Abstract
Inflammatory factors in the peripheral blood, such as the C-reactive protein level and neutrophil-to-lymphocyte ratio (NLR), are prognostic markers in multiple types of cancer, including non-small cell lung cancer (NSCLC). However, the association between inflammatory factors and prognosis based on histological types has not been adequately reported. In addition, the relationship between these factors and the immune condition of the tumor microenvironment (TME) is unclear. In this single center, retrospective study, we first investigated the relationship between preoperative inflammatory markers and clinical outcomes in 176 patients with NSCLC who underwent surgery. Lung adenocarcinoma (LUAD) showed no significant prognostic marker, whereas for lung squamous cell carcinoma (LUSC), a multivariate analysis showed that a high NLR was significantly associated with postoperative recurrence. In LUSC patients, the median time of postoperative recurrence-free survival in patients with a low NLR was longer than that in patients with a high NLR. We then compared the tumor-infiltrating lymphocyte (TIL) profile with inflammatory markers in peripheral blood and found that the NLR was negatively correlated with the frequencies of T cells and B cells in LUSC tissues. Thus, the NLR is a useful predictive biomarker for postoperative recurrence and may reflect the immune condition of the TME in LUSC.
Similar content being viewed by others
Introduction
Lung cancer is histologically classified into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). SCLC accounts for approximately 15% of all lung cancers and is characterized by its neuroendocrine function. In contrast, NSCLC accounts for approximately 85% of lung cancers and includes multiple histological types, such as adenocarcinoma (LUAD), squamous cell carcinoma (LUSC), and large cell carcinoma. Among NSCLCs, LUAD and LUSC are the major histological subgroups and they have different genetic drivers, control networks, and prognostic profiles. In addition, clinical trials for NSCLC have shown different responses to chemotherapy, kinase mutation-targeted drugs, and immune checkpoint inhibitors in LUAD and LUSC1,2. Therefore, LUAD and LUSC are considered different diseases at the molecular, pathological, and clinical levels.
In multiple tumors, including lung cancer, inflammatory markers such as C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in peripheral blood have been reported to be significantly correlated with patient prognosis3,4,5,6,7,8. In SCLC and NSCLC patients, several reports examined the relationship between peripheral blood markers and prognosis3,4,7. However, to date, NSCLC has often been studied as a mixture of LUAD and LUSC to explore prognostic biomarkers, and few reports have compared the findings for many cases by dividing them into LUAD and LUSC.
The tumor-infiltrating lymphocyte (TIL) profile in the tumor microenvironment (TME) is associated with the prognosis of patients with NSCLC9. However it is not clear whether peripheral blood markers reflect TIL profiles in NSCLC tissues8,11,12,13,14,15,16,17,18. In this study, we first examined the association between inflammatory markers in peripheral blood and the prognosis in LUAD and LUSC. We then investigated the relationship between indicators of inflammation, which are prognostic predictors in peripheral blood, and the TIL profile, which reflects the immune condition of the TME.
Results
Clinicopathological characteristics of NSCLC patients
The baseline clinicopathological characteristics of the 176 NSCLC patients are summarized in Table 1. The median patient age was 70 years (range, 28–88 years), and 115 (65.3%) patients were male. Meanwhile, 137 (77.8%) patients had a history of smoking. According to the eighth edition of the TNM staging system, 66 (37.5%), 51 (29.0%), and 59 (33.5%) patients were categorized into stages I, II, and III, respectively. Of these patients, 101 (57.4%) had adenocarcinoma, 60 (34.1%) had squamous cell carcinoma, and 15 (8.5%) had other histological types. No patients received neoadjuvant therapy. 40 (22.7%) patients received postoperative chemotherapy, 2 (1.1%) patients received postoperative radiation therapy, and 1(0.6%) patient received postoperative chemoradiation. 56 (31.8%) patients showed recurrence while 120 (68.2%) patients did not. The median RFS was 24 months (range, 1–56 months). A total of 159 (90.3%) patients were alive and 17 (9.7%) patients died. The median OS was 28 months (range, 1 month; max, 56 months). On preoperative blood tests, the median CRP level and NLR, LMR, and PLR values for the study population were 0.13 (range, 0.01–9.22), 2.68 (0.67–48.66), 4.37 (1.48–9.25), and 154.2 (56.16–491.5), respectively.
