Abstract
Thyroid hormone metabolism can be closely associated with cardiovascular disorders. We examined the relationship between low triiodothyronine (T3) levels and heart failure status, including B-type natriuretic peptide (BNP) levels, in 625 patients with cardiovascular disorders who underwent cardiac catheterization. A multiple regression analysis revealed that the left ventricular ejection fraction (LVEF), hemoglobin (Hb) levels, sex (male), free T3 (FT3) levels, and estimated glomerular filtration rate (eGFR) were significantly negatively associated with the log BNP value, while age was significantly positively associated with the log BNP value (P < 0.001 each). Furthermore, the log BNP and age were significantly negatively associated with the FT3 levels, while the Hb and body mass index (BMI) were significantly positively associated with the FT3 levels (P < 0.001 each). Theoretically constructed structure equation modeling (SEM) revealed an inverse association between FT3 and BNP (β = −0.125, P = 0.002), and the same relationship remained in the patient group with normal-range BNP values (β = −0.198, P = 0.008). We demonstrated a significant relationship between high BNP and low serum FT3 levels, and this relationship remained significant in patients with normal BNP levels. These results indicate that low T3 is associated with high plasma BNP levels rather than worsening of hemodynamics.
Similar content being viewed by others
Introduction
The synthesis and secretion of thyroid hormone are largely regulated by the hypothalamus-pituitary-thyroid axis1. Under the regulation of thyroid-stimulating hormone (TSH), the thyroid gland synthesizes and secretes mainly thyroxine (T4) (approximately 85%) and a smaller percentage of 3,5,3’-triiodothyronine (T3). T4 is converted to T3, which is the active form of thyroid hormone, by 5'-monodeiodination in the liver, kidney, and skeletal muscle2,3. T3 and T4 also exert a negative feedback effect that suppresses the synthesis and secretion of TSH in the pituitary gland2,4.
T3 shows various biological effects systemically, including stimulation of tissue thermogenesis, skeletal development, modulation of the appetite and food intake, and regulation of the body weight5,6,7. The serum T3 concentration decreases under conditions of critical illness, such as severe systemic infection, trauma, and starvation8,9,10. This low T3 syndrome is a beneficial adaptation and protective response that decreases energy consumption under pathological conditions11.
In the heart, T3 plays an important role in modulating myocardial contractility and hemodynamics by regulating the expression of the cardiac gene encoding cardiac protein12. An altered thyroid hormone metabolism closely reflects the pathological condition of cardiovascular systems13,14,15,16. Indeed, a low T3 syndrome has been reported in patients with heart failure, and the magnitude of this decrease in T3 is related to the prognosis of cardiovascular disorders17,18,19,20,21,22. Furthermore, previous studies have reported that the serum T3 level was inversely associated with the level of B-type natriuretic peptide (BNP) or N-terminal pro BNP (NT-pro BNP), a biomarker of heart failure severity23,24,25,26. However, thyroid hormone reportedly stimulates the release of BNP from both cultured atrial and ventricular myocytes in a dose-dependent manner27. In addition, thyroid hormone may be involved in the process of left ventricle (LV) remodeling, even in euthyroid patients28. For these reasons, the relationship between BNP and T3 levels needs to be reconsidered.
In the present study, we investigated the relationship between BNP and free T3 (FT3) levels in patients with cardiovascular disorders, taking into account other clinical factors that have the potential to affect the BNP or FT3 levels.
Results
Patient characteristics
Table 1 shows the clinical characteristics of the patients in this study. The average FT3 and free T4 (FT4) levels were 2.33 ± 0.32 pg/mL and 1.25 ± 0.23 ng/dL, respectively. The median TSH level was 1.43 μIU/mL (interquartile range [IQR] 0.91–2.22 μIU/mL), the median BNP level was 36.0 pg/mL (IQR 15.9–96.3 pg/mL), and the median LV ejection fraction (LVEF) was 60.8% (IQR 50.6–66.1%).
