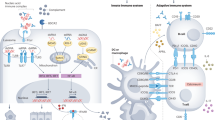
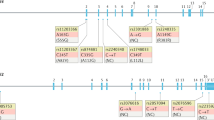
Abstract
Relapsing polychondritis is a rare inflammatory disease characterized by recurrent inflammation of cartilaginous structures, mainly of the ears, nose and respiratory tract, with a broad spectrum of accompanying systemic features. Despite its rarity, prompt recognition and accurate diagnosis of relapsing polychondritis is crucial for appropriate management and optimal outcomes. Our understanding of relapsing polychondritis has changed markedly in the past couple of years with the identification of three distinct patient clusters that have different clinical manifestations and prognostic outcomes. With the progress of pangenomic sequencing and the discovery of new somatic and monogenic autoinflammatory diseases, new differential diagnoses have emerged, notably the vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome, autoinflammatory diseases and immune checkpoint inhibitor-related adverse events. In this Review, we present a detailed update of the newly identified clusters and highlight red flags that should raise suspicion of these alternative diagnoses. The identification of these different clusters and mimickers has a direct impact on the management, follow-up and prognosis of patients with relapsing polychondritis and autoinflammatory syndromes.
Key points
-
Studies in the past few years have allowed improved clustering of relapsing polychondritis phenotypes, with relevant clinical and prognostic implications.
-
Somatic mutations in UBA1 have been linked to the vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic syndrome, as well as to a specific relapsing polychondritis phenotype.
-
Screening for UBA1 mutations is indicated in patients with a compatible clinical presentation, regardless of age and sex.
-
In patients with cancer, immune-checkpoint inhibitors can induce relapsing polychondritis features and require specific management.
-
Other autoinflammatory conditions can mimic relapsing polychondritis, thus warranting consideration of genetic testing, particularly in paediatric-onset cases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Cheng, L. et al. Bibliometric analysis of the global publication activity in the field of relapsing polychondritis during 1960–2023. Clin. Rheumatol. 42, 3201–3212 (2023).
Mathew, S. D., Battafarano, D. F. & Morris, M. J. Relapsing polychondritis in the Department of Defense population and review of the literature. Semin. Arthritis Rheum. 42, 70–83 (2012).
Hazra, N., Dregan, A., Charlton, J., Gulliford, M. C. & D’Cruz, D. P. Incidence and mortality of relapsing polychondritis in the UK: a population-based cohort study. Rheumatology 54, 2181–2187 (2015).
Horváth, A. et al. A nationwide study of the epidemiology of relapsing polychondritis. Clin. Epidemiol. 8, 211–230 (2016).
Belot, A. et al. Pediatric-onset relapsing polychondritis: case series and systematic review. J. Pediatr. 156, 484–489 (2010).
Alqanatish, J. T. & Alshanwani, J. R. Relapsing polychondritis in children: a review. Mod. Rheumatol. 30, 788–798 (2020).
Dion, J. et al. Relapsing polychondritis can be characterized by three different clinical phenotypes: analysis of a recent series of 142 patients. Arthritis Rheumatol. 68, 2992–3001 (2016).
Trentham, D. E. & Le, C. H. Relapsing polychondritis. Ann. Intern. Med. 129, 114–122 (1998).
Arnaud, L., Mathian, A., Haroche, J., Gorochov, G. & Amoura, Z. Pathogenesis of relapsing polychondritis: a 2013 update. Autoimmun. Rev. 13, 90–95 (2014).
Taneja, V., Griffiths, M., Behrens, M., Luthra, H. S. & David, C. S. Auricular chondritis in NOD.DQ8.Aβo (Ag7−/−) transgenic mice resembles human relapsing polychondritis. J. Clin. Invest. 112, 1843–1850 (2003).
Lang, B. et al. Susceptibility to relapsing polychondritis is associated with HLA-DR4. Arthritis Rheum. 36, 660–664 (1993).
Luo, Y. et al. Ultra-rare genetic variation in relapsing polychondritis: a whole-exome sequencing study. Ann. Rheum. Dis. 83, 253–260 (2024).
Buckner, J. H., Van Landeghen, M., Kwok, W. W. & Tsarknaridis, L. Identification of type II collagen peptide 261–273-specific T cell clones in a patient with relapsing polychondritis. Arthritis Rheum. 46, 238–244 (2002).