We then compared the clinicopathological characteristics between patients with LUAD (n = 101) and LUSC (n = 60). LUSC patients were older (p = 0.023), predominantly male (p < 0.001), and had a higher smoking status (p < 0.001), higher CRP levels (p < 0.001), and higher mortality rates (p < 0.001) than LUAD patients (Table 2). The LMR was higher (p = 0.001) in patients with LUAD than in those with LUSC.
Analysis of prognostic value for patient outcome
Univariate analysis of clinicopathological factors, inflammatory markers, and peripheral blood cell types showed that pathological stage, PLR, WBC and platelet counts, and neutrophil (%), lymphocyte (%), and monocyte (%) levels were correlated with RFS in NSCLC patients (Table 3). In patients with LUAD, only the pathological stage was correlated with RFS. In patients with LUSC, pathological stage, NLR, WBC count, neutrophil count, and neutrophil (%), lymphocyte (%), monocyte (%), and basophil levels (%) were correlated with RFS. Smoking status in patients with LUSC was indicated as not available (NA) because the number of nonsmokers was too small to be suitable for statistical analysis. Similarly, in Tables 4 and 5, the values for which the number of events was small and could not be analyzed are also indicated as NA.
In univariate analysis of the prognostic value for OS, we found that there was no factor correlated with OS in NSCLC and LUAD, and only the basophil (%) were correlated with OS in patients with LUSC.
In the multivariate analysis, worse RFS was associated with a high pathological stage (hazard ratio [HR], 3.377; 95% CI 1.897–6.012; q < 0.001; p < 0.001) in LUAD patients (Table 5A). In LUSC patients, RFS was significantly worse in patients with high pathological stage (HR, 3.694; 95% CI, 1.512–9.023; q = 0.032; p = 0.004) and NLR (HR, 2.262; 95% CI 1.23–4.16; q = 0.036, p = 0.009) (Table 5A).
In LUAD patients, multivariate analysis for OS was not performed because of the small number of patients who died, and no factor was correlated with OS in patients with LUSC (Table 5B).
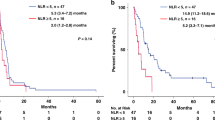
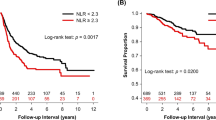
We then examined the cutoff NLR value to assess its clinical performance. The ROC curve showed that the best NLR cut-off value was 4.787 (Fig. 1). For this cutoff value, the sensitivity was 38.9%, specificity was 92.9%, and the area under the curve (AUC) was 0.634. The cutoff value for NLR was set at 4.8 as an approximation, and 51 (85%) LUSC patients were categorized into the high NLR group (NLR ≥ 4.8), while the remaining 9 (15%) LUSC patients were stratified into the low NLR group (NLR < 4.8). LUSC patients with a low NLR had a longer RFS than those with a high NLR (NA vs. 8 months, p < 0.001) (Fig. 2A). Similarly, patients with LUSC with a low NLR had better OS than those with a high NLR (NA vs. 23 months, p = 0.015) (Fig. 2B).
Association between NLR and the TIL profile in lung cancer
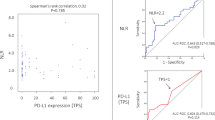
To examine whether NLR reflects the immune condition of the TME, we evaluated the correlations between NLR and the TIL profile. To analyze the TIL profile, we employed a flow cytometry (FCM) panel of 26 markers to identify 13 unique immune cell types and functional subpopulations from 140 NSCLC samples (82 LUAD samples, 49 LUSC samples, and 9 other histological subtype samples). Flow cytometry showed that NLR was negatively correlated with the frequencies of T cells/CD45+ cells (r = − 0.374, p = 0.008) and B cells/CD45+ cells (r = − 0.287, p = 0.046) in the tumor tissues of LUSC patients (Fig. 3). NLR showed a negative correlation with the frequency of CD8+ T cells/CD45+ cells (r = − 0.265, p = 0.066) and a positive correlation propensity with macrophages/CD45+ cells (r = 0.259, p = 0.072) (Table 6). No obvious correlation was found between other TIL immune cells and NLR in patients with LUSC (Table 6). NLR showed a positive correlation with non-Tregs (Fr. III)/CD45+ cells (r = 0.364, p < 0.001) in patients with LUAD10.
