Abstract
If the brain structure is assessed at neonatal intensive care units, covert clinical events related with subtle brain injury might be identified. The reduced scattering coefficient of near-infrared light (μS’) obtained using time-resolved near-infrared spectroscopy from the forehead of infants is associated with gestational age, body weight and Apgar scores, presumably reflecting subtle changes of the brain related to foetal growth and birth transition. One hundred twenty-eight preterm and term infants were studied to test whether μS’ obtained from the head at term-equivalent age is associated with foetal growth, birth transition and nutritional status after birth, which are key independent variables of developmental outcomes. As potential independent variables of μS’, birth weight, Apgar scores, age at full enteral feeding and post-conceptional age at the study were assessed to represent foetal growth, birth transition and nutritional status after birth. Subsequently, higher μS’ values were associated with higher Apgar scores (p = 0.003) and earlier establishment of enteral feeding (p < 0.001). The scattering property of near-infrared light within the neonatal brain might reflect changes associated with birth transition and nutritional status thereafter, which might be used as a non-invasive biomarker to identify covert independent variables of brain injury in preterm infants.
Similar content being viewed by others
Introduction
Advances in neonatal intensive care have significantly improved the survival rate of preterm infants1,2. However, a considerable fraction of extremely preterm infants develop cognitive impairments even in the absence of major cerebral lesions, such as intracranial haemorrhage and periventricular leukomalacia3,4. Magnetic resonance imaging (MRI) studies in preterm infants have demonstrated the relationship between subtle brain lesions at term equivalent age and long-term cognitive impairments5,6,7. However, because of the cost, time and safety associated with the scan, MRI is usually performed only once before discharge from the hospital, causing difficulty in identification of the upstream events associated with subtle brain lesions. Reliable tools for the assessment of subtle change of the brain structure, which can be assessed before and after clinical events at the cot-side, may help distinguish the upstream events responsible for subtle cerebral lesions and cognitive impairments in preterm infants.
Near-infrared spectroscopy (NIRS) is a handy, non-invasive tool, which has been used to analyse the tissue oxygen metabolism in the brains of newborn infants8,9,10,11. Near-infrared light penetrates the intact scalp, skull and cerebral tissue more efficiently than visible light, and is mainly absorbed by blood haemoglobin, the level of which depends on the binding of haemoglobin to oxygen12. Thus, fractions of oxygenated and deoxygenated haemoglobin are calculated using light absorption coefficient (μa) obtained from the near-infrared light of different wavelengths13. Time-resolved near-infrared spectroscopy (TR-NIRS) is a relatively new technique, which enables simultaneous quantification of μa and reduced scattering coefficient (μS’)14,15. Unlike μa predominantly provides information regarding tissue oxygenation, μS’ is an index of light scattering, which is theoretically determined by the structural complexity of tissue13. When preterm infants were studied shortly after birth, μS’ values obtained from the forehead showed a positive linear correlation with gestational age16. Our study in preterm and term infants further confirmed that μS’ values obtained shortly after birth were associated with variables, such as antenatal glucocorticoid, emergency delivery, gestational age, body size, Apgar scores, requirement for mechanical ventilation and blood gas data at birth, suggesting the possibility that μS’ might reflect subtle structural changes in the brain associated with antenatal growth, peripartum stress and birth transition17. However, little is known regarding the relationship between μS’ values obtained from the head of newborn infants and their downstream clinical outcomes.
The aim of this study was to test the association of μS’ measured at term-equivalent period with intrauterine growth, birth transition and nutrition after birth, which are short-term surrogate markers for neurodevelopmental outcomes of hospitalised newborn infants18,19,20,21.
Results
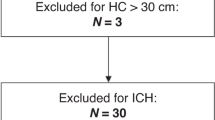
Four infants, who developed grade III/IV intraventricular haemorrhage, and one infant, who developed hypoxic-ischaemic encephalopathy, were excluded, leaving 128 infants within the final study cohort (Fig. 1). These infants had a gestation period of 32.0 ± 4.2 weeks and weighed 1564 ± 688 g at birth, and were studied on 44.8 ± 28.3 days of age or 38.6 ± 2.1 weeks post-conceptional age (Table 1).
For the left and right temporal regions and the posterior region, data were not obtained for 8, 8 and 21 infants, respectively, because of insufficient signals from the head (n = 15), poor probe contact (n = 4) and the use of a cap device for non-invasive respiratory support (n = 2). No further data were excluded because of their poor quality or reproducibility. The mean μa and μS’ values for all wavelengths and head positions were 0.126 ± 0.025 cm−1 and 6.453 ± 1.416 cm−1, respectively.