Serratrice, J. et al. Severe relapsing polychondritis occurring after ear piercing. J. Rheumatol. 30, 2716–2717 (2003).
Ogimoto, T., Yoshida, H., Mizuta, M. & Hirai, T. Relapsing polychondritis after treatment with PD-1 blockade. Invest. New Drugs 40, 389–391 (2022).
Rednic, S. et al. Relapsing polychondritis: state of the art on clinical practice guidelines. RMD Open 4, e000788 (2018).
Vitale, A. et al. Relapsing polychondritis: an update on pathogenesis, clinical features, diagnostic tools, and therapeutic perspectives. Curr. Rheumatol. Rep. 18, 3 (2016).
Arnaud, L. et al. The relapsing polychondritis disease activity index: development of a disease activity score for relapsing polychondritis. Autoimmun. Rev. 12, 204–209 (2012).
Arnaud, L. et al. French practical guidelines for the diagnosis and management of relapsing polychondritis. Rev. Med. Interne 44, 282–294 (2023).
Mathian, A. et al. Relapsing polychondritis: a 2016 update on clinical features, diagnostic tools, treatment and biological drug use. Best. Pract. Res. Clin. Rheumatol. 30, 316–333 (2016).
Shimizu, J., Yamano, Y., Kawahata, K. & Suzuki, N. Elucidation of predictors of disease progression in patients with relapsing polychondritis at the onset: potential impact on patient monitoring. BMC Rheumatol. 4, 41 (2020).
Dubey, S. et al. Respiratory subtype of relapsing polychondritis frequently presents as difficult asthma: a descriptive study of respiratory involvement in relapsing polychondritis with 13 patients from a single UK centre. ERJ Open Res. 7, 00170–02020 (2021).
Wang, D., Guan, L., Dong, X., Zhu, X. & Tong, Z. Comparison of relapsing polychondritis patients with and without respiratory involvement based on chest computed tomography: a retrospective cohort study. BMC Pulm. Med. 22, 222 (2022).
Jalaber, C. et al. Differentiating tracheobronchial involvement in granulomatosis with polyangiitis and relapsing polychondritis on chest CT: a cohort study. Arthritis Res. Ther. 24, 241 (2022).
Damian, L. et al. Rare within rare. Necrotising scleritis and peripheral ulcerative keratitis: eye-threatening complications of relapsing polychondritis. Clin. Exp. Rheumatol. 40, 86–92 (2022).
Gallagher, K., Al-Janabi, A. & Wang, A. The ocular manifestations of relapsing polychondritis. Int. Ophthalmol. 43, 2633–2641 (2023).
Ferrada, M. et al. Defining clinical subgroups in relapsing polychondritis: a prospective observational cohort study. Arthritis Rheumatol. 72, 1396–1402 (2020).
Malik, M. U. et al. Spectrum of immune-mediated inner ear disease and cochlear implant results. Laryngoscope 122, 2557–2562 (2012).
Shimizu, J., Oka, H., Yamano, Y., Yudoh, K. & Suzuki, N. Cardiac involvement in relapsing polychondritis in Japan. Rheumatology 55, 583–584 (2016).
Yin, R. et al. Relapsing polychondritis: focus on cardiac involvement. Front. Immunol. 14, 1218475 (2023).
D’Cruz, D. P. & Ferrada, M. A. Relapsing polychondritis and large-vessel vasculitis. J. Rheumatol. 47, 1732–1733 (2020).
Le Besnerais, M. et al. Aortic involvement in relapsing polychondritis. Joint Bone Spine 85, 345–351 (2018).
Stone, J. R. et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc. Pathol. 24, 267–278 (2015).
Francès, C. et al. Dermatologic manifestations of relapsing polychondritis. A study of 200 cases at a single center. Medicine 80, 173–179 (2001).
Chen, K.-R. Cutaneous vasculitis in autoinflammatory diseases. J. Dermatol. 51, 150–159 (2023).
Luo, Y. et al. A prospective observational cohort study and systematic review of 40 patients with mouth and genital ulcers with inflamed cartilage (MAGIC) syndrome. Semin. Arthritis Rheum. 52, 151924 (2022).