Discussion
NLR has been reported to be associated with the systemic inflammatory status11,12,13,14,15,16, which increases the number of neutrophils and affects tumor growth and progression17. Lymphocytes are essential immune cells in both humoral and cellular antitumor immune responses, and low lymphocyte counts are associated with an immunosuppressive condition in patients with cancer18,19,20. Therefore, a high NLR has been considered to be a predictor of poor prognosis in many cancers, including NSCLC3,8. However, few studies have reported comparisons of prognostic factors in relation to the histological type of NSCLC. This is the first study to show that prognostic markers in the peripheral blood of patients showing postoperative recurrence differ by histologic type and to suggest that NLR, as a prognostic marker, reflects the tumor microenvironment in LUSC, which was clarified by comparing the findings for LUAD and LUSC21.
TILs are associated with the prognosis of multiple cancers. CD8+ T cells ultimately differentiate into cytotoxic T cells, which have cell-killing functions22,23. CD4+ T cells are required for nearly all functions in tumor immunity24. Recent studies have also identified a key role of B lymphocytes in immunotherapy, and their presence has been associated with an improved prognosis across different cancer types25,26. Since peripheral blood obtained using a minimally invasive technique is an ideal biomarker, identification of the peripheral blood factors that correlate with T and B cells in the TME will be of value. Several reports have shown a negative correlation between NLR and CD3+ cells in TILs in multiple cancers, including NSCLC7,27. Our study showed that the NLR is negatively correlated with T cells as well as B cells in TILs for LUSC but not for LUAD patients, explaining that NLR is a poor prognostic factor in LUSC patients.
TIL composition and activation status were associated with patient outcomes28. TILs were mainly evaluated by immunohistochemical analyses focusing on CD3+ cells and CD8+ T cells7,29,30 and morphological evaluation by HE staining31,32. However, TIL analysis using FCM has been described in only a few reports33. In this study, we examined multiple unique immune cell types and functional subpopulations by FCM, which allowed simultaneous comparison of multiple TIL immune cell types with NLR.
Although not analyzed in this study, tumor-associated neutrophils (TANs) were present in the TME. TANs occur in two states: antitumor (N1) and tumor growth-promoting (N2)34. Because of the positive correlation between TANs and peripheral blood neutrophil counts, a high NLR may be a poor prognostic factor, reflecting N2 neutrophils in the TME35.
For the treatment of lung cancer, in addition to anti-PD-1/PD-L1 antibody therapy, combined therapy with chemotherapy and immunotherapy has become the standard of care36,37,38,39,40,41. The development of predictive biomarkers for the efficacy of immune-checkpoint inhibitors (ICBs) in the peripheral blood is awaited. NLR has been reported to predict the efficacy of immune checkpoint therapy in NSCLC patients42,43,44. Since TIL compositions influence the efficacy of ICBs, this study may contribute to understanding the reason why NLR is a prognosis predictor for ICBs.
This study had several limitations: the sample size was not large, the observation period was not long, and the relationship with the efficacy of ICBs was not examined. More research is needed to confirm the usefulness of the NLR as a prognostic factor and ICB-effect predictor in a large cohort. To understand why prognostic factors in the peripheral blood differ between LUAD and LUSC, the differences in the TME between LUAD and LUSC and the relationship between peripheral blood factors and the TME require elucidation. Furthermore, there are also possible limitations regarding the statistical analysis. For the NLR cutoff, the ROC curve does not account for time factors. It may have been more desirable to perform the analysis using survival-ROC analysis45.
In conclusion, a high NLR was significantly associated with poor prognosis in LUSC but not in LUAD patients, reflecting the frequencies of T and B cells in the TME.
