Abstract
We evaluated the role of prostate health index (PHI) in predicting Gleason score (GS) upgrading in International Society of Urological Pathology Grade Group (ISUP GG) 1 & 2 prostate cancer (PCa) or adverse pathologic outcomes at radical prostatectomy (RP). A total of 300 patients with prostate specific antigen ≥ 3 ng/mL, PHI and prostate biopsy (71 patients with RP included) were retrospectively included in the study. The primary study outcomes are PCa and clinically significant PCa (csPCa, defined as ISUP GG ≥ 2) diagnostic rate of PHI, and GS upgrading rate at RP specimen. The secondary outcomes are the comparison between GS upgrading and non-upgrading group, GS upgrading and high-risk PCa (ISUP GG ≥ 3 or ≥ pT3a) predictability of preoperative clinical factors. Overall, 139 (46.3%) and 92 (30.7%) were diagnosed with PCa and csPCa, respectively. GS upgrading rate was 34.3% in all patients with RP. Significant differences were shown in the total prostate volume (p = 0.047), the distribution of ISUP GG at biopsy (p = 0.001) and RP (p = 0.032), respectively. PHI values ≥ 55 [Odds ratio (OR): 3.64 (95% confidence interval (CI) = 1.05–12.68, p = 0.042] and presence of PI-RADS lesion ≥ 4 (OR: 7.03, 95% CI = 1.68–29.51, p = 0.018) were the significant predictors of GS upgrading in RP specimens (AUC = 0.737). PHI values ≥ 55 (OR: 9.05, 5% CI = 1.04–78.52, p = 0.046) is a significant factor for predicting adverse pathologic features in RP specimens (AUC = 0.781). PHI could predict GS upgrading in combination with PIRADS lesions ≥ 4 in ISUP GG 1 & 2. PHI alone could evaluate the possibility of high-risk PCa after surgery as well.
Similar content being viewed by others
Introduction
A major challenge in prostate cancer (PCa) treatment is to detect those which should not be managed with active surveillance (AS) 1. To overcome the limitations of total prostate-specific antigen (tPSA) for diagnosis, [−2]proPSA (p2PSA) derivatives, such as Prostate Health Index (PHI), percentage of p2PSA (%p2PSA) and PHI density (PHID) have been suggested. %p2PSA and PHI have been associated with improved overall detection and aggressive PCa detection over tPSA. and free PSA (fPSA)/tPSA ratio (%fPSA) in several studies 2,3,4.
Currently, no single biomarker has the ideal performance characteristics necessary for the detection and risk stratification of PCa. The PHI seems to be a simple and inexpensive test for a multivariant approach to PCa screening and management. The PHI improves cancer prediction at initial and extended biopsy stages and even has some capability to predict disease recurrence after radical prostatectomy (RP) 3. As the age-specific PCa incidence rates increase with age in Asian countries, better markers are needed to differentiate aggressive PCa from indolent, in order to better counsel patients as to appropriate treatment options ranging from radical treatment to AS 4,5. In this study, we aimed to evaluate the role of PHI in localized PCa and whether it can function as an independent predictor of Gleason score (GS) upgrading at RP specimen in GS 3 + 3 PCa, of unfavorable or high risk PCa, and of adverse pathologic features in RP specimens.
Patients and methods
Patient selection and evaluation
All patients with transrectal prostate biopsy, PSA ≥ 3 ng/mL, and clinical stage ≤ cT3aN0 between 04/2019 and 07/2020 were included in this retrospective cohort analysis. Among them, 139 (46.3%) had a histologically confirmed diagnosis of PCa from transrectal ultrasound guided biopsy (TRUS-Bx) or magnetic resonance imaging-guided biopsy (MRI-GB) within the 3 months before surgery. Due to the referral nature of our practice, genitourinary pathologists with more than 15 years of experience reviewed all the biopsy slides. Thus, the biopsy GS reported represents the result of our internal review, and detailed biopsy information was available for all patients. Prior to prostate biopsy, blood was drawn to measure the prebiopsy tPSA, fPSA, and p2PSA levels. Blood samples were processed using the Access 2 immunoassay kit (Beckman Coulter, Brea, CA, USA) 6,7.