Yokota, K., Tachibana, H., Miyake, A., Yamamoto, T. & Mimura, T. Relapsing polychondritis and aseptic meningoencephalitis. Intern. Med. 62, 481–486 (2023).
Michalaki, V. et al. Limbic encephalitis as a late complication of relapsing polychondritis: a case report and review of the literature. Mediterr. J. Rheumatol. 34, 229–237 (2023).
Husein, S., Murayama, Y., Koo, A., Wakefield, M. & Buccoliero, R. Relapsing polychondritis presenting with sero-negative limbic encephalitis. Clin. Med. 23, 618–620 (2023).
Cao, X. et al. Comparison of relapsing polychondritis patients with and without central nervous system involvement: a retrospective study of 181 patients. Int. J. Immunopathol. Pharmacol. 35, 20587384211000547 (2021).
Isaak, B. L., Liesegang, T. J. & Michet, C. J. Ocular and systemic findings in relapsing polychondritis. Ophthalmology 93, 681–689 (1986).
Ferrada, M. A. et al. Somatic mutations in UBA1 define a distinct subset of relapsing polychondritis patients with VEXAS. Arthritis Rheumatol. 73, 1886–1895 (2021).
Khitri, M.-Y. et al. Comparison between idiopathic and VEXAS-relapsing polychondritis: analysis of a French case series of 95 patients. RMD Open 8, e002255 (2022).
Ferrada, M. A. et al. Translation of cytoplasmic UBA1 contributes to VEXAS syndrome pathogenesis. Blood 140, 1496–1506 (2022).
Beck, D. B. et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 383, 2628–2638 (2020).
Mascaro, J. M. et al. Spanish cohort of VEXAS syndrome: clinical manifestations, outcome of treatments and novel evidences about UBA1 mosaicism. Ann. Rheum. Dis. 82, 1594–1605 (2023).
Beck, D. B. et al. Estimated prevalence and clinical manifestations of UBA1 variants associated with VEXAS syndrome in a clinical population. J. Am. Med. Assoc. 329, 318 (2023).
Beck, D. B., Werner, A., Kastner, D. L. & Aksentijevich, I. Disorders of ubiquitylation: unchained inflammation. Nat. Rev. Rheumatol. 18, 435–447 (2022).
Kosmider, O. et al. VEXAS syndrome is characterized by inflammasome activation and monocyte dysregulation. Nat. Commun.15, 910 (2024).
Barba, T. et al. VEXAS syndrome in a woman. Rheumatology 60, e402–e403 (2021).
Stubbins, R. J. et al. VEXAS syndrome in a female patient with constitutional 45, X (Turner syndrome). Haematologica 107, 1011 (2022).
McHugh, J. Pathogenic UBA1 variants define a subset of relapsing polychondritis. Nat. Rev. Rheumatol. 17, 312 (2021).
Tsuchida, N. et al. Pathogenic UBA1 variants associated with VEXAS syndrome in Japanese patients with relapsing polychondritis. Ann. Rheum. Dis. 80, 1057–1061 (2021).
Sánchez-Hernández, B. E., Calderón-Espinoza, I. & Martín-Nares, E. Challenging the paradigm: a case of early-onset VEXAS syndrome. Rheumatology 63, e99–e100 (2023).
Duan, S. et al. Dynamic monitoring of UBA1 somatic mutations in patients with relapsing polychondritis. Orphanet J. Rare Dis. 19, 1 (2024).
Huang, Q., Cui, D., Chen, J., Ren, H. & Yang, M. Intermittent fever and cough in a 56-year-old patient: relapsing polychondritis and extranodal NK/T-cell lymphoma. Rheumatol. Immunol. Res. 4, 40–43 (2023).
Chang-Miller, A. et al. Renal involvement in relapsing polychondritis. Medicine 66, 202–217 (1987).
Espinoza, L. R. et al. Immune complex-mediated renal involvement in relapsing polychondritis. Am. J. Med. 71, 181–183 (1981).
Papo, T. et al. Antineutrophil cytoplasmic antibodies in polychondritis. Ann. Rheum. Dis. 52, 384–385 (1993).