The comparison of clinical data between groups separated by the median level of FT3 (2.33 pg/mL)
To investigate the relationship between the FT3 level and various clinical data, we divided all patients into 2 groups by the median level of FT3 (2.33 pg/mL) and compared the two groups. As shown in Table 2, the number of male patients was significantly smaller (P < 0.001), the age and TSH level were significantly higher (each P < 0.001), and the body mass index (BMI), diastolic blood pressure (DBP), hemoglobin (Hb) level, estimated glomerular filtration rate (eGFR), total bilirubin level, albumin level, and triglyceride level were significantly lower in the low-T3 group (FT3 ≤ 2.33 pg/mL) than in the high-T3 group (FT3 > 2.33 pg/mL) (total bilirubin level: P = 0.01, and the others: P < 0.001, respectively). Furthermore, although there was no marked difference between the groups with regard to the left ventricular ejection fraction (LVEF), left ventricular end-systolic volume index (LVESVI), or left ventricular end-diastolic volume index (LVEDVI), the BNP level was significantly higher in the low-T3 group than in the high-T3 group (P < 0.001).
The correlation of the plasma BNP levels with various clinical factors
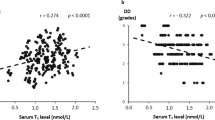
Table 3 shows the Spearman rank correlation coefficients between the plasma BNP level and various clinical factors. The plasma BNP level was significantly and positively correlated with the age, FT4 level, TSH level, LVESVI, and LVEDVI (age: r = 0.349, P < 0.001; FT4: r = 0.206, P < 0.001; TSH: r = 0.124, P = 0.002; LVESVI: r = 0.445, P < 0.001; LVEDVI: r = 0.341, P < 0.001) and negatively correlated with the sex (male), BMI, DBP, Hb level, eGFR, FT3 level, and LVEF (sex: r = −0.216, P < 0.001; BMI: r = −0.168, P < 0.001; DBP: r = −0.204, P < 0.001; Hb: r = −0.298, P < 0.001; eGFR: r = −0.312, P < 0.001; FT3: r = −0.280, P < 0.001; LVEF: r = −0.420, P < 0.001).
The correlation of the serum FT3 levels with various clinical factors
Table 4 shows the Spearman correlation coefficients between the serum FT3 level and various clinical factors. The serum FT3 level was significantly and positively correlated with the sex (male), BMI, DBP, Hb level, eGFR, and FT4 level (sex: r = 0.188, P < 0.001; BMI: r = 0.281, P < 0.001; DBP: r = 0.179, P < 0.001; Hb: r = 0.346, P < 0.001; eGFR: r = 0.197, P < 0.001; FT4: r = 0.091, P = 0.022) and negatively correlated with the age, TSH level, and BNP level (age: r = −0.290, P < 0.001; TSH: r = −0.160, P < 0.001; BNP: r = −0.280, P < 0.001), but LVEF, LVESVI, and LVEDVI were not significant.
The multiple regression analysis to determine the factors associated with the plasma BNP and serum FT3 levels in the whole group
Based on the statistical comparison of the two groups and results of a bivariate analysis, the multiple regression analysis for the BNP level included FT3, sex (male), age, BMI, DBP, Hb, eGFR, and LVEF as independent variables. As shown on Table 5, a multiple regression analysis revealed that LVEF, Hb, sex (male), FT3, and eGFR were significantly negatively, and age was positively associated with the log BNP (sex: P = 0.002, FT3 = 0.003, eGFR: P = 0.008, others: P < 0.001, respectively). In the same manner as the analysis for BNP, a multiple regression analysis for the FT3 level included BNP, sex (male), age, BMI, DBP, Hb, eGFR, and LVEF as independent variables. As shown on Table 6, a multiple regression analysis revealed that the log BNP and age were significantly negatively associated with the FT3 level, while the Hb value and BMI were positively associated with the FT3 level (age: P = 0.015, others: P < 0.001, respectively).