RP was performed using a robot-assisted approach by experienced urologists. Pelvic lymph node dissection was carried out according to the operating surgeons’ preferences. Surgical specimens were processed and analyzed using a standardized technique by the same genitourinary pathologists who reviewed biopsy slides. Men with previous prostate surgery, active urinary tract infection or those using medications that affect PSA levels (e.g., 5-α reductase inhibitors) were excluded. The primary study outcomes were PCa and clinically significant PCa (csPCa) diagnostic rate of PHI and GS upgrading rate at RP specimen. The secondary study outcomes were significant clinical factors predicting GS upgrading or adverse pathologic features (International Society of Urological Pathology Grade Group [ISUP GG] ≥ 3 or ≥ pT3a) at RP specimen. The institutional review board approved the study (IRB number: B-2011-648-104). A written informed patient consent was waived by the Seoul National University Bundang Hospital Institutional Review Board due to the retrospective nature of study. All methods were conducted in accordance with relevant guidelines and regulations (the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards).
Transrectal ultrasound guided biopsy and MRI guided biopsy
Transrectal prostate biopsies were obtained under local anesthesia using an automatic biopsy gun and an 18-G needle under TRUS guidance. In all, 12 cores (six in the peripheral zone and six in the transitional zone) were taken in all patients. In the case of MRI guided biopsy, at least two or more cognitive fusion-targeted or MRI/Ultrasound image fusion (BioJet MRI-Ultrasound Fusion system with bk5000 ultrasound, BK Medical, United States) biopsy cores were added for each lesion in patients with suspicious or equivocal lesions in mpMRI 8. Two uroradiologists with more than 10-years of experience including more than a thousand pelvic MRIs read, graded the level of suspicion for clinically significant cancer from mpMRI mapping images using the Prostate Imaging Reporting and Data System Version 2 (PIRADS V2) scale 1–5 9.
Pathological analysis
The same expert pathologists from Department of Pathology in our center assessed specimens from needle biopsies and RP. We followed the recommendations of the 2014 ISUP consensus for GS. The highest-grade pattern was recorded if there were multiple scores at multiple biopsy sites or multiple cancer nodules in the RP specimen. We excluded patients presenting minor tertiary pattern 5 on prostatectomy. GS upgrading was defined as any increased total sum in the pathological GS compared with that of the biopsy GS. In addition, an increase in the main structure score without a change in the total sum (ex. ISUP GG 2 → 3) was also defined as GS upgrading 10. Adverse pathologic feature was defined as non-organ-confined disease (pT3 or higher) or GS ≥ 4 + 3 (ISUP GG ≥ 3) after RP 11,12.
Data collection
The clinical and biopsy variables included age at surgery, preoperative initial PSA series (tPSA, fPSA, p2PSA with the latest collected right before biopsy), clinical stage, primary and secondary (highest) Gleason grading on biopsy, number of positive cores, number of total cores and percentage of positive/total cores, maximum percentage of surface specimen tumor involvement, presence of perineural invasion, and/or high-grade prostatic intraepithelial neoplasia in biopsy specimens. Pathologic variables were primary and secondary (highest) GS, pT and pN stage, extraprostatic extension, seminal vesicle invasion, and lymph node metastasis. The American Joint Committee on Cancer TNM 8th edition (2018) was used for pathologic staging, and GS was assigned according to the 2014 ISUP modified Gleason scoring system 13.
fPSA and PHI percentages were calculated using the formulas:
CsPCa was defined as the presence of at least one sample with a Gleason four or five grade lesion, or ISUP GG ≥ 2.
Statistical analysis
In addition to descriptive statistics, we used the chi-square test for comparing categorical variables and the Students t test, Wilcoxon rank sum test, Mann Whitney U-test, or Kruskal–Wallis test for comparison of continuous variables. Clinical data and detailed biopsy information were analyzed in multivariable prediction models using logistic regression 14. The univariate and multivariate logistic regression analyses were performed to detect risk factors for GS upgrading in ISUP GG 1 & 2, adverse pathologic features at all RP specimens. Multivariate analysis using variables with a p value < 0.05 in univariate analysis was performed to identify which variables were independently predictive of outcomes (GS upgrading and adverse pathologic features). The predictive models’ accuracy was compared using area under the receiver operating curve (AUC). All tests were two sided with a value of 0.05. The statistical analysis was performed using IBM Statistical Package for the Social Science Statistics for Windows (SPSS) version 22.0 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA https://www.ibm.com/products/spss-statistics).
Ethical statements
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-2011-648-104 and date of approval: 9th/November/2020).