Dependence of μa and μS’ on wavelengths and head positions
The wavelength of 836 nm was associated with higher μa values, whereas the wavelength of 791 nm was associated with lower μa values compared to those of 761 nm (both p < 0.001) (Table 2). The right temporal and posterior regions of the head were associated with higher μa values compared to those of the anterior region (both p < 0.001).
The wavelengths of 791 and 836 nm were associated with lower μS’ values compared to those of 761 nm (both p < 0.001). The left and right temporal and posterior regions of the head were associated with higher μS’ values compared to the anterior region (all p < 0.001).
Dependence of μa and μS’ on clinical variables: univariate analysis
The higher μa values were positively associated with gestational age (p = 0.001), body weight at birth (p < 0.001), blood haemoglobin level at study (p < 0.001) and μS’ values (p < 0.001), and negatively associated with antenatal glucocorticoid (p < 0.001), cord blood pH (p = 0.003) and postnatal age at study (p = 0.001); relationships with multiple pregnancy (p = 0.016), head circumference at birth (p = 0.005) and body weight at study (p = 0.036) were lost after correction for multiple comparisons (all adjusted for the wavelengths and head positions; Table 2 and Fig. 2).
Dependence of μS’ on µa in four head regions. Scatter plots demonstrating relationships between µa and μS’ obtained from the anterior (A), left-temporal (B), right-temporal (C) and posterior (D) head regions for wavelengths of 761 nm (circle), 791 nm (triangle) and 836 nm (cross). µa, absorption coeffieicnt. μS’, reduced scattering coefficient.
The μS’ level was positively associated with gestational age (p < 0.001) and μa values (p < 0.001), and negatively associated with indomethacin for patent ductus arteriosus (p < 0.001) and postnatal age to achieve full enteral feeding (p < 0.001); relationships with antenatal glucocorticoid (p = 0.013), body weight and head circumference at birth (both p = 0.012), Apgar scores at 1 and 5 min (p = 0.039 and 0.029, respectively) and postnatal age at study (p = 0.003) were lost after correction for multiple comparisons (all adjusted for the wavelengths and head positions; Table 2). See Online Supplemental Tables S1–S3 for findings from analyses performed for each wavelength.
Dependence of μS’ and μa on clinical variables: multivariate analysis
Higher μa values were associated with greater age to achieve full enteral feeding (p = 0.049), greater post-conceptional age at study (p = 0.015), higher blood haemoglobin levels at study (p < 0.001) and higher μS’ values (p < 0.001) (Table 3). Higher μS’ values were associated with higher Apgar scores at 5 min (p = 0.003), smaller age to achieve full enteral feeding (p < 0.001) and higher μa values (p < 0.001). See Online Supplemental Tables S4–S6 for findings from analyses performed for each wavelength.
Discussion
Building on previous studies of TR-NIRS, which suggested that the light scattering within the brain shortly after birth is dependent on variables related to foetal growth, antenatal stress and birth transition, we have demonstrated that higher μS’ values obtained at term-equivalent age were associated with higher Apgar scores and earlier establishment of enteral nutrition. μS’ can be a unique and clinically useful biomarker of subtle changes in the brains of newborn infants with respect to antenatal stress, birth transition and nutritional status after birth.
Light scattering within a tissue theoretically increases with relatively more complex microstructures due to increased reflection and path length of near-infrared light14. Thus μS’ has a potential to provide microstructural information of the brain. Ijichi and colleagues first reported that μS’ values of near-infrared light obtained shortly after birth from the foreheads of newborn infants with a gestation age of 30–41 weeks depended on gestational age16. Our previous study confirmed that μS’ values obtained from the foreheads of preterm and term infants assessed shortly after birth were dependent on body size and Apgar scores, as well as on gestational age. These findings suggest the possible utility of μS’ values as a non-invasive marker to evaluate subtle differences in the brain subsequent to foetal maturation, antenatal stress and birth transition17. Our current study further verified that the μS’ value obtained at term equivalent period is associated with both clinical variables at birth and those related to the nutritional status of the infant after birth. Intrauterine growth and maturation, intrapartum stress and response and postpartum nutrition and growth constitute key independent variables of the neurodevelopmental outcomes of the infant18,19,20,21. If the consequence of the intrinsic maturity, extrinsic stress, birth transition and nutritional status of the infant can be assessed using μS’ values obtained from the heads of newborn infants, along with other substantiations, μS’ might serve as a clinically useful biomarker of cerebral maturation and damage. Future studies need to address the contribution of other potential independent variables of light scattering as measured from the scalp, such as the gyration of the brain and developmental changes in the layer of cerebrospinal fluid.