The concept and results of the proposed path model (A) in the whole group
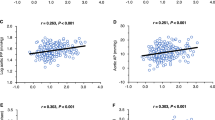
Based on multiple linear regression analyses and the findings of previous studies29,30,31,32, we selected sex (male), age, BMI, LVEF, eGFR, and Hb as independent variables and entered them into the path model. The theoretical path model (A) (Fig. 1) was proposed by positioning TSH, FT3, and BNP in parallel. The association between two factors was linked by two-way arrows. The sex (male), age, BMI, LVEF, eGFR, and Hb were placed in parallel, following arrows to TSH, FT3, and BNP, respectively. Paths were drawn from independent to dependent variables, with directional arrows for each regression model. As shown in Fig. 1 and Supplementary Table S1, path model (A) revealed that the age, BMI, LVEF, and Hb were associated with FT3 (age: standardized regression coefficient [St. β] = −0.109, P = 0.011, BMI: St. β = 0.161, P < 0.001, LVEF: St. β = 0.114, P = 0.002, Hb: St. β = 0.244, P = < 0.001). Path model (A) also revealed that sex (male), age, LVEF, and eGFR were associated with BNP (male: St. β = −0.142, P < 0.001, age: St. β = 0.107, P = 0.009, LVEF: St. β = −0.409, P < 0.001, eGFR: St. β = −0.117, P = 0.002). In addition, path model (A) showed an inverse association between FT3 and BNP (St. β = −0.125 P = 0.002), between TSH and FT3 (St. β = −0.114, P = 0.005), and between TSH and BNP (St. β = 0.084, P = 0.036).
Path model (A) (the whole BNP group). The path model theoretically proposed to clarify the contribution of sex (male), age, BMI, LVEF, eGFR, or Hb to TSH, FT3 or BNP. The analysis was performed on all cases. Each path has a coefficient representing the standardized coefficient of a regressing independent variable on a dependent variable of the relevant path. These variables represent the standardized regression coefficients (direct effect) and squared multiple correlations (in narrow italics) as well as the correlations among exogenous variables (green). BMI, body mass index; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; BNP, B-type natriuretic peptide.
The relationship between BNP and FT3 in the normal BNP level group
We defined the patient group that demonstrated BNP levels within the normal range as the “normal BNP level group”, and additional analyses were performed in the normal BNP level group (n = 185). As shown in Fig. 2 and Supplementary Table S2, path model (B) revealed that the BMI and eGFR were significantly associated with FT3 (BMI: St. β = 0.153, P = 0.034, eGFR: St. β = 0.197, P = 0.010), but LVEF was not significantly associated with FT3 (St. β = −0.002, P = 0.978). Path model (B) also revealed that the Hb was the only independent variable associated with BNP (St. β = −0.179, P = 0.022). In addition, path model (B) showed no significant association between TSH and FT3 or between TSH and BNP but retained a significant inverse association between FT3 and BNP (St. β = −0.198, P = 0.008).
Path model (B) (the normal BNP level group). The path model theoretically proposed to clarify the contribution of sex (male), age, BMI, LVEF, eGFR, or Hb to TSH, FT3 or BNP. The analysis was performed only on patients in whom the BNP levels were within the normal range (BNP ≤ 18.4 pg/mL). Each path has a coefficient representing the standardized coefficient of a regressing independent variable on a dependent variable of the relevant path. These variables represent the standardized regression coefficients (direct effect) and squared multiple correlations (in narrow italics) as well as the correlations among exogenous variables (green). BMI, body mass index; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; BNP, B-type natriuretic peptide.
Discussion
The present study showed that a decrease in the LVEF was significantly associated with a low serum T3 level, and there was a significant relationship between high BNP levels and low serum FT3 levels. Of note, in the normal BNP level group, the relationship between a high BNP level and low serum T3 level remained significant, while the LVEF was not significantly correlated with the serum FT3 level. However, whether a high BNP level led to the development of a low T3 level or vice versa remains unclear.