Results
Patients
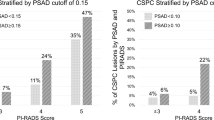
Of 300 patients who were evaluated for PHI and had prostate biopsy, 92 (30.7%) were diagnosed with csPCa. The baseline characteristics according to biopsy outcomes are summarized in supplementary Table 1. There were significant differences in PSA level (p = 0.014), PSA density (PSAD) (p = 0.002), PHID (p < 0.001), cancer core number at prostate biopsy (p = 0.001), cancer positive core rate (p < 0.001), PCa detection rate (p < 0.001), csPCa detection rate (p < 0.001), distribution of ISUP GG at biopsy (p < 0.001), and distribution of ISUP GG (p < 0.001) at RP among groups if classified by PHI level range (0–26.9/27.0–35.9/36.0–54.9/ ≥ 55.0).
Baseline characteristics according to Gleason score (GS) upgrading in RP specimens
Subgroup analysis, classified by GS upgrading, was performed and is described in Table 1. 71 patients underwent RP and GS upgrading rate was 33.8% (24/71) in all patients with RP. Significant differences were shown in the preoperative prostate volume (p = 0.014), the distribution of ISUP GG at biopsy (p = 0.001), and the distribution of ISUP GG (p = 0.016) at RP pathology.
Predictor of GS upgrading at RP
24 patients with RP had GS upgrading at RP specimen. Multivariate logistic regression analysis (Table 2) revealed PHI values ≥ 55 (Odds ratio (OR): 3.64 [95% Confidence interval [CI] = 1.05–12.68, p = 0.042) and presence of PI-RADS lesion ≥ 4 (OR: 7.03, 95% CI = 1.68–29.51, p = 0.018) were the significant predictors of GS upgrading in RP specimens (AUC = 0.737).
Predictor of adverse pathologic features at RP (adverse pathologic features: ≥ pT3a or GS ≥ 4 + 3)
13 patients had adverse pathologic features at RP. PHI values ≥ 55 (OR: 9.05, 5% CI = 1.04–78.52, p = 0.046) is a significant factor for predicting adverse pathologic features in RP specimens (Table 3) (AUC = 0.781).
Discussion
Although numerous studies on PHI have been reported, relatively little has been revealed about whether PHI can predict high-risk PCa. This might be due to recent trends in which PHI has been used to gain more certainty on PCa for people with grey zone PSA levels, and the relatively low probability that high-risk PCas are actually detected in the PSA grey zone. Based on the fact that biopsy has a significant role in the diagnosis and staging of PCa 15, AS has recently been seen as an option for low-risk groups of patients. However, selection criteria are not yet definitively established 16,17,18. Tsang et al. reported the necessity of PSAD for AS and a cutoff for PSAD in identifying adverse pathological outcomes in an Asian cohort. They concluded that PSAD with a cutoff at 0.19 ng/ml/ml provided the best balance between sensitivity and specificity in predicting adverse pathological disease 19. Compared to that study, our results showed that a PSAD level ≥ 0.15 ng/ml/ml could also significantly predict adverse pathologic features at RP (OR: 7.32, 95% CI = 1.19–45.08, p = 0.032).
A large-scale study reported the frequency of GS upgrading and downgrading in 7643 patients were 36.3 and 12.0%, respectively, revealing a stronger tendency for biopsies to underestimate rather than overestimate the true GS 20. Detection of GS upgrading at RP specimen is fundamental. It may potentially lead to reduction of probability for undertreatment of undergraded patients at the initial biopsy, or has been associated with adverse pathological outcomes, such as positive surgical margin status and biochemical recurrence (BCR) 21,22. GS upgrading can help predict those high risk patients that should not be managed with AS. Imnadze et al. demonstrated that adverse pathologic features at RP are associated with an increased BCR risk which is influenced substantially by pretreatment factors, and pathologic features in isolation cannot dictate treatment 23. For counseling a patient who is considering AS, clinicians should share meaningful information about chances of having disease with adverse pathology, and the oncologic risk associated with such findings in the specific context of preoperative risk including PHI results. Furthermore, PHI results might also be used to aid a clinician in the selection of high PHI with ISUP GG ≥ 3 patients, if upgraded at RP specimen, for post-RP adjuvant therapy.
From our multivariate analysis, the combination of PHI and mpMRI is better at predicting GS upgrading than mpMRI alone (Table 2). Although PHI, (considered as a new medical technology in South Korea), is not yet reimbursed by national health insurance, a combination of PHI and MRI-GB can be strongly recommended for patients with PSA grey zone levels, to increase the diagnostic rate of PCa and csPCa. It is believed that the insurance drawback can be overcome through clear communication during consultations, including stressing the need for PHI to patients retaining private insurance. In one study, the PHI also improved the cost-effectiveness of PCa detection with a 17% reduction in costs of diagnosis and a 1% reduction in the total costs for treatment of PCa. These cost savings were due to a reduction in the number of unnecessary biopsies 17. Another study 24 reported the use of the PHI in the PCa detection (19.1% diagnosed as PCa) in 157 Asian males with a tPSA of 4–10 ng/mL. At a PHI cutoff level of 55, 42.9% of patients had PCa, and all of them were at GS > 6. The PCa detection rate was 55.5% in our study, which was higher than theirs, and use of PHI demonstrated superior performance over PSA in PCa detection (AUC—PHI: 0.672 vs tPSA: 0.594) 24.
