Abstract
Heart failure (HF) is a multiple hormonal deficiency syndrome which includes alterations in the serum concentration of thyroid hormones (TH). This cross-sectional study enrolled 215 male patients hospitalised for acute HF. Data on cardiovascular risk factors, chronic medications, cardiac function assessed by echocardiography, and clinical parameters of HF were prospectively collected. The independent predictive association of TH with all investigated parameters of the HF severity were assessed. The patient’s mean age was 74.4 years, 57.2% had arterial hypertension, 54.0% were consuming alcohol, and 42.3% were diabetics. Multivariate analysis revealed that total triiodothyronine (TT3) was an independent predictor of greater left ventricular ejection fraction (LVEF; β = 0.223, p = 0.008), less progressed left ventricular diastolic dysfunction (LVDD; β = − 0.271, p = 0.001) and lower N-terminal pro-brain natriuretic peptide (NT-proBNP; β = − 0.365, p < 0.001). None of the TH other than TT3 was associated with LVDD or NT-proBNP, whereas free triiodothyronine (β = − 0.197, p = 0.004), free thyroxine (β = − 0.223, p = 0.001) and total thyroxine (β = − 0.140, p = 0.041) were inversely associated with LVEF. The present study suggests that, among TH, serum TT3 level is most closely associated with echocardiographic, laboratory and clinical parameters of the severity of HF in men.
Similar content being viewed by others
Introduction
Heart failure (HF), a common final pathway of numerous cardiac disorders, is characterised by a complex pathophysiology where hormonal imbalance plays an important role1, 2. This multiple hormonal deficiency syndrome includes lower circulating levels of growth hormone, insulin-like growth factor-1, dehydroepiandrosterone sulphate, and testosterone3,4,5,6. Alterations in the serum concentration of thyroid hormones (TH) have also been associated with HF.
The non-thyroidal illness syndrome (NTIS) is a set of changes in the circulating concentrations of TH commonly seen in advanced HF. NTIS is represented by a decrease in serum triiodothyronine (T3) levels which is, in more severe and prolonged cases of the syndrome, associated with a reduction in serum thyroxine (T4). These changes are not followed by an expected increase in the serum concentrations of thyroid-stimulating hormone (TSH)7. It has been suggested that between 18 and 30% of patients with congestive HF develop a drop in serum levels of T38,9,10,11. This drop has been associated with a reduced left ventricular ejection fraction (LVEF), a higher degree of diastolic dysfunction of the left ventricle (LVDD), and an increased risk of death12,13,14. All these cardiac abnormalities may be reversible with the restoration of normal thyroid hormone values15.
There are important differences between men and women in almost all aspects of HF due to gender-specific factors, primarily involving sex hormones16. Testosterone, the primary sex hormone in men, has been associated with LVDD and clinical parameters of HF in male patients17,18,19,20. The peripheral effects of testosterone may explain most of its favourable effects on the pathophysiology of HF, but its central effects need further exploration21.
So far, the relationship between TH and the parameters of HF has not been investigated in greater detail in male patients with HF. The present study aims to investigate the association of five TH, namely free and total T3 (fT3 and TT3), free and total T4 (fT4 and TT4), and TSH, with LVEF and LVDD, and other clinical parameters of HF severity in men, with controlling for the effect of serum testosterone levels and other clinical factors.
Methods
This cross-sectional study enrolled male patients admitted for acute HF at the Department of Cardiology and Angiology, University Hospital Centre Split, between February 2016 and October 2019. Data on baseline characteristics, the existence of cardiovascular risk factors, cardiovascular drugs from the patient's chronic therapy, blood determinations, and echocardiographic parameters were prospectively collected during the patient's hospital stay. The participant’s height and weight, wearing hospital clothes without shoes, were measured during the clinical examination at the beginning of the hospitalization, and body mass index (BMI, in kg/m2) was calculated. The Ethics Committee from the Science Department of the University Hospital Centre Split approved the study protocol, and each participant gave his written informed consent (2181-147-10-01/01-M. J). The study complies with the Helsinki Declaration.
Inclusion criteria were: (1) clinical presentation typical for HF; (2) LVEF equal to or less than 50% (using the Simpson method), and/or LVDD established by transthoracic echocardiography; (3) unchanged medical therapy during at least one month. Exclusion criteria were: (1) acute or chronic systemic illness that could affect hormonal metabolism (i.e., a primary endocrine disorder, autoimmune or malignant disease, infection, terminal phase of renal failure, primary liver disease and liver cirrhosis), including patients with the BMI less than 18.5 kg/m2 for potential frailty; (2) any hormonal treatment or drugs significantly influencing TH levels at the time of the study or in the past (i.e., antithyroid drugs, synthetic TH, corticosteroids, dopamine, dobutamine); (3) cardiac surgery, acute coronary syndrome or coronary revascularization within six months before the study; (4) C-reactive protein levels above 15 mg/dL. C-reactive protein levels under 10 mg/L may be interpreted as of minor clinical importance22 and considering a low-grade systemic inflammation that accompanies HF23, we established a maximum of 15 mmol/L, i.e., a 50% increase, as a cut-off value for inclusion24.