Material and methods
Patients
This retrospective study enrolled 176 patients with pathological stage I-III primary NSCLC who underwent surgery at the National Cancer Center Hospital (Tokyo, Japan) between October 2016 and June 2019. Patients with metastatic or pathological stage IV disease were excluded. This study protocol was approved by the National Cancer Center Ethics Committee (2016–124, dated: August 5th, 2016). All patients provided written informed consent before sampling. The study also abided by the principles of the Declaration of Helsinki.
Adjuvant therapies and neoadjuvant therapies were examined by multidisciplinary discussions for each individual patient based on pathologic findings, patient performance status, age, comorbidity and patient’s intension.
Clinical follow-up
Recurrence-free survival (RFS) was defined as the interval between surgery and disease progression or death, whichever occurred first. Patients without any of these events were censored at the final follow-up, without documented progression. Overall survival (OS) was defined as the time from surgery to death from any cause. Patients without any of these events were censored at the final follow-up visit. The median follow-up period for all patients was 24 months (range, 1–56 months). The median follow-up period for the surviving patients was 28 months (range, 1–56 months).
Blood inflammatory markers
Blood samples were collected the day before surgery. The NLR was defined as the number of neutrophils divided by the number of lymphocytes. LMR was defined as the lymphocyte count divided by the monocyte count, and PLR was defined as the platelet count divided by the lymphocyte count.
Flow cytometry
Viable cells from tumor suspensions were counted and incubated with Fixable Viability Dye eFluor™ 506 (BioLegend, San Diego, CA, USA) for 30 min at 4 °C for dead cell staining, followed by FcR blocking using the FcR blocking reagent (Miltenyi Biotech) for 10 min at 4 °C. The cells were then stained with the fluorescently labeled antibodies listed in Table S1 at 4 °C for 30 min. Next, intracellular staining was performed with intracellular antibodies and a Foxp3/Transcription Factor Staining Buffer set (Thermo Fisher Scientific) according to the manufacturer's instructions.
After washing, the cells were analyzed using an LSR Symphony instrument (BD Biosciences, Franklin Lakes, NJ, USA). At least 50,000 live events were collected per sample (BD LSR II cytometer). Data were analyzed using the FlowJo software (BD Biosciences). The gating of each sample was based on plots of SSC-Height versus SSC-Width and FSC-Height versus FSC-Width to eliminate aggregates. FVD staining was used to identify and eliminate dead cells as evaluated using contour plots. A propidium iodide overlay was used to validate cell viability in the training set.
The 13 immune cells are defined as shown in Supplementary Table 2. The frequency of each immune cell in the TILs was calculated as the number of transformed cells divided by the number of CD45+ cells.
Statistical analysis
All statistical analyses were conducted using R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at p < 0.05. Cox proportional hazards regression models were used to evaluate the prognostic factors in univariate and multivariate analyses. The p value was adjusted by the False Discovery Rate with 5% significant level. Cutoff values were calculated using receiver operating characteristic (ROC) curves for factors considered significant prognostic markers. RFS and OS curves were analyzed using the Kaplan–Meier method, and statistical differences were determined using the log-rank test. Correlations between immune cells in TILs and inflammatory markers in peripheral blood were calculated using Spearman's rank correlation coefficient test.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Relli, V., Trerotola, M., Guerra, E. & Alberti, S. Abandoning the notion of non-small cell lung cancer. Trends Mol. Med. 25, 585–594. https://doi.org/10.1016/j.molmed.2019.04.012 (2019).
de Sousa, V. M. L. & Carvalho, L. Heterogeneity in lung cancer. Pathobiology 85, 96–107. https://doi.org/10.1159/000487440 (2018).
Gu, X. B., Tian, T., Tian, X. J. & Zhang, X. J. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: A meta-analysis. Sci. Rep. 5, 12493. https://doi.org/10.1038/srep12493 (2015).
Shrotriya, S., Walsh, D., Bennani-Baiti, N., Thomas, S. & Lorton, C. C-Reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: A systematic review. PLoS One 10, e0143080. https://doi.org/10.1371/journal.pone.0143080 (2015).