With regard to the absorption of near-infrared light, only modest relationships were observed between higher μa values and longer time to achieve full enteral feeding and greater post-conceptional age at the time of the study; robust correlations were only observed between μa values and priori covariates of the wavelengths of light, head position and blood haemoglobin concentration at the time of the study. Given that absorption of near-infrared light within the range of 750–850 nm is primarily determined by the tissue haemoglobin concentrations14,15, μa values might reflect the maturation of the cerebral tissue via increased complexity of the cerebral vessels and subsequent blood volume. Progression of anaemia and increase in the cerebral blood flow and volume with increasing postnatal age might also affect the dependence of μa values on clinical variables12.
Strengths and limitations
We were able to elucidate the clinical variables potentially determining the property of light absorption and scattering within the brain in a relatively large cohort of newborn infants. However, we were unable to present a direct association between μS’ values and microstructure of the brain. As described in the previous section, the observed relationships between μS’, μa and clinical variables can be affected by a range of clinical biases. For example, extremely preterm infants are relatively anaemic at birth and the anaemia progresses with postnatal age without transfusion, potentially leading to lower blood haemoglobin and μa levels with greater gestational age at birth and greater postnatal age at the time of TR-NIRS study. Although we carefully selected independent variables and covariates to minimise the bias, the findings might still be affected by the bias derived from the collinearity between the variables. Our study cohort comprised newborn infants, who were hospitalised at a tertiary neonatal intensive care unit. Although the observed μa and μS’ values were comparable to those reported in healthy newborn infants22, extrapolation of our findings into physiological transition and growth in healthy newborn infants must be done cautiously. Finally, the longitudinal follow-up study of the study population is still underway, resulting in the lack of outcome information in association with the light absorption and scattering properties.
Conclusions
The μS’ values of the near-infrared light obtained at term-equivalent period from the heads of newborn infants were associated with Apgar scores and postnatal age when full enteral feeding was achieved, suggesting a correlation between the light scattering property and stress-response at birth and nutritional status of the infant thereafter. With further validations, μS’ might serve as a biomarker to distinguish the variation of the microstructural complexity of the brain tissue subsequent to different maturational stage, antenatal stress, tissue damage and repair, nutritional status and growth. Associations between the μS’ values and detailed clinical courses, macro- and microstructural MRI findings and neuro-developmental outcomes need to be addressed to assess the clinical utility of this non-invasive cot-side tool.
Materials and methods
This study was conducted in compliance with the Declaration of Helsinki under the approval of the Ethics Committee of Kurume University School of Medicine (reference number: 12128). Informed parental consent was obtained for each participating newborn infant. All methods were carried out in accordance with relevant guidelines and regulations.
Study population
This study was performed as a secondary analysis of a prospective longitudinal study, which was performed between June 2009 and January 2015 to serially collected the TR-NIRS data of preterm and term infants hospitalised at a tertiary neonatal intensive care centre of Kurume University Hospital (Kurume, Fukuoka, Japan). Independent variables of μS’ values obtained shortly after birth from a part (n = 60) of the current cohort have been reported in a previous study17. Of 136 newborn infants within the original study cohort, 132 infants, who had TR-NIRS data obtained between 34 and 42 weeks postconceptional age, were considered. Infants with chromosomal aberration, malformation syndrome, grade III/IV intraventricular haemorrhage, hypoxic-ischaemic encephalopathy, congenital hydrocephalus and other major cerebral anomalies were excluded.
Data collection
The μa and μS’ values were obtained from the heads of the infants for three wavelengths, 761, 791 and 836 nm, using a TR-NIRS system (TRS-10, Hamamatsu Photonics K.K., Hamamatsu, Shizuoka, Japan)17. This system employs the time-correlated single photon counting method to create time response profiles of pulsed laser light penetrating an object. The time response profiles were then fitted into a photon diffusion equation using the nonlinear least square fitting method to obtain μa and μS’ for each wavelength14. Although the data acquisition for the original study was repeated with intervals of approximately 1 week from birth to discharge, for the current study, a particular value obtained between 34 and 42 weeks of post-conceptional age (closest to 40 weeks gestation if there were multiple records) was used to represent each infant.
