryngoscope 122, 1291–1296. https://doi.org/10.1002/lary.23249 (2012).
Urabe, Y. et al. Metachronous multiple esophageal squamous cell carcinomas and lugol-voiding lesions after endoscopic mucosal resection. Endoscopy 41, 304–309 (2009).
Hori, K. et al. Lugol-voiding lesions are an important risk factor for a second primary squamous cell carcinoma in patients with esosphageal cancer or head and neck cancer. Am. J. Gastroenterol. 106, 858–866. https://doi.org/10.1038/ajg.2010.489 (2011).
Kuo, C. Y. et al. Implementing precision medicine in endoscopy practice. J. Gastroenterol. Hepatol. 37, 1455–1468. https://doi.org/10.1111/jgh.15933 (2022).
Ishihara, R. et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig. Endosc. Off. J Japan Gastroenterol. Endosc. Soc. 32, 452–493. https://doi.org/10.1111/den.13654 (2020).
Pimentel-Nunes, P. et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European society of gastrointestinal endoscopy (ESGE) guideline–update 2022. Endoscopy 54, 591–622. https://doi.org/10.1055/a-1811-7025 (2022).
Todoric, J., Antonucci, L. & Karin, M. Targeting Inflammation in cancer prevention and therapy. Cancer Prev. Res. (Phila. Pa.) 9, 895–905. https://doi.org/10.1158/1940-6207.Capr-16-0209 (2016).
Donskov, F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin. Cancer Biol. 23, 200–207. https://doi.org/10.1016/j.semcancer.2013.02.001 (2013).
Bar-Ad, V. et al. Neutrophil to lymphocyte ratio associated with prognosis of lung cancer. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mexico 19, 711–717. https://doi.org/10.1007/s12094-016-1593-y (2017).
Caputo, D. et al. Neutrophil to lymphocyte ratio (NLR) and derived neutrophil to lymphocyte ratio (d-NLR) predict non-responders and postoperative complications in patients undergoing radical surgery after neo-adjuvant radio-chemotherapy for rectal adenocarcinoma. Cancer Invest. 34, 440–451. https://doi.org/10.1080/07357907.2016.1229332 (2016).
Chua, W., Charles, K. A., Baracos, V. E. & Clarke, S. J. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br. J. Cancer 104, 1288–1295. https://doi.org/10.1038/bjc.2011.100 (2011).
Gao, G. D., Sun, B., Wang, X. B. & Wang, S. M. Neutrophil to lymphocyte ratio as prognostic indicator for patients with esophageal squamous cell cancer. Int. J. Biol. Markers 32, e409–e414. https://doi.org/10.5301/ijbm.5000294 (2017).
Anand, S. et al. Does neutrophil-to-lymphocyte ratio (NLR) predict pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma?. J. Gastrointest. Cancer 52, 659–665. https://doi.org/10.1007/s12029-020-00445-5 (2021).
Zheng, Z., Yang, C., Cai, C. & Zhu, H. The preoperative neutrophil lymphocyte ratio and platelet lymphocyte ratio predicts disease-free survival in resectable esophageal squamous cell carcinoma. Cancer Manag. Res. 13, 7511–7516. https://doi.org/10.2147/cmar.S321326 (2021).
Lv, X., Han, S., Xu, B., Deng, Y. & Feng, Y. The value of complete blood count for the prognosis analysis of preoperative esophageal squamous cell carcinoma. BMC Cancer 21, 1072. https://doi.org/10.1186/s12885-021-08789-2 (2021).
Ajani, J. A. et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 17, 855–883. https://doi.org/10.6004/jnccn.2019.0033 (2019).
Balkwill, F. & Mantovani, A. Inflammation and cancer: Back to Virchow?. Lancet 357, 539–545. https://doi.org/10.1016/s0140-6736(00)04046-0 (2001).
Kidane, D. et al. Interplay between DNA repair and inflammation, and the link to cancer. Crit. Rev. Biochem. Mol. Biol. 49, 116–139. https://doi.org/10.3109/10409238.2013.875514 (2014).
Mierke, C. T. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Rep. Prog. Phys. Phys. Soc. (G. B.) 77, 076602. https://doi.org/10.1088/0034-4885/77/7/076602 (2014).
Labiano, S., Palazon, A. & Melero, I. Immune response regulation in the tumor microenvironment by hypoxia. Semin. Oncol. 42, 378–386. https://doi.org/10.1053/j.seminoncol.2015.02.009 (2015).
Mohammed, Z. M. et al. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br. J. Cancer 107, 864–873. https://doi.org/10.1038/bjc.2012.347 (2012).
Rabinowich, H., Cohen, R., Bruderman, I., Steiner, Z. & Klajman, A. Functional analysis of mononuclear cells infiltrating into tumors: Lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. 47, 173–177 (1987).
Monreal, M. et al. Platelet count and survival in patients with colorectal cancer—a preliminary study. Thromb. Haemost. 79, 916–918 (1998).
Sharma, D., Brummel-Ziedins, K. E., Bouchard, B. A. & Holmes, C. E. Platelets in tumor progression: A host factor that offers multiple potential targets in the treatment of cancer. J. Cell. Physiol. 229, 1005–1015. https://doi.org/10.1002/jcp.24539 (2014).
Pollard, J. W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78. https://doi.org/10.1038/nrc1256 (2004).
Torisu-Itakura, H. et al. Monocyte-derived IL-10 expression predicts prognosis of stage IV melanoma patients. J. Immunother. (Hagerstown Md. 1997) 30, 831–838 (2007).
Li, B., Xiong, F., Yi, S. & Wang, S. Prognostic and clinicopathologic significance of neutrophil-to-lymphocyte ratio in esophageal cancer: An update meta-analysis. Technol. Cancer Res. Treat. 21, 15330338211070140 (2022).
Acknowledgements
This work was supported by grants from the Ministry of Health and Welfare (MOHW111-TDU-B-221-114006) and the Ministry of Science and Technology, Taiwan (MOST-110-2314-B-650-003) and E-Da Hospital (EDPJ104051, EDPJ106067, EDPJ107058, EDPJ108062, EDAHS111018, EDAHS111031).
Author information
Authors and Affiliations
Contributions
C.-J.C. prepared the manuscript and analyzed the data. W.-L.W. initiated and coordinated the study. W.-L.W., C.-T.L., C.-J.C., Y.-N.T., M.-H.H., C.-M.T., C.-H.C. and C.-C.W. enrolled the study subjects and followed up the endoscopies for all patients. All authors have approved the final draft submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, CJ., Lee, CT., Tsai, YN. et al. Prognostic significance of systemic inflammatory response markers in patients with superficial esophageal squamous cell carcinomas. Sci Rep 12, 18241 (2022). https://doi.org/10.1038/s41598-022-21974-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21974-y
This article is cited by
-
CT-based radiomics combined with hematologic parameters for survival prediction in locally advanced esophageal cancer patients receiving definitive chemoradiotherapy
Insights into Imaging (2024)
-
A modified melanoma-molGPA scoring model: assessment of survival after and efficacy of different radiotherapy modalities in patients with melanoma brain metastases
Discover Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.