Blood samples for blood count and biochemical analysis were taken on admission, (i.e., during the patient’s evaluation in the emergency department), from the anterior cubital vein. Circulating TH and total testosterone levels were assessed within the first three days of hospitalization using commercially available radioimmunoassay kits (Roche Diagnostics GmbH, Mannheim, Germany). The inter-assay coefficient for all hormones ranged between 8 and 13%. For all patients, these blood samples were taken between 8 and 9 a.m. to account for circadian variation. According to the manufacturer's instructions in our laboratory, normal reference intervals were 1.34 to 2.73 nmol/L for TT3, 3.8–6.0 pmol/L for fT3, 78–157 nmol/L for TT4, 7.90–14.40 pmol/L for fT4, and 0.34–5.60 mIU/L for TSH. The estimated glomerular filtration rate (eGFR, in mL/min/1.73 m2) was calculated using the Modification of Diet in Renal Disease equation25.
Transthoracic echocardiography was performed by certified cardiologists using a Vivid 9E device (GE Medical System, Milwaukee WI, USA) and interpreted according to the standard guidelines of the European Society of Cardiology (ESC) for LVEF26 and LVDD assessment27. The LVEF was calculated using the Simpson biplane method. The LVDD was assessed by measuring the mitral valve inflow on pulsed-wave Doppler in the four-chamber view (E and A velocity waves, E/A ratio, E-wave deceleration time, isovolumic relaxation time) and septal and lateral peak annular tissue velocities (é and á waves, the é/á ratio, E/é ratio) obtained by tissue-Doppler and pulsed-wave Doppler imaging. The pulsed-wave Doppler sample volumes were positioned within one centimetre of the septal and lateral insertion of the mitral valve leaflets. According to LVEF, we stratified our patients into two categories, normal, with LVEF greater than 50%, and reduced, with LVEF equal to or less than 50%. LVDD was assessed according to the ESC recommendations and divided into three basic grades28. However, to provide a more detailed assessment, we divided the restrictive pattern of mitral inflow into reversible and irreversible types and included intermediate grades where appropriate17.
Data were expressed as percentages for dichotomous variables, as mean value ± standard deviation (SD) for normally distributed continuous variables, and through medians and interquartile ranges (IQR) for nonuniformly distributed continuous variables. The normality of data distribution was tested using the Kolmogorov–Smirnov test. In the univariate analysis, differences between the groups were tested using the χ2 test, t-test, or Mann–Whitney U test, as appropriate. Multiple regression analysis was used to assess the independent predictive association of the TH, total testosterone levels, age, BMI, eGFR, traditional risk factors, previous myocardial infarction (MI), and cardiovascular medications with the echocardiographic parameters (LVEF and LVDD), N-terminal pro-brain natriuretic peptide (NT-proBNP), New York Heart Association (NYHA) functional class and HF duration. These results were expressed through β and p-values. The statistical significance of the test was determined by a p-value less than 0.05. Data were analysed with IBM SPSS Statistics 26 (26.0.0.0; 2019; Armonk, New York; USA).
Results
Characteristics of the study population
Tables 1 and 2 display the baseline characteristics, prehospital medication, and laboratory findings of the 215 included men. The median duration of HF was 24.0 months, and the patient’s mean age was 74.4 years. The average LVEF was 46.1%; 41.4% of the included patients had preserved LVEF, 23.7% had mid-range LVEF, and 34.9% had reduced LVEF. All patients had LVDD with an average grade of 2.7 ± 0.6. More than half of the patients had arterial hypertension or were currently consuming alcohol, while 42.3% of them were diabetics. Approximately two-thirds of the patients used loop diuretics in chronic therapy.
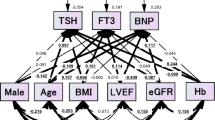
The analysis of interrelations among TH revealed that there were significant positive correlations among fT3 and both fractions of T4 as well as among fT4, TT3 and TT4. In addition, there was a borderline positive correlation between fT3 and TT3 and a significant inverse correlation between TSH and both fT4 and TT4 (Supplementary Table S1).
TT3 and parameters of HF
In the univariate analysis, serum TT3 levels were significantly associated with the echocardiographic parameters of cardiac function showing a positive correlation with LVEF and an inverse correlation with LVDD (p < 0.0001; Fig. 1). TT3 level also inversely correlated with NT-proBNP level (p < 0.0001; Fig. 2) and NYHA class (r = − 0.207, p = 0.002), whereas there was no significant relationship with the duration of HF (r = − 0.47, p = 0.56). Multivariate analysis confirmed that all the aforementioned associations, except for NYHA class and HF duration, are independent of other clinical factors, including serum testosterone levels (Tables 3, 4, 5, Supplementary Tables S2 and S3).