In the present study, a low FT3 level was significantly associated with an increase in the BNP level in patients with heart failure. Some previous reports have shown that a decrease in T3 is significantly related to an increase in BNP in patients with heart failure23,24,25,26. In humans, peripheral thyroid hormone metabolism is regulated by three iodothyronine deiodinases: D1, D2, and D3. D3 is present in the brain, skin, placenta, pregnant uterus, and various fetal tissues and catalyzes the conversion of T4 to reverse T3 (rT3) and the conversion of T3 to 3,3'-diiodothyronine (3,3'-T2), both of which are biologically inactive. Under pathological conditions, such as cancer, chronic inflammation, and critical illness, D3 is activated, the conversion of T3 to 3,3`-T2 is accelerated, and the serum T3 level consequently decreases33,34,35. In particular, the remarkable activation of D3 and a decrease in the serum T3 level are observed in patients with cardiovascular disorders34,36,37. However, in the present study, low FT3 levels associated with an increase in BNP were observed even in patients with normal BNP levels, thus indicating that low T3 is associated with high plasma BNP levels rather than worsening of hemodynamics. Perhaps a low T3 level might accelerate an increase in the BNP level. An unknown factor related to low T3 (e.g. thyroid hormone metabolites) might cause an increase in the BNP level. Further experiments will be needed to clarify what this unknown factor is.
In addition, Pinelli et al. showed an inverse correlation between FT3 and log NT-proBNP in non-cardiac patients, but it was a small-sample study (n = 52)38. In our study, 625 patients were involved, and high BNP levels were inversely correlated with low serum FT3 levels in patients with heart failure according to structural equation models (SEM), a relationship that was retained even in cases with normal BNP levels.
Several limitations associated with the present study warrant mention. First, this study was a retrospective one conducted at a single university hospital. Second, this study included patients with various cardiovascular diseases, and not all patients had apparent structural heart disease. Some patients in Stage A according to the Guidelines of Heart Failure39,40 were also included. Third, in the present study, the cardiac function of most patients was preserved, and the number of patients with heart failure with mid-range ejection fraction (HFmrEF) (n = 76) or heart failure with reduced ejection fraction (HFrEF) (n = 74) was small. Therefore, it was difficult to analyze the relationship between BNP and FT3 levels accurately in patients with HFmrEF or HFrEF. Based on an analysis of this current dataset, although the results were feasible in the patients with a preserved cardiac function, the results cannot be applied to those with HFmrEF or HFrEF. Finally, although we briefly checked the cardiac function of each patient by echocardiography before cardiac catheterization, we did not regularly register the precise echocardiography data and were thus unable to analyze the echocardiography data in detail in the present study.
In conclusion, there was a significant relationship between high plasma BNP levels and low serum FT3 levels in patients with heart failure, and this relationship was maintained even in cases with normal BNP levels. These findings indicate that low T3 is associated with high plasma BNP levels rather than worsening of hemodynamics.
Methods
Patient population
The study population consisted of 712 patients who were consecutively admitted to our institution with cardiovascular disorders and who underwent elective cardiac catheterization from September 2017 to February 2021. We excluded patients who underwent dialysis (n = 60). In addition, we excluded patients with already-known thyroid disorders and/or who were treated with thyroid medications (n = 27). Finally, we analyzed 625 patients in the present study (Supplementary Figure).
This study was conducted in accordance with the principles expressed in the Declaration of Helsinki and approved by the medical ethics committee of Jikei University School of Medicine [24–355(7121)]. All methods were carried out in accordance with relevant guideline and regulations. The Ethics Committee waived the need for informed written consent, since it was a retrospective study. Instead of obtaining informed consent from each patient, we posted a notice about the study design and contact information at a public location in our institution according to our routine ethical regulations.
Data collection
The clinical characteristics of patients were retrospectively collected from their medical records. Blood samples were collected just before cardiac catheterization with the patient in a fasting condition. The LVEF, LVESVI, and LVEDVI were measured at the time of left ventriculography. Biochemical analyses of the plasma and serum were performed in our hospital’s central laboratory during the study period. To measure the plasma BNP level, blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA) and then immediately centrifuged at 3000 rpm for 5 min at 14 °C. Thereafter, the plasma BNP levels were immediately measured by a chemiluminescent enzyme immunoassay (CLEIA) with an AIA-CL2400 (TOSOH Corporation, Tokyo, Japan) as described in the previous report41. The normal range of plasma BNP level was set at ≤ 18.4 pg/mL. The serum FT3, FT4, and TSH levels were measured by the CLEIA method using a CL AIA-PACK FT3 TEST CUP, CL AIA-PACK FT4 TEST CUP, and CL AIA-PACK TSH TEST CUP, respectively (TOSOH Corporation).