In this study, adverse pathology definition was T3a or higher or ISUP GG 3, to confirm that PHI can significantly predict adverse pathology. Though varying definitions of adverse pathology exist, the criteria defined by either primary Gleason pattern ≥ 4 or pT3-4 disease, like ours, appears to most accurately predict adverse pathology or BCR in patients with lower risk PCa at the time of diagnosis 25.
The limitation of this study is that it is a retrospective design with a small sample size and a single ethnicity. Our sample size might be considered too low to solve the main question of the study. Since AS can be considered as a first treatment option for low-grade PCa, there may be a non-negligible selection bias in our study. In fact, in South Korea, where PHI is not yet reimbursed by national insurance, the total number of PHI tests are not high, as it was initiated less than five years ago and there is scarce long-term follow-up data. In addition, csPCa as defined in this study does not have a standard global definition, which may make it difficult to apply in real-world clinical practice. Our predictive model may be considered as a model that analyzes the association between PHI and high-risk PCa rather than formally evaluating the predictability of total PCa. Another limitation is that clinicians need to be cautious in applying our results in real world practice considering that GS upgrading at prostatectomy may instead indicate under-scoring at biopsy. Finally, it was not possible to generate a comparative analysis with other available blood biomarkers, including PCA3 and genetic markers. In the near future, these limitations are expected to be overcome to some extent if a multicenter prospective randomized controlled trial is implemented. Nevertheless, this study is the first to provide clues that PHI can predict simultaneously GS upgrading in potential active surveillance candidates and high-risk PCa even from a small Korean cohort, and shows that PHI is reliable and can be added to existing evaluation tools for PCa diagnosis and prediction. The important clinical question in our study is whether PHI or PHI density improves upon PSA/PSA density in predicting GS upgrading or adverse pathology. Though with small numbered group and PHI analyzed as dichotomous variable, multivariate analysis showed relatively more associations of PHI with those features than PSA or PSAD. Moreover, relevant data for Koreans are scarce. Therefore, our results are worth applying to Korean all over the world and also satisfy unmet needs for clinicians counseling them. In addition, our results are expected to be utilized as supportive evidence for PCa patients who need radical treatments such as surgery or radiation therapy while potentially preventing excessive prostate biopsy.
Conclusion
PHI could predict GS upgrading in combination with PIRADS lesions ≥ 4 in ISUP GG 1 & 2. PHI alone could evaluate the possibility of unfavorable or high-risk PCa with adverse pathologic features after surgery as well. PHI appears to improve csPCa detection and provide prognostic value. These results provide an opportunity to define appropriate treatment strategies for AS candidates and also can be of significant assistance for the pre-treatment counseling of all potential PCa patients. Our study findings could be helpful for the management of both patients in AS and patients considered for RP. Further studies are still needed on the predictiveness of PHI on several aspects on PCa in the future.
References
Heidegger, I. et al. ProPSA and the Prostate Health Index as predictive markers for aggressiveness in low-risk prostate cancer-results from an international multicenter study. Prostate Cancer Prostatic Dis. 20(3), 271–275 (2017).
Barisiene, M. et al. Prostate health index and prostate health index density as diagnostic tools for improved prostate cancer detection. Biomed. Res. Int. 2020, 9872146 (2020).
Lepor, A., Catalona, W. J. & Loeb, S. The prostate health index: Its utility in prostate cancer detection. Urol. Clin. N. Am. 43(1), 1–6 (2016).
Chiu, P. K. et al. Prostate health index and %p2PSA predict aggressive prostate cancer pathology in chinese patients undergoing radical prostatectomy. Ann. Surg. Oncol. 23(8), 2707–2714 (2016).
Ha Chung, B., Horie, S. & Chiong, E. The incidence, mortality, and risk factors of prostate cancer in Asian men. Prostate Int. 7(1), 1–8 (2019).
Jansen, F. H. et al. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur. Urol. 57(6), 921–927 (2010).
Catalona, W. J. et al. A multicenter study of [−2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range [published correction appears in J Urol. 2011 Jul;186(1):354]. J. Urol. 185(5), 1650–1655 (2011).