Correlation between serum total triiodothyronine (T3) level and the echocardiographic parameters of cardiac function. Scatterplots depicting the correlation between serum total triiodothyronine (T3) level and the echocardiographic parameters of cardiac systolic and diastolic function in the study population. Significant correlations were observed for both the left ventricular ejection fraction (LVEF; panel (a), linear regression equation: Y = 10.34X + 33.14) and the grade of diastolic dysfunction (DD; panel (b), linear regression equation: Y = − 0.57X + 3.43).
Correlation between serum total triiodothyronine (T3) level and N-terminal pro-brain natriuretic peptide (NT-proBNP) level. Scatterplot depicting the correlation between serum total triiodothyronine (T3) level and serum N-terminal pro-brain natriuretic peptide (NT-proBNP) level in the study population. Linear regression equation: Y = − 1628.1X + 2980.1. Regression equation, r and p-values were obtained from the linear regression analysis.
Other TH and parameters of HF
Univariate analysis showed that TSH, fT3 and fT4 were inversely related to LVEF, while none of the TH other than TT3 was associated with LVDD (Supplementary Table S4). A positive correlation was observed between TSH and both NT-proBNP and NYHA class, while there was an inverse correlation between TSH and HF duration (Supplementary Table S4). Multivariate analysis confirmed the independent association only between TSH and HF duration (Supplementary Table S5) whereas there was no independent association between TSH and LVEF, LVDD, NT-proBNP or NYHA class (Supplementary Tables S6–S9). Independent predictors of LVEF were lower levels of fT3 (Supplementary Table S10), fT4 (Supplementary Table S11) and TT4 (Supplementary Table S12). Also, after adjustment for other clinical variables, fT3, fT4, and TT4 were not associated with LVDD (Supplementary Tables S13, S4 and S15) or NT-proBNP (Supplementary Tables S16, S17 and S18). There were independent associations of fT4 with HF duration (Supplementary Table S19) and TT4 with NYHA class (Supplementary Table S20) whereas there were no predictive associations between fT3, fT4, and TT4 and the rest of the observed parameters (Supplementary Tables S21–S24).
Other variables and parameters of HF
In all multivariate analyses, total testosterone also showed substantial associations with LVEF (Table 3, Supplementary Tables S6, S10–S12), LVDD (Table 4, Supplementary Tables S7, S13–S15) and NT-proBNP (Table 5, Supplementary Tables S8, S16–S18); those associations were of similar nature and strength as the associations observed for TT3. Age and non-use of alcohol were independently associated with HF duration in all multiple regression analyses (Supplementary Tables S3, S5, S19, S22 and S24).
Discussion
Our study showed that, among the five investigated TH, serum TT3 levels had the closest correlation with echocardiographic, laboratory and clinical parameters of the HF severity in men. These associations were independent of other clinical variables and factors, including serum testosterone levels.
TH are important modulators of cardiac function through cardiac and extra-cardiac mechanisms. They influence the heart rate, heart rhythm, myocardial contraction, and blood pressure, and have an impact on cardiovascular risk factors, such as hyperlipidaemia, arterial hypertension and thrombogenesis15. The main alteration of TH in HF, the NTIS, is characterised by changes in the TH pathophysiology both at the level of the hypothalamic–pituitary–thyroid axis and at the organ and tissue level7. In the acute phase of a critical illness, concomitant with a drop in serum T3 level, a transient rise in serum T4 level may be seen29. The disruption of the thyroid axis occurs when the illness is prolonged, which may result in a decrease in serum T4 and TSH levels29. Changes in TH levels that accompany NTIS may have prognostic significance for the worsening of HF and may represent independent predictors of cardiac and overall mortality12, 30,31,32,33.
The T3 tissue availability is regulated through the expression of TH membrane transporters (monocarboxylate transporters 8 and 10, and organic anion transporting polypeptide family 1C1), iodothyronine deiodinases (DIO1, DIO2 and DIO3), intracellular thyroid receptors (α1, β1 and β2) and hypothalamus thyrotropin-releasing hormone (TRH)7, 34,35,36. In NTIS, it has been reported that factors such as systemic inflammation36, glucocorticoid serum rise37 or energy status38 may alter the expression of the above-mentioned regulators, resulting in the T3 serum and tissue reduction. Experimental studies have shown that proinflammatory cytokines (interleukins 6, 1 and 1β, and tumour necrosis factor α) downregulate various components of TH synthesis and metabolism39, 40, whereas high glucocorticoid levels are responsible for suppressing the pituitary response to TRH in men37, 41. During food deprivation, as an example of low energy status, a drop in serum leptin is present, which induces a decrease in TRH expression in the hypothalamus42. Different components of TH level regulation are altered differently depending on the type of tissue, duration, severity, and type of the illness7, 43. However, regardless of the important advances, there are multiple gaps in the current knowledge of the pathophysiology of NTIS.