Statistical analyses
Data are expressed as the mean ± standard deviation (SD) or as the median (25th, 75th percentile) for significantly skewed variables. For continuous variables, differences between the two groups were evaluated either by an unpaired Student’s t-test or the Mann -Whitney rank-sum test. For discrete variables, which were expressed as counts and percentages, any differences between the two groups were analyzed by the chi-square test, unless the expected values in any cells were less than 5, in which case Fisher’s exact test was used. Correlations between the clinical parameters and the BNP or FT3 levels were assessed using Spearman’s rank correlation coefficient. First, to exclude the effect of other factors in clarifying the relationship between BNP and FT3, a step-wise multiple linear regression analysis was performed with BNP as the dependent variable. The independent variables were selected based on theoretical grounds, the results of the statistical comparison of two groups and a bivariate analysis. Similarly, a step-wise multiple regression analysis was repeated with FT3 as the dependent variable using the same independent variables. All statistical analyses were performed using the SPSS Statistics software program (version 27.0; SPSS Inc., Chicago, IL, USA). P values of < 0.05 were considered to indicate statistical significance.
In addition, a path model based on a SEM was used to investigate the relationships between the BNP and FT3 levels. The path model defined some hierarchical regression models among clinical factors and the BNP and FT3 levels. A path analysis was performed using the IBM SPSS AMOS software program (version 27; Amos Development Corporation, Meadville, PA, USA). The SEM that were obtained were tested and confirmed at a significance level of P < 0.05.
The following points should be noted regarding SEM: First, SEM is an effective analytical method for confirming hypotheses, but analysts need to consider sufficient hypotheses before and after building a path model. By maximizing the analyst's knowledge, the hypothesis approaches the appropriate model. In other words, it takes trial and error to create a proper path diagram. Second, "causality" is an issue that needs to be addressed. Strictly speaking, in order to build a causal relationship, it is necessary to discuss the pre-occurrence priority in terms of the temporal priority in which the causal relationship occurs. Care must be taken to conclude that there was an exact causal relationship without such consideration. If the temporal relationship is unclear, it should simply be called a relationship. In the present study, we planned to devise an optimal path diagram in consideration of these points. However, we may need to look at other path diagrams in the future.
References
Fliers, E., Kalsbeek, A. & Boelen, A. Beyond the fixed setpoint of the hypothalamus-pituitary-thyroid axis. Eur. J. Endocrinol. 171, R197-208. https://doi.org/10.1530/eje-14-0285 (2014).
Klein, I. & Danzi, S. Thyroid disease and the heart. Circulation 116, 1725–1735. https://doi.org/10.1161/circulationaha.106.678326 (2007).
Marsili, A., Zavacki, A. M., Harney, J. W. & Larsen, P. R. Physiological role and regulation of iodothyronine deiodinases: A 2011 update. J. Endocrinol. Invest. 34, 395–407. https://doi.org/10.1007/bf03347465 (2011).
Larsen, P. R. Thyroid-pituitary interaction: Feedback regulation of thyrotropin secretion by thyroid hormones. N. Engl. J. Med. 306, 23–32. https://doi.org/10.1056/nejm198201073060107 (1982).
Bassett, J. H. & Williams, G. R. Critical role of the hypothalamic-pituitary-thyroid axis in bone. Bone 43, 418–426. https://doi.org/10.1016/j.bone.2008.05.007 (2008).
Silva, J. E. & Larsen, P. R. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305, 712–713. https://doi.org/10.1038/305712a0 (1983).