Wolchok, J. Putting the immunologic brakes on cancer. Cell 175, 1452–1454 (2018).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Hirsch, L., Zitvogel, L., Eggermont, A. & Marabelle, A. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer 120, 3–5 (2019).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Kostine, M. et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann. Rheum. Dis. 80, 36–48 (2021).
Kuba, K. et al. Nivolumab-related tracheobronchial chondritis: extremely rare manifestation of an immune-related adverse effect. Head Neck 42, E43–E48 (2020).
Asoh, T., Yanagihara, T., Tanaka, R. & Yoneda, R. Tracheobronchial chondritis associated with immune checkpoint blockade. Intern. Med. 60, 2517–2518 (2021).
Someya, M. et al. Tracheobronchial chondritis as an immune-related adverse event occurring during the administration of nivolumab for recurrent hypopharyngeal squamous cell carcinoma. Ear Nose Throat J. https://doi.org/10.1177/01455613221081912 (2022).
Hamada-Ode, K., Taniguchi, Y., Osaki, M., Yoshimatsu, R. & Nitta, N. Clinical Images: nivolumab-induced tracheobronchial chondritis in a patient with hypopharyngeal cancer. Arthritis Rheumatol. 75, 121 (2023).
Mutoh, T. et al. Relapsing polychondritis following PD-1 blockade by an immune checkpoint inhibitor. JMA J. 6, 552–555 (2023).
Zhang, L. et al. Diagnosing relapsing polychondritis remains a common challenge: experience from a Chinese retrospective cohort. Clin. Rheumatol. 39, 2179–2184 (2020).
McAdam, L. P., O’Hanlan, M. A., Bluestone, R. & Pearson, C. M. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine 55, 193–215 (1976).
Damiani, J. M. & Levine, H. L. Relapsing polychondritis — report of ten cases. Laryngoscope 89, 929–946 (1979).
Michet, C. J., McKenna, C. H., Luthra, H. S. & O’Fallon, W. M. Relapsing polychondritis. Survival and predictive role of early disease manifestations. Ann. Intern. Med. 104, 74–78 (1986).
Foidart, J. M. et al. Antibodies to type II collagen in relapsing polychondritis. N. Engl. J. Med. 299, 1203–1207 (1978).
Buckner, J. H., Wu, J. J., Reife, R. A., Terato, K. & Eyre, D. R. Autoreactivity against matrilin-1 in a patient with relapsing polychondritis. Arthritis Rheum. 43, 939–943 (2000).
Kempta Lekpa, F. et al. Serum cartilage oligomeric matrix protein (COMP) level is a marker of disease activity in relapsing polychondritis. Clin. Exp. Rheumatol. 28, 553–555 (2010).
Patel, N., Dulau-Florea, A. & Calvo, K. R. Characteristic bone marrow findings in patients with UBA1 somatic mutations and VEXAS syndrome. Semin. Hematol. 58, 204–211 (2021).
Finetti, M., Omenetti, A., Federici, S., Caorsi, R. & Gattorno, M. Chronic infantile neurological cutaneous and articular (CINCA) syndrome: a review. Orphanet J. Rare Dis. 11, 167 (2016).
Belot, A. et al. Protein kinase cδ deficiency causes mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum. 65, 2161–2171 (2013).
De Ravin, S. S. et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood 116, 1263–1271 (2010).
Zimmer, J. et al. Clinical and immunological aspects of HLA class I deficiency. QJM 98, 719–727 (2005).
Munoz, J. et al. Stimulator of interferon genes-associated vasculopathy with onset in infancy: a mimic of childhood granulomatosis with polyangiitis. JAMA Dermatol. 151, 872–877 (2015).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Rice, G. et al. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am. J. Hum. Genet. 80, 811–815 (2007).
van Wijck, R. T. A., Swagemakers, S. M. A., van der Spek, P. J., van Hagen, P. M. & van Daele, P. L. A. A CDC42 stop-loss mutation in a patient with relapsing polychondritis and autoinflammation. J. Clin. Immunol. 43, 69–71 (2023).
El Masri, R. & Delon, J. RHO GTPases: from new partners to complex immune syndromes. Nat. Rev. Immunol. 21, 499–513 (2021).
Takenouchi, T., Kosaki, R., Niizuma, T., Hata, K. & Kosaki, K. Macrothrombocytopenia and developmental delay with a de novo CDC42 mutation: yet another locus for thrombocytopenia and developmental delay. Am. J. Med. Genet. A 167A, 2822–2825 (2015).