Selvaraj et al. have reported that in HF with preserved LVEF, serum TT3 has been negatively associated with NT-proBNP and severe LVDD44. In patients suffering from idiopathic LV dysfunction, TT3 has been suggested as the predictor of NYHA class only, without association with either LVEF or NT-proBNP45. In neonatal rat ventricular myocytes, exposure to T3 increased gene expression of brain natriuretic peptide (BNP)46. However, it has also been reported that BNP gene expression is generally increased during the fetal period, as an important regulator of myocyte growth47. The expression of BNP genes and other genes typically expressed during the fetal period is enhanced during HF48, 49. In a recent study, adult rats49, one group with propylthiouracil-induced hypothyroidism and another one with MI-induced HF, have been investigated. The results have suggested that the BNP expression is negatively regulated by cardiac tissue T3 levels following oral T3 treatment in both groups. In addition, the increasing serum TT3 showed a strong inverse correlation with serum BNP, which is in accordance with our results. Therefore, both that49 and our study suggest that BNP could serve as a serum biomarker of cardiac tissue T3 activity.
For the first time, our results revealed strong predictive associations of TT3 and all three important parameters of HF severity, namely LVEF, LVDD and NT-proBNP. It is important to stress that all of these associations are independent of the effects of other relevant clinical variables, including testosterone levels. Our study strongly supports the role of TT3 as the chief TH involved in the modulation of cardiac function in terms of HF mechanisms and progression. This is in line with the principal role of TT3 in preserving LVEF50, probably through the favourable influence of T3 on cardiac contractility and reduction of myocardial damage15, 51,52,53,54,55. The beneficial role on LVDD may also be explained by the reduction of myocardial fibrosis56,57,58 through cardioprotective mechanisms of T3, such as activation of cytoprotective mechanisms, metabolic adaptation and neoangiogenesis15.
In addition, T3 has been implicated in the reduction of peripheral vascular resistance59, 60. An increase in peripheral vascular resistance has been proposed as a contributor to HF progression through deleterious and irreversible effects on the heart and circulation61. The combined effect of all of the aforementioned mechanisms may be responsible for the overall beneficial effect of TT3 in HF, expressed through a strong inverse relation with NT-proBNP in our patients.
Despite the significant impact of T3 on cardiac function, previous data have shown no clear association of fT3 with the parameters of HF severity. One previous study has reported a positive relationship of fT3 with NT-proBNP levels32, whereas several other studies have reported no relationship with LVEF33, 62. However, none of these associations have been adjusted for important clinical confounders. Experimental data on intravenous T3 therapy have shown that fT3 levels, raised by infusion, are associated with an improved stroke volume and end-diastolic LV volume, lower NT-proBNP levels and signs of deactivation of neuroendocrine mechanisms63.
Our multivariate analysis revealed an inverse relationship between fT3 and LVEF, whereas there was no independent relationship between fT3 and LVDD or NT-proBNP. In the context of the above-described data63, this may represent a compensatory mechanism of enhanced increase of fT3 aiming to improve LVEF.
The evidence on the relationship of TT4 and fT4 with cardiac function parameters is scarce. In patients with dilated cardiomyopathy, a positive relationship with NT-proBNP and no relationship with the grade of LVDD has been reported for fT444. Randomised controlled studies on relatively small populations of patients with dilated cardiomyopathy have demonstrated an improvement in LVEF and parameters of longitudinal and circumferential strain after receiving substitutional therapy with levothyroxine, the synthetic form of T464,65,66.
Our results showed a negative association of TT4 and fT4 with LVEF and no independent association with LVDD and NT-proBNP. Also, TT4 was a predictor of NYHA class, whereas fT4 was a predictor of HF duration. During NTIS, the conversion of T4 to T3 is reduced7. In general, TH nuclear receptors bind T3 with a 10 times greater affinity than T467. Accordingly, it has been suggested that cardiac TH-responsive genes are expressed as a function of serum T3, whereas, regardless of its serum levels, T4 may have a lower impact on cardiac function15. The fact that we observed an inverse association of TT4 and fT4 with TSH may suggest that this part of the thyroid feedback loop is less disrupted by the pathological mechanisms of HF. The observed associations may also be interpreted as a compensatory rise of TT4 and fT4 in patients with more severe HF, as in the case of fT3. In this context, higher T4 levels combined with lower T3 levels may indicate a lower peripheral conversion of TH45, 68. Since both T4 and T3 substitutional therapy have been related to the improvement of cardiac function in several studies63,64,65,66, the T3/T4 ratio could be useful in monitoring future therapy options.