Mullur, R., Liu, Y. Y. & Brent, G. A. Thyroid hormone regulation of metabolism. Physiol. Rev. 94, 355–382. https://doi.org/10.1152/physrev.00030.2013 (2014).
Kaptein, E. M., Robinson, W. J., Grieb, D. A. & Nicoloff, J. T. Peripheral serum thyroxine, triiodothyronine and reverse triiodothyronine kinetics in the low thyroxine state of acute nonthyroidal illnesses. A noncompartmental analysis. J. Clin. Invest. 69, 526–535. https://doi.org/10.1172/jci110478 (1982).
Mocchegiani, E. et al. Thyroid and thymic endocrine function and survival in severely traumatized patients with or without head injury. Intensive Care Med. 21, 334–341. https://doi.org/10.1007/bf01705412 (1995).
Davidson, M. B. & Chopra, I. J. Effect of carbohydrate and noncarbohydrate sources of calories on plasma 3,5,3’-triiodothyronine concentrations in man. J. Clin. Endocrinol. Metab. 48, 577–581. https://doi.org/10.1210/jcem-48-4-577 (1979).
Utiger, R. D. Altered thyroid function in nonthyroidal illness and surgery. To treat or not to treat?. N. Engl. J. Med. 333, 1562–1563. https://doi.org/10.1056/nejm199512073332310 (1995).
Klein, I. & Ojamaa, K. Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 344, 501–509. https://doi.org/10.1056/nejm200102153440707 (2001).
Janssen, R., Muller, A. & Simonides, W. S. Cardiac thyroid hormone metabolism and heart failure. Eur. Thyroid J. 6, 130–137. https://doi.org/10.1159/000469708 (2017).
Tang, Y. D. et al. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation 112, 3122–3130. https://doi.org/10.1161/circulationaha.105.572883 (2005).
Liu, Z. & Gerdes, A. M. Influence of hypothyroidism and the reversal of hypothyroidism on hemodynamics and cell size in the adult rat heart. J. Mol. Cell. Cardiol. 22, 1339–1348. https://doi.org/10.1016/0022-2828(90)90979-c (1990).
Campbell, S. E. & Gerdes, A. M. Regional changes in myocyte size during the reversal of thyroid-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 20, 379–387. https://doi.org/10.1016/s0022-2828(88)80129-9 (1988).
Hamilton, M. A., Stevenson, L. W., Luu, M. & Walden, J. A. Altered thyroid hormone metabolism in advanced heart failure. J. Am. College Cardiol. 16, 91–95. https://doi.org/10.1016/0735-1097(90)90462-x (1990).
Opasich, C. et al. Sick euthyroid syndrome in patients with moderate-to-severe chronic heart failure. Eur. Heart J. 17, 1860–1866. https://doi.org/10.1093/oxfordjournals.eurheartj.a014804 (1996).
Iervasi, G. et al. Low-T3 syndrome: A strong prognostic predictor of death in patients with heart disease. Circulation 107, 708–713. https://doi.org/10.1161/01.cir.0000048124.64204.3f (2003).
Sato, Y. et al. Low T3 syndrome is associated with high mortality in hospitalized patients with heart failure. J. Cardiac. Fail. 25, 195–203. https://doi.org/10.1016/j.cardfail.2019.01.007 (2019).
Brozaitiene, J., Mickuviene, N., Podlipskyte, A., Burkauskas, J. & Bunevicius, R. Relationship and prognostic importance of thyroid hormone and N-terminal pro-B-Type natriuretic peptide for patients after acute coronary syndromes: A longitudinal observational study. BMC Cardiovasc. Disord. 16, 45. https://doi.org/10.1186/s12872-016-0226-2 (2016).
Du, J. B. et al. The role of brain natriuretic peptide and serum triiodothyronine in the diagnosis and prognosis of chronic heart failure. Acta Cardiol. 67, 291–296. https://doi.org/10.1080/ac.67.3.2160717 (2012).
Selvaraj, S. et al. Association of serum triiodothyronine with B-type natriuretic peptide and severe left ventricular diastolic dysfunction in heart failure with preserved ejection fraction. Am. J. Cardiol. 110, 234–239. https://doi.org/10.1016/j.amjcard.2012.02.068 (2012).