Kim, T. J., Lee, M. S., Hwang, S. I., Lee, H. J. & Hong, S. K. Outcomes of magnetic resonance imaging fusion-targeted biopsy of prostate imaging reporting and data system 3 lesions. World J. Urol. 37(8), 1581–1586 (2019).
Weinreb, J. C. et al. PI-RADS prostate imaging—Reporting and data system: 2015, version 2. Eur. Urol. 69(1), 16–40 (2016).
Xu, N. et al. Risk of upgrading from prostate biopsy to radical prostatectomy pathology: Is magnetic resonance imaging-guided biopsy more accurate?. J. Cancer. 9(19), 3634–3639 (2018).
Falagario, U. G. et al. Defining prostate cancer at favorable intermediate risk: the potential utility of magnetic resonance imaging and genomic tests. J. Urol. 202(1), 102–107 (2019).
Falagario, U. G. et al. Does multiparametric magnetic resonance of prostate outperform risk calculators in predicting prostate cancer in biopsy naïve patients?. Front Oncol. 10, 603384 (2021).
Epstein, J. I. et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 40(2), 244–252 (2016).
Kleinbaum, D. G., Kupper, L. L., Nizam, A. & Muller, K. E. Applied Regression Analysis and Other Multivariable Methods (Duxbury Applied) 4th edn. (Duxbury Press, 2008).
Mottet, N. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 71(4), 618–629 (2017).
Cimino, S. et al. Active surveillance for low-risk prostate cancer: Are all criteria similar?. Anticancer Agents Med. Chem. 18(7), 958–963 (2018).
Sriplakich, S. et al. Prospective performance of the Prostate Health Index in prostate cancer detection in the first prostate biopsy of men with a total prostatic specific antigen of 4–10 ng/mL and negative digital rectal examination. Prostate Int. 6(4), 136–139 (2018).
Gandaglia, G. et al. Identification of pathologically favorable disease in intermediate-risk prostate cancer patients: implications for active surveillance candidates. Prostate 75(13), 1484–1491 (2015).
Tsang, C. F. et al. Is prostate specific antigen (PSA) density necessary in selecting prostate cancer patients for active surveillance and what should be the cutoff in the Asian population?. Prostate Int. 7(2), 73–77 (2019).
Epstein, J. I., Feng, Z., Trock, B. J. & Pierorazio, P. M. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: Incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur. Urol. 61(5), 1019–1024 (2012).
Davies, J. D. et al. Prostate size as a predictor of Gleason score upgrading in patients with low risk prostate cancer. J. Urol. 186(6), 2221–2227 (2011).
Freedland, S. J. et al. Upgrading and downgrading of prostate needle biopsy specimens: Risk factors and clinical implications. Urology 69(3), 495–499 (2007).
Imnadze, M., Sjoberg, D. D. & Vickers, A. J. Adverse pathologic features at radical prostatectomy: Effect of preoperative risk on oncologic outcomes. Eur. Urol. 69(1), 143–148 (2016).
Loeb, S. & Catalona, W. J. The Prostate Health Index: A new test for the detection of prostate cancer. Ther. Adv. Urol. 6(2), 74–77 (2014).
Kozminski, M. A. et al. Standardizing the definition of adverse pathology for lower risk men undergoing radical prostatectomy. Urol. Oncol. 34(9), 415.e1-415.e4156 (2016).
Funding
This study was supported by institutional grant (02-2020-021) from the SNUBH Research Fund.
Author information
Authors and Affiliations
Contributions
Conceptualization, H.K. and S.K.H.; methodology, H.K. and S.K.H.; software, H.K.; validation, G.J., J.H.K., and S.S.B.; formal analysis, H.K., G.J. and S.K.H.; investigation, H.K. and S.K.H.; resources, G.J., J.H.K., S.S.B. and S.K.H.; data curation, H.K.; writing—original draft preparation, H.K. and S.K.H.; writing—review and editing, H.K., G.J., J.H.K., S.S.B., and S.K.H.; visualization, H.K.; supervision S.K.H.; project administration, H.K., and S.K.H.; funding acquisition, H.K. and S.K.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, H., Jung, G., Kim, J.H. et al. Role of prostate health index to predict Gleason score upgrading and high-risk prostate cancer in radical prostatectomy specimens. Sci Rep 11, 17447 (2021). https://doi.org/10.1038/s41598-021-96993-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96993-2
This article is cited by
-
Relationship between Proclarix and the Aggressiveness of Prostate Cancer
Molecular Diagnosis & Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.