Data were acquired when the infant was clinically stable and asleep or calmly awake. The TR-NIRS probes were inserted into a rubber holder, with an inter-optode distance of 3 cm, and was applied to a relatively flat part of the head. Data acquisition (10 s) was repeated five times for each of the frontal, left and right temporo-parietal and occipital regions by repositioning the probe each time. In our previous study, which acquired TR-NIRS data using the same protocol to the current one17, standard deviations of μa and μS’ values for five successionally obtained data within the same head position and infant were, in average, 2.4% and 2.7%, respectively. Based on these small intra-individual and intra-regional differences in μa and μS’ values, five readings each of μa and μS’ were averaged for each brain region. We confirmed the degree of fit to the photon diffusion equation using the conversion chi-square value index of between 0.8 and 1.223. Data were not collected for brain regions with poor probe contact (typically due to the lack of flat surfaces or use of cap devices for non-invasive respiratory support), poor fit to the photon diffusion equation or insufficient signal-to-noise ratio with the count rate < 100 K counts/s or relative dark- to peak-count ratio of > 0.1. The data were retrospectively assessed to identify those with poor quality or intra-regional reproducibility before being processed for further analysis.
Clinical information
The clinical background information was obtained from the electronic records of the patients, including (1) maternal and antenatal variables (antenatal glucocorticoid, multiple pregnancies and emergency caesarean delivery), (2) variables at and shortly after birth (sex, cord blood pH, Apgar scores at 1 min and 5 min, gestational age, body weight, head circumference, hypoglycaemia within 48 h of birth, indomethacin for the treatment of the patent ductus arteriosus, grade I/II intraventricular haemorrhage and periventricular leukomalacia, (3) variables associated with clinical variables of infants after the transitional period (body weight on the day of study, age when full enteral feeding of > 100 ml/kg/d was achieved and chronic lung disease assessed 36 weeks post-conceptional age or on day 28, whichever was later). In order to assess the influence of intrauterine growth on μS’ values, body weight and head circumference at birth were expressed as z-scores in accordance with the New Japanese Neonatal Anthropometric Charts for Gestational Age at Birth24.
Data analysis
To minimise biases owing to missing data, multiple imputation of the missing values of less than 10% (excluding for μa and μS’) was performed (n = 5 imputations), based on the correlation between variables with missing values and other characteristics of the participants (SPSS ver. 22.0, IBM, Armonk, NY, U.S.A.). Although the property of μa was out of our study scope, independent variables of both μa and μS’ were assessed to clarify the possible influence of light absorption to the relationship between μS’ values and clinical variables. The generalised estimating equation with a linear model was used to account for repeated sampling of TR-NIRS data for three near-infrared light wavelengths and four head regions. Although the influence of the wavelength is much greater on μa than on μS’16,17, the three wavelengths were incorporated within the model for consistency in the analytical procedure. Crude effects of clinical variables on μa and μS’ values were assessed using the univariate model adjusting for the wavelengths and head positions. p values < 0.002 were assumed to be significant, correcting multiple comparisons of 25 variables. The final models to explain μa and μS’ values were developed based on our hypothesis, which employed the body weight at birth, Apgar scores at 5 min, age to achieve full enteral feeding and post-conceptional age at study; the model was also adjusted for priori covariates, which were known independent variables of clinical outcomes (antenatal glucocorticoid, multiple pregnancies and sex), μa (wavelength, position of the head and μS’) and μS’ (wavelength, position of the head and μa). Data were presented as mean ± standard deviation unless specified otherwise.
References
Kono Y, Yonemoto N, Nakanishi H, Kusuda S, Fujimura M (2018) Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks' gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatr Open 2(1):211.
Norman, M. et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004–2007 and 2014–2016. JAMA 321(12), 1188–1199 (2019).
Iwata, S. et al. Subtle white matter injury is common in term-born infants with a wide range of risks. Int. J. Dev. Neurosci. 28(7), 573–580 (2010).
Volpe, J. J. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J. Pediatr. 153(2), 160–163 (2008).
Iwata S, Iwata O, Matsuishi T. Sleep patterns of Japanese preschool children and their parents: implications for co-sleeping. Acta Paediatr. 2013.