In patients with dilated cardiomyopathy, TSH level has been associated with NT-proBNP levels44, whereas no significant relationship between TSH and echocardiographic parameters of cardiac size and function has been observed62. In patients with subclinical hypothyroidism, significant negative correlations of TSH with cardiac index, stroke volume and end-diastolic volume, as well as a positive relationship of TSH with systemic vascular resistance have been described69. However, for all of the reported relationships, no adjustments were made for relevant clinical variables.
TRH and TSH levels are regulated by a typical endocrine feedback loop70. Nevertheless, there is no compensatory rise in serum TSH in NTIS following a reduction in serum levels of other TH7. It has been suggested that factors such as age, sex, body mass index, race, smoking habits, iodine intake, the timing of the sample acquisition, as well as accompanying medical conditions and medications can affect serum TSH levels15. In our study, after adjusting for total testosterone levels, age, BMI, eGFR, traditional cardiovascular disease risk factors, previous MI and prehospital cardiovascular medications, no association between TSH and LVEF, LVDD and NT-proBNP was found. Therefore, our study suggests that serum TSH levels are not helpful in the assessment of HF progression and are not associated with cardiac function parameters.
It has been hypothesised that the beneficial effect of testosterone may be exerted only on a relatively healthy heart unaffected by pathological remodelling17,71. Total testosterone has been suggested as an independent predictor of NT-proBNP, LVDD and NYHA class24, 72, 73. The present study confirmed that the associations of testosterone with HF parameters are independent of TH.
HF is a long-term syndrome that has a more severe form in older patients74. Excessive consumption of alcohol has been related to alcoholic cardiomyopathy75, 76. In contrast, the beneficial effects of light-to-moderate alcohol consumption in HF have been well-established77,78,79,80. In our study, the average age of the patients was 74.4 years with a median duration of HF of 24.0 months. Age and non-use of alcohol were independent predictors of HF duration in all adjusted analyses. We may only speculate that younger patients may have had more rapidly progressed forms of HF and died sooner. In the same manner, shorter HF duration in patients consuming alcohol could suggest predominantly adverse effects of alcohol associated with a worse overall prognosis.
The main strength of our study is the extensive exclusion criteria covering conditions that could have had an impact on the variables of interest. Moreover, in contrast to the majority of previous clinical studies, in the present study, we adjusted the associations of TH with HF parameters for a number of important clinical confounders.
Since we investigated a highly selected population, created to obtain as representative sample as possible, our sample was not suitable for the assessment of specific roles of traditional cardiovascular risk factors and cardiovascular medications. Additionally, we did not record the exact doses of the medicines in chronic therapy, nor did we detail the amounts of alcohol consumption or the iodine intake. We measured serum levels of TH, whereas the exact cardiac tissue TH levels remained uncertain. We did not collect data on the aetiology of HF. Furthermore, there is a possibility of a measurement bias considering that the echocardiography exams were not performed by the same person.
Despite the possible impact of T3 on cardiac function and the experimental data showing an improvement in cardiac function following a rise in fT3 serum levels, we observed a discrepancy between fT3 and TT3 in their correlation with HF parameters. We may assume that multiple clinical factors in the real-world setting coupled with the complex TH metabolism could have underlined these observations. Another limitation of the present study is its cross-sectional nature, which prevents observing the relationship between cause and effect. Since we only included male HF patients, whether the results can be generalised to female HF patients and other patient populations is questionable.
At the moment, there are no recommendations for the type and dose of the TH substitutional therapy, nor for the monitoring of the therapy effect in patients with NTIS. For example, inconclusive results have been obtained for the efficacy of the T4 + T3 combination as a treatment option in clinical hypothyroidism and for restoring euthyroidism81, 82. This therapy has been suggested by the European Thyroid Association for patients with persistent complaints despite previous substitutional levothyroxine therapy and serum TSH values within the reference range, provided they have received the needed psychological support and have no autoimmune disorders83. As we observed that all investigated TH, except for TSH, were associated with LVEF, there is a possibility that T4 + T3 therapy, through the complex pathways of metabolic effects of TH, may be useful for patients with clinical HF. Additional experimental and clinical studies are needed to explore whether this or some other treatment option may produce clinically meaningful improvement of TT3 associated with an improvement in the clinical course, symptom severity and functional status of HF patients.
Conclusion
There is still a considerable lack of knowledge about the role of TH in the pathophysiology and prognosis of patients with HF. Our study suggests that among TH, serum TT3 level is most closely associated with echocardiographic, laboratory and clinical parameters of the severity of HF in men, independently of other clinical variables and factors, including circulating testosterone levels.
Data availability
The dataset analysed in the present study is available from the corresponding author upon reasonable request.
References
Jessup, M. & Brozena, S. Heart failure. N. Engl. J. Med. 348, 2007–2018 (2003).
Bozkurt, B. et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 23, 352–380 (2021).
Broglio, F. et al. Patients with dilated cardiomyopathy show reduction of the somatotroph responsiveness to GHRH both alone and combined with arginine. Eur. J. Endocrinol. 142, 157–163 (2000).