Chen, Y. et al. Prognostic significance of combined preoperative platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in patients undergoing surgery with stage IB non-small-cell lung cancer. Cancer Manag. Res. 10, 5411–5422. https://doi.org/10.2147/CMAR.S177320 (2018).
Li, B. et al. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta 483, 48–56. https://doi.org/10.1016/j.cca.2018.04.023 (2018).
Shirasawa, M. et al. Prognostic impact of peripheral blood neutrophil to lymphocyte ratio in advanced-stage pulmonary large cell neuroendocrine carcinoma and its association with the immune-related tumour microenvironment. Br. J. Cancer 124, 925–932. https://doi.org/10.1038/s41416-020-01188-7 (2021).
Xie, D. et al. Nomograms predict overall survival for patients with small-cell lung cancer incorporating pretreatment peripheral blood markers. J. Thorac. Oncol. 10, 1213–1220. https://doi.org/10.1097/JTO.0000000000000585 (2015).
Yan, Q. et al. Prognostic value of immune-related genes in the tumor microenvironment of lung adenocarcinoma and lung squamous cell carcinoma. Aging 12, 4757–4777. https://doi.org/10.18632/aging.102871 (2020).
Chen, Z. Y. et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br. J. Cancer 112, 1088–1097. https://doi.org/10.1038/bjc.2015.61 (2015).
Cannon, N. A. et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J. Thorac. Oncol. 10, 280–285. https://doi.org/10.1097/JTO.0000000000000399 (2015).
Yao, Y., Yuan, D., Liu, H., Gu, X. & Song, Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol. Immunother. 62, 471–479. https://doi.org/10.1007/s00262-012-1347-9 (2013).
Szkandera, J. et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int. J. Cancer 135, 362–370. https://doi.org/10.1002/ijc.28677 (2014).
Stotz, M. et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br. J. Cancer 109, 416–421. https://doi.org/10.1038/bjc.2013.332 (2013).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444. https://doi.org/10.1038/nature07205 (2008).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899. https://doi.org/10.1016/j.cell.2010.01.025 (2010).
Bronte, V., Serafini, P., Mazzoni, A., Segal, D. M. & Zanovello, P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 24, 301–305. https://doi.org/10.1016/s1471-4906(03)00132-7 (2003).
Ray-Coquard, I. et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 69, 5383–5391. https://doi.org/10.1158/0008-5472.CAN-08-3845 (2009).
Lin, E. Y. & Pollard, J. W. Role of infiltrated leucocytes in tumour growth and spread. Br. J. Cancer 90, 2053–2058. https://doi.org/10.1038/sj.bjc.6601705 (2004).
Dong, Y. et al. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 33, 4632–4642. https://doi.org/10.1038/onc.2013.409 (2014).
Inomata, M. et al. Clinical parameters for predicting the survival in patients with squamous and non-squamous-cell NSCLC receiving PD-1 inhibitor therapy. Pathol. Oncol. Res. 26, 327–333. https://doi.org/10.1007/s12253-018-0473-x (2020).
Geng, Y. et al. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: A meta-analysis. Cell. Physiol. Biochem. 37, 1560–1571. https://doi.org/10.1159/000438523 (2015).
Ohtaki, Y. et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocytes in large cell neuroendocrine carcinoma of lung. Am. J. Transl. Res. 10, 3243–3253 (2018).
Borst, J., Ahrends, T., Babala, N., Melief, C. J. M. & Kastenmuller, W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647. https://doi.org/10.1038/s41577-018-0044-0 (2018).
Paijens, S. T., Vledder, A., de Bruyn, M. & Nijman, H. W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 18, 842–859. https://doi.org/10.1038/s41423-020-00565-9 (2021).
Wang, S. S. et al. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell. Mol. Immunol. 16, 6–18. https://doi.org/10.1038/s41423-018-0027-x (2019).
Dirican, N., Karakaya, Y. A., Gunes, S., Daloglu, F. T. & Dirican, A. Association of intra-tumoral tumour-infiltrating lymphocytes and neutrophil-to-lymphocyte ratio is an independent prognostic factor in non-small cell lung cancer. Clin. Respir. J. 11, 789–796. https://doi.org/10.1111/crj.12417 (2017).