Woodward, L. J., Anderson, P. J., Austin, N. C., Howard, K. & Inder, T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 355(7), 685–694 (2006).
Anderson, P. J. et al. Associations of newborn brain magnetic resonance imaging with long-term neurodevelopmental impairments in very preterm children. J. Pediatr. 187, 58–65 (2017).
Meek, J. H., Tyszczuk, L., Elwell, C. E. & Wyatt, J. S. Cerebral blood flow increases over the first three days of life in extremely preterm neonates. Arch. Dis. Child Fetal Neonatal Ed. 78(1), F33–F37 (1998).
Kusaka, T. et al. Noninvasive optical imaging in the visual cortex in young infants. Hum. Brain Mapp. 22(2), 122–132 (2004).
Iwata, S. et al. Dual role of cerebral blood flow in regional brain temperature control in the healthy newborn infant. Int. J. Dev. Neurosci. 37, 1–7 (2014).
Uchida-Ota, M. et al. Maternal speech shapes the cerebral frontotemporal network in neonates: A hemodynamic functional connectivity study. Dev. Cogn. Neurosci. 39, 100701 (2019).
Mintzer, J. P. & Moore, J. E. Regional tissue oxygenation monitoring in the neonatal intensive care unit: Evidence for clinical strategies and future directions. Pediatr. Res. 86(3), 296–304 (2019).
Kusaka, T. et al. Evaluation of cerebral circulation and oxygen metabolism in infants using near-infrared light. Brain Dev. 36(4), 277–283 (2014).
Ohmae, E. et al. Clinical evaluation of time-resolved spectroscopy by measuring cerebral hemodynamics during cardiopulmonary bypass surgery. J. Biomed. Opt. 12(6), 062112 (2007).
Dunne, L., Hebden, J. & Tachtsidis, I. Development of a near infrared multi-wavelength, multi-channel, time-resolved spectrometer for measuring brain tissue haemodynamics and metabolism. Adv. Exp. Med. Biol. 812, 181–186 (2014).
Ijichi, S. et al. Developmental changes of optical properties in neonates determined by near-infrared time-resolved spectroscopy. Pediatr. Res. 58(3), 568–573 (2005).
Kurata, T. et al. Physiological and pathological clinical conditions and light scattering in brain. Sci. Rep. 6, 31354 (2016).
Kato, T. et al. Extremely preterm infants small for gestational age are at risk for motor impairment at 3 years corrected age. Brain Dev. 38(2), 188–195 (2016).
Shankaran, S. et al. Outcome of extremely-low-birth-weight infants at highest risk: Gestational age < or =24 weeks, birth weight < or =750 g, and 1-minute Apgar < or =3. Am. J. Obstet. Gynecol. 191(4), 1084–1091 (2004).
Ehrenkranz, R. A. et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117(4), 1253–1261 (2006).
Franz, A. R. et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 123(1), e101–e109 (2009).
Andresen, B. et al. Cerebral oxygenation and blood flow in normal term infants at rest measured by a hybrid near-infrared device (BabyLux). Pediatr. Res. 86(4), 515–521 (2019).
Grinvald, A. & Steinberg, I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal. Biochem. 59(2), 583–598 (1974).
Itabashi, K., Miura, F., Uehara, R. & Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 56(5), 702–708 (2014).
Acknowledgements
The authors thank the newborn infants and their families for their participation and cooperation with the study, and Ms. Chiho Yoshii and Chiaki Ueno for their consistent support. This work was supported by the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research 20H00102, 16K09005, 18K07795 and 18K15722).
Author information
Authors and Affiliations
Contributions
O.I., S.I., T.K., M.O. and K.T. designed the study. S.I., T.K., K.T., K.K., S.T., M.K., M.S. and Y.A. participated in the patient recruitment and data collection. S.I., YC.L. and Y.A. performed the statistical analyses. O.I., S.I., T.K., K.T., E.O. and S.S. contributed to the interpretation of the findings. O.I. and S.I. drafted the manuscript, which was critically reviewed by T.K., K.T., K.K., M.K., Y.C.L., M.S., Y.A., S.T., E.O. and S.S. O.I., S.I., T.K., K.T., K.K., M.K., YC.L., M.S., Y.A., S.T., E.O. and S.S. approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iwata, O., Iwata, S., Kurata, T. et al. Foetal growth, birth transition, enteral nutrition and brain light scattering. Sci Rep 11, 21318 (2021). https://doi.org/10.1038/s41598-021-00624-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00624-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.