Kontoleon, P. E. et al. Hormonal profile in patients with congestive heart failure. Int. J. Cardiol. 87, 179–183 (2003).
Jankowska, E. A. et al. Anabolic deficiency in men with chronic heart failure: Prevalence and detrimental impact on survival. Circulation 114, 1829–1837 (2006).
Saccà, L. Heart failure as a multiple hormonal deficiency syndrome. Circ. Heart Fail. 2, 151–156 (2009).
Fliers, E. & Boelen, A. An update on non-thyroidal illness syndrome. J. Endocrinol. Investig. 44, 1597–1607 (2021).
Hamilton, M. A., Stevenson, L. W., Luu, M. & Walden, J. A. Altered thyroid hormone metabolism in advanced heart failure. J. Am. Coll. Cardiol. 16, 91–95 (1990).
Opasich, C. et al. Sick euthyroid syndrome in patients with moderate-to-severe chronic heart failure. Eur. Heart J. 17, 1860–1866 (1996).
Ascheim, D. D. & Hryniewicz, K. Thyroid hormone metabolism in patients with congestive heart failure: The low triiodothyronine state. Thyroid 12, 511–515 (2002).
Wang, B. et al. Non-thyroidal illness syndrome in patients with cardiovascular diseases: A systematic review and meta-analysis. Int. J. Cardiol. 226, 1–10 (2017).
Pingitore, A. et al. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am. J. Med. 118, 132–136 (2005).
Biondi, B. et al. Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 84, 2064–2067 (1999).
Mitchell, J. E. et al. Thyroid function in heart failure and impact on mortality. JACC Heart Fail. 1, 48–55 (2013).
Razvi, S. et al. Thyroid hormones and cardiovascular function and diseases. J. Am. Coll. Cardiol. 71, 1781–1796 (2018).
Lam, C. S. P. et al. Sex differences in heart failure. Eur. Heart J. 40, 3859–3868c (2019).
Čulić, V. & Bušić, Ž. Testosterone may influence left ventricular diastolic function depending on previous myocardial infarction and smoking. Int. J. Cardiol. 186, 67–71 (2015).
Jin, Q. et al. Lower free testosterone level is correlated with left ventricular diastolic dysfunction in asymptomatic middle-aged men with type 2 diabetes mellitus. Int. J. Clin. Pract. 68, 1454–1461 (2014).
Tinetti, M. et al. Left ventricular filling pressure in male patients with type 2 diabetes and normal versus low total testosterone levels. Cardiol. J. 22, 206–211 (2015).
Čulić, V. Androgens in cardiac fibrosis and other cardiovascular mechanisms. Int. J. Cardiol. 179, 190–192 (2015).
Bušić, Ž & Čulić, V. Central and peripheral testosterone effects in men with heart failure: An approach for cardiovascular research. World J. Cardiol. 7, 504 (2015).
Black, S., Kushner, I. & Samols, D. C-reactive protein. J. Biol. Chem. 279, 48487–48490 (2004).
Anker, S. D. Inflammatory mediators in chronic heart failure: An overview. Heart 90, 464–470 (2004).
Čulić, V., Bušić, Ž & Bušić, M. Circulating sex hormones, alcohol consumption and echocardiographic parameters of cardiac function in men with heart failure. Int. J. Cardiol. 224, 245–251 (2016).
Levey, A. S. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 130, 461 (1999).
Cosyns, B., Edvardsen, T., Hristova, K. & Kim, H. K. Left ventricle: systolic function. In The EACVI Textbook of Echocardiography 2nd edn (eds Lancelotti, P. et al.) 131–146 (Oxford University Press, 2016).
Smiseth, O. A., Galderisi, M. & Oh, J. K. Left ventricle: Diastolic function. In The EACVI Textbook of Echocardiography 2nd edn (eds Lancelotti, P. et al.) 147–161 (Oxford University Press, 2016).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 29, 277–314 (2016).
Mebis, L. & Van den Berghe, G. Thyroid axis function and dysfunction in critical illness. Best Pract. Res. Clin. Endocrinol. Metab. 25, 745–757 (2011).
Maldonado, L. S., Murata, G. H., Hershman, J. M. & Braunstein, G. D. Do thyroid function tests independently predict survival in the critically ill?. Thyroid 2, 119–123 (1992).
Kanaan, L. et al. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ. Heart Fail. 11, e005266 (2018).
Zhang, B., Peng, W., Wang, C., Li, W. & Xu, Y. A low fT3 level as a prognostic marker in patients with acute myocardial infarctions. Intern. Med. 51, 3009–3015 (2012).
Iervasi, G. et al. Low-T3 syndrome: A strong prognostic predictor of death in patients with heart disease. Circulation 107, 708–713 (2003).
Visser, W. E., Friesema, E. C. & Visser, T. J. Minireview: Thyroid hormone transporters: The knowns and the unknowns. Mol. Endocrinol. 25, 1–14 (2011).