Lam, M. T. et al. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J. Exp. Med. 216, 2778–2799 (2019).
Gernez, Y. et al. Severe autoinflammation in 4 patients with C-terminal variants in cell division control protein 42 homolog (CDC42) successfully treated with IL-1β inhibition. J. Allergy Clin. Immunol. 144, 1122–1125.e6 (2019).
de Montmollin, N. et al. Tracheobronchial involvement of relapsing polychondritis. Autoimmun. Rev. 18, 102353 (2019).
Lee, K. S. et al. Relapsing polychondritis: prevalence of expiratory CT airway abnormalities. Radiology 240, 565–573 (2006).
Miyazu, Y. et al. Endobronchial ultrasonography in the diagnosis and treatment of relapsing polychondritis with tracheobronchial malacia. Chest 124, 2393–2395 (2003).
Tang, J. et al. Extracorporeal high-frequency combined with contrast-enhanced ultrasound: a novel imaging method for detection and treatment evaluation of patients with cervical trachea-associated relapsing polychondritis. Ann. Transl. Med. 9, 1785 (2021).
Sato, R. et al. Advantage of magnetic resonance imaging in detecting tracheal involvement and evaluation of the therapeutic response in relapsing polychondritis with asthma-like symptoms. J. Clin. Rheumatol. 27, e90–e91 (2021).
Sharma, A. et al. Fluorodeoxyglucose positron emission tomography/computed tomography in the diagnosis, assessment of disease activity and therapeutic response in relapsing polychondritis. Rheumatology 59, 99–106 (2020).
Okuda, S. et al. FDG-PET/CT and auricular cartilage biopsy are useful for diagnosing with relapsing polychondritis in patients without auricular symptoms. Life 11, 956 (2021).
Nakatsubo, D. et al. A case of relapsing polychondritis localized to the laryngeal cartilage in which FDG-PET/CT was helpful for diagnosis. Scand. J. Rheumatol. 52, 102–104 (2023).
Zeng, Y. et al. Is 18F-FDG PET/CT useful for diagnosing relapsing polychondritis with airway involvement and monitoring response to steroid-based therapy? Arthritis Res. Ther. 21, 282 (2019).
Yoshida, M., Taniguchi, Y., Yoshida, T., Nishikawa, H. & Terada, Y. Ultrasonography of auricular cartilage is a potential tool for diagnosing relapsing polychondritis and monitoring disease activity. Int. J. Rheum. Dis. 25, 201–209 (2022).
Dawudi, Y. et al. B-cell lymphoma mimicking relapsing polychondritis. Br. J. Haematol. 198, 222 (2022).
Rose, E. et al. Physician global assessment as a disease activity measure for relapsing polychondritis. Arthritis Care Res. 74, 1269–1276 (2022).
Rose, E. et al. Discordance in patient and physician global assessment in relapsing polychondritis. Rheumatology 61, 2025–2033 (2022).
Cao, X. et al. Three new inflammatory markers C reactive protein to albumin ratio, neutrophil to lymphocyte ratio, and platelet to lymphocyte ratio correlated with relapsing polychondritis disease activity index. Clin. Rheumatol. 40, 4685–4691 (2021).
Liu, Y. et al. Development and validation of diagnostic and activity-assessing models for relapsing polychondritis based on laboratory parameters. Front. Immunol. 14, 1274677 (2023).
Mertz, P. et al. The relapsing polychondritis damage index (RPDAM): development of a disease-specific damage score for relapsing polychondritis. Joint Bone Spine 86, 363–368 (2019).
Zhai, S.-Y. et al. Clinical analysis of relapsing polychondritis with airway involvement. J. Laryngol. Otol. 137, 96–100 (2023).
Yoshida, T. et al. Risk factors for the recurrence of relapsing polychondritis. Arthritis Res. Ther. 24, 127 (2022).
Papo, T. et al. Pregnancy in relapsing polychondritis: twenty-five pregnancies in eleven patients. Arthritis Rheum. 40, 1245–1249 (1997).
Chen, N. & Zheng, Y. Characteristics and clinical outcomes of 295 patients with relapsing polychondritis. J. Rheumatol. 