Wang, K. et al. BNP as a new biomarker of cardiac thyroid hormone function. Front. Physiol. 11, 729. https://doi.org/10.3389/fphys.2020.00729 (2020).
She, J. et al. Correlation of triiodothyronine level with in-hospital cardiac function and long-term prognosis in patients with acute myocardial infarction. Dis. Markers 2018, 5236267. https://doi.org/10.1155/2018/5236267 (2018).
Pfister, R. et al. The relationship and prognostic impact of low-T3 syndrome and NT-pro-BNP in cardiovascular patients. Int. J. Cardiol. 144, 187–190. https://doi.org/10.1016/j.ijcard.2009.03.137 (2010).
Kohno, M. et al. Stimulation of brain natriuretic peptide release from the heart by thyroid hormone. Metab.: Clin. Exp. 42, 1059–1064. https://doi.org/10.1016/0026-0495(93)90023-h (1993).
Iida, M. et al. Thyroid hormone within the normal range is associated with left ventricular mass in patients with hypertension. J. Am. Soc. Hypertens. 6, 261–269. https://doi.org/10.1016/j.jash.2012.04.002 (2012).
Takami, Y. et al. Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am. J. Kidney Dis. 44, 420–428 (2004).
Tominaga, M. et al. Association between plasma B-type natriuretic peptide and anaemia in heart failure with or without ischaemic heart disease: A retrospective study. BMJ Open 9, e024194. https://doi.org/10.1136/bmjopen-2018-024194 (2019).
Yasue, H. et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 90, 195–203. https://doi.org/10.1161/01.cir.90.1.195 (1994).
Mehra, M. R. et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J. Am. College Cardiol. 43, 1590–1595. https://doi.org/10.1016/j.jacc.2003.10.066 (2004).
Huang, S. A. & Bianco, A. C. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat. Clin. Pract. Endocrinol. Metab. 4, 148–155. https://doi.org/10.1038/ncpendmet0727 (2008).
Peeters, R. P. et al. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J. Clin. Endocrinol. Metab. 88, 3202–3211. https://doi.org/10.1210/jc.2002-022013 (2003).
Huang, S. A. et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N. Engl. J. Med. 343, 185–189. https://doi.org/10.1056/nejm200007203430305 (2000).
Olivares, E. L. et al. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology 148, 4786–4792. https://doi.org/10.1210/en.2007-0043 (2007).
Wassen, F. W. et al. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology 143, 2812–2815. https://doi.org/10.1210/endo.143.7.8985 (2002).
Pinelli, M. et al. Relationship between low T3 syndrome and NT-proBNP levels in non-cardiac patients. Acta Cardiol. 62, 19–24. https://doi.org/10.2143/ac.62.1.2019366 (2007).
Tsutsui, H. et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure-digest version. Circ. J.: Off. J. Jpn. Circ. Soc. 83, 2084–2184 (2019).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128, e240-327. https://doi.org/10.1161/CIR.0b013e31829e8776 (2013).
Kashiwagi, Y. et al. Close linkage between blood total ketone body levels and B-type natriuretic peptide levels in patients with cardiovascular disorders. Sci. Rep. 11, 6498. https://doi.org/10.1038/s41598-021-86126-0 (2021).
Acknowledgements
We would like to thank Brian Quinn, Japan Medical Communication, for reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
H.T., Y.K., T.N. and M.Y. were responsible for the conception and design of the study. H.T., Y.K. and M.Y. were responsible for the data analysis, writing of the manuscript, and production of the figures and tables. H.K., Y.K., T.N., Y.T., Y.O. and K.M. contributed to the collection and interpretation of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takahashi, H., Kashiwagi, Y., Nagoshi, T. et al. Low triiodothyronine levels correlate with high B-type natriuretic peptide levels in patients with heart failure. Sci Rep 11, 21865 (2021). https://doi.org/10.1038/s41598-021-01454-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01454-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.