Bianco, A. C. & Kim, B. W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 116, 2571–2579 (2006).
Boelen, A., Kwakkel, J. & Fliers, E. Beyond low plasma T3: Local thyroid hormone metabolism during inflammation and infection. Endocr. Rev. 32, 670–693 (2011).
Nicoloff, J. T., Fisher, D. A. & Appleman, M. D. Jr. The role of glucocorticoids in the regulation of thyroid function in man. J. Clin. Investig. 49, 1922–1929 (1970).
Schütz, P., Bally, M., Stanga, Z. & Keller, U. Loss of appetite in acutely ill medical inpatients: Physiological response or therapeutic target?. Swiss Med. Wkly. 144, w13957 (2014).
de Vries, E. M., Fliers, E. & Boelen, A. The molecular basis of the non-thyroidal illness syndrome. J. Endocrinol. 225, R67–R81 (2015).
Kwakkel, J., Wiersinga, W. M. & Boelen, A. Differential involvement of nuclear factor-kappaB and activator protein-1 pathways in the interleukin-1beta-mediated decrease of deiodinase type 1 and thyroid hormone receptor beta1 mRNA. J. Endocrinol. 189, 37–44 (2006).
Ikemade, A. et al. Neuroanatomical pathways for thyroid hormone feedback in the human hypothalamus. J. Clin. Endocrinol. Metab. 90, 4322–4334 (2005).
Chan, J. L., Heist, K., Depaoli, A. M., Veldhuis, J. D. & Mantzoros, C. S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 111, 1409–1421 (2003).
Beigneux, A. P., Moser, A. H., Shigenaga, J. K., Grunfeld, C. & Feingold, K. R. Sick euthyroid syndrome is associated with decreased TR expression and DNA binding in mouse liver. Am. J. Physiol. Endocrinol. Metab. 284, E228–E236 (2003).
Selvaraj, S. et al. Association of serum triiodothyronine with B-type natriuretic peptide and severe left ventricular diastolic dysfunction in heart failure with preserved ejection fraction. Am. J. Cardiol. 110, 234–239 (2012).
Pingitore, A. et al. Early activation of an altered thyroid hormone profile in asymptomatic or mildly symptomatic idiopathic left ventricular dysfunction. J. Card. Fail. 2, 520–526 (2006).
Liang, F., Webb, P., Marimuthu, A., Zhang, S. & Gardner, D. G. Triiodothyronine increases brain natriuretic peptide (BNP) gene transcription and amplifies endothelin-dependent BNP gene transcription and hypertrophy in neonatal rat ventricular myocytes. J. Biol. Chem. 278, 15073–15083 (2003).
Cameron, V. A. & Ellmers, L. J. Minireview: Natriuretic peptides during development of the fetal heart and circulation. Endocrinology 144, 2191–2194 (2003).
Man, J., Barnett, P. & Christoffels, V. M. Structure and function of the Nppa-Nppb cluster locus during heart development and disease. Cell Mol. Life Sci. 75, 1435–1444 (2018).
Wang, K. et al. BNP as a new biomarker of cardiac thyroid hormone function. Front. Physiol. 11, 729 (2020).
Lymvaios, I. et al. Thyroid hormone and recovery of cardiac function in patients with acute myocardial infarction: A strong association?. Eur. J. Endocrinol. 165, 107–114 (2011).
Forini, F. et al. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology 155, 4581–4590 (2014).
Matsumoto, S. et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ. Res. 113, 322–326 (2013).
Forini, F. et al. Early long-term L-T3 replacement rescues mitochondria and prevents ischemic cardiac remodelling in rats. J. Cell. Mol. Med. 15, 514–524 (2011).
Pantos, C. et al. Enhanced tolerance of the rat myocardium to ischemia and reperfusion injury early after acute myocardial infarction. Basic Res. Cardiol. 102, 327–333 (2007).
Pantos, C. et al. Thyroid hormone improves postischaemic recovery of function while limiting apoptosis: A new therapeutic approach to support hemodynamics in the setting of ischaemia–reperfusion?. Basic Res. Cardiol. 104, 69–77 (2009).
Kinugawa, K., Jeong, M. Y., Bristow, M. R. & Long, C. S. Thyroid hormone induces cardiac myocyte hypertrophy in a thyroid hormone receptor a1-specific manner that requires TAK1 and p38 mitogen-activated protein kinase. Mol. Endocrinol. 19, 1618–1628 (2005).
van Rooij, E. et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 17, 662–673 (2009).
Wang, W. et al. Free triiodothyronine level correlates with myocardial injury and prognosis in idiopathic dilated cardiomyopathy: Evidence from cardiac MRI and SPECT/PET imaging. Sci. Rep. 6, 39811 (2016).
Jabbar, A. et al. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 14, 39–55 (2017).
Carrillo-Sepúlveda, M. A. et al. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc. Res. 85, 560–570 (2010).