Bremnes, R. M. et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J. Thorac. Oncol. 11, 789–800. https://doi.org/10.1016/j.jtho.2016.01.015 (2016).
Schalper, K. A. et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J. Natl. Cancer Inst. https://doi.org/10.1093/jnci/dju435 (2015).
Kim, M. Y. et al. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 88, 24–33. https://doi.org/10.1016/j.lungcan.2015.01.016 (2015).
Kilic, A., Landreneau, R. J., Luketich, J. D., Pennathur, A. & Schuchert, M. J. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J. Surg. Res. 167, 207–210. https://doi.org/10.1016/j.jss.2009.08.029 (2011).
Horne, Z. D. et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J. Surg. Res. 171, 1–5. https://doi.org/10.1016/j.jss.2011.03.068 (2011).
Xiang, Z. J. et al. Neutrophil-lymphocyte ratio (NLR) was associated with prognosis and immunomodulatory in patients with pancreatic ductal adenocarcinoma (PDAC). Biosci. Rep https://doi.org/10.1042/BSR20201190 (2020).
Coffelt, S. B., Wellenstein, M. D. & de Visser, K. E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446. https://doi.org/10.1038/nrc.2016.52 (2016).
Han, S. et al. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer 15, 617. https://doi.org/10.1186/s12885-015-1629-7 (2015).
Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378, 2288–2301. https://doi.org/10.1056/NEJMoa1716948 (2018).
Paz-Ares, L. et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 22, 198–211. https://doi.org/10.1016/s1470-2045(20)30641-0 (2021).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092. https://doi.org/10.1056/NEJMoa1801005 (2018).
Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379, 2040–2051. https://doi.org/10.1056/NEJMoa1810865 (2018).
Horn, L. et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379, 2220–2229. https://doi.org/10.1056/NEJMoa1809064 (2018).
Paz-Ares, L. et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. The Lancet 394, 1929–1939. https://doi.org/10.1016/s0140-6736(19)32222-6 (2019).
Alessi, J. V. et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J. Immunother. Cancer https://doi.org/10.1136/jitc-2021-003536 (2021).
Bagley, S. J. et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 106, 1–7. https://doi.org/10.1016/j.lungcan.2017.01.013 (2017).
Diem, S. et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 111, 176–181. https://doi.org/10.1016/j.lungcan.2017.07.024 (2017).
Frencesco, J. R. et al. Neutrophil-to-lymphocyte ratio is a major prognostic factor in non-small cell lung carcinoma patients undergoing first line immunotherapy with pembrolizumab. Cancer Diagn. Progn. 3, 44–52 (2023).
Acknowledgements
This work was supported by the Funding for Research to Expedite Effective Drug Discovery by Government, Academia, and Private partnership (GAPFREE) grants JP19ak0101044h0104 and JP19ak0101043h0105A; Grants-in-Aid for Practical Research for Innovative Cancer Control grants 21ck0106532h0002 and 21ck010640h0002 from the Japan Agency for Medical Research and Development (AMED), National Cancer Center Research and Development Fund (2020-J-2), and Grants-in-Aid for Scientific Research C 18K07036, 21K06900, and 21K07951.
Author information
Authors and Affiliations
Contributions
K.O.: Conceptualization, writing-original draft, writing-review and editing. Y.N.: Conceptualization, formal analysis, investigation. H.F.: Methodology. R.S.: Methodology. Y.Y.: Resources, investigation. S.-i.W.: Resources, investigation. N.M.: Methodology. Y.S.: Methodology. H.M.: Formal analysis, supervision, investigation. H.T.: Funding acquisition, writing-review and editing. K.T.: Supervision. T.S.: Supervision. A.O.: Funding acquisition, writing-review and editing. K.A.: Conceptualization, funding acquisition, writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohashi, K., Nishito, Y., Fukuda, H. et al. Neutrophil-to-lymphocyte ratio is a prognostic factor reflecting immune condition of tumor microenvironment in squamous cell lung cancer. Sci Rep 14, 429 (2024). https://doi.org/10.1038/s41598-023-50378-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50378-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.