Hasenfuss, G. & Mann, D. L. Pathophysiology of heart failure. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine 11th edn (eds Zipes, D. P. et al.) 442–461 (Elsevier, 2019).
Kozdag, G. et al. Relation between free triiodothyronine/free thyroxine ratio, echocardiographic parameters and mortality in dilated cardiomyopathy. Eur. J. Heart Fail. 7, 113–118 (2005).
Pingitore, A. et al. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: A randomized, placebo-controlled study. J. Clin. Endocrinol. Metab. 93, 1351–1358 (2008).
Moruzzi, P., Doria, E., Agostoni, P. G., Capacchione, V. & Sganzerla, P. Usefulness of l-thyroxine to improve cardiac and exercise performance in idiopathic dilated cardiomyopathy. Am. J. Cardiol. 73, 374–378 (1994).
Moruzzi, P., Doria, E. & Agostoni, P. G. Medium-term effectiveness of l-thyroxine treatment in idiopathic dilated cardiomyopathy. Am. J. Med. 101, 461–467 (1996).
Badran, H. M., Faheem, N., Zidan, A., Yacoub, M. & Soltan, G. Effect of short-term l-thyroxine therapy on left ventricular mechanics in idiopathic dilated cardiomyopathy. J. Am. Soc. Echocardiogr. 33, 1234–1244 (2020).
Sandler, B. et al. Thyroxine-thyroid hormone receptor interactions. J. Biol. Chem. 279, 55801–55808 (2004).
Sabatino, L. et al. Is the low tri-iodothyronine state a crucial factor in determining the outcome of coronary artery bypass patients? Evidence from a clinical pilot study. J. Endocrinol. 175, 577–586 (2002).
Ripoli, A. et al. Does subclinical hypothyroidism affect cardiac pump performance?. J. Am. Coll. Cardiol. 45, 439–445 (2005).
Larsen, P. R. Thyroid-pituitary interaction: Feedback regulation of thyrotropin secretion by thyroid hormones. N. Engl. J. Med. 306, 23–32 (1982).
Yeo, Y., Park, S. W., Lee, S. C. & Song, Y. M. The relationship between serum sex hormone and cardiac echocardiographic findings in healthy men. Sci. Rep. 12, 12787 (2022).
Čulić, V. & Bušić, Ž. The severity of acute heart failure in men according to diabetes mellitus: The role of testosterone and renal dysfunction. Int. J. Cardiol. 168, 5039–5041 (2013).
Čulić, V. & Bušić, Ž. Testosterone levels and heart failure in obese and non-obese men. Int. J. Cardiol. 176, 1163–1166 (2014).
Roger, V. L. et al. Trends in heart failure incidence and survival in a community-based population. JAMA 292, 344–350 (2004).
Laonigro, I., Correale, M., Di Biase, M. & Altomare, E. Alcohol abuse and heart failure. Eur. J. Heart Fail. 11, 453–462 (2009).
George, A. & Figueredo, V. M. Alcoholic cardiomyopathy: A review. J. Card. Fail. 17, 844–849 (2011).
Gonçalves, A. et al. Alcohol consumption and risk of heart failure: The atherosclerosis risk in communities study. Eur. Heart J. 36, 939–945 (2015).
Dorans, K. S. et al. Alcohol and incident heart failure among middle-aged and elderly men: Cohort of Swedish men. Circ. Heart Fail. 8, 422–427 (2015).
Gémes, K. et al. Light-to-moderate drinking and incident heart failure—The Norwegian HUNT study. Int. J. Cardiol. 203, 553–560 (2016).
Larsson, S. C., Wallin, A. & Wolk, A. Contrasting association between alcohol consumption and risk of myocardial infarction and heart failure: Two prospective cohorts. Int. J. Cardiol. 231, 207–210 (2017).
Wiersinga, W. M. T4+T3 combination therapy: An unsolved problem of increasing magnitude and complexity. Endocrinol. Metab. (Seoul) 36, 938–951 (2021).
Grozinsky-Glasberg, S., Fraser, A., Nahshoni, E., Weizman, A. & Leibovici, L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: Meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 91, 2592–2599 (2006).
Wiersinga, W. M., Duntas, L., Fadeyev, V., Nygaard, B. & Vanderpump, M. P. ETA guidelines: The use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur. Thyroid J. 1, 55–71 (2012).
Author information
Authors and Affiliations
Contributions
V.Č. was responsible for the study conception and design. I.T., I.V. and Ž.B. participated in the data acquisition. I.T. and V.Č. analysed and interpreted the data and wrote the manuscript. All authors contributed to the intellectual value of the study and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Turić, I., Velat, I., Bušić, Ž. et al. Circulating thyroid hormones and clinical parameters of heart failure in men. Sci Rep 13, 20319 (2023). https://doi.org/10.1038/s41598-023-47391-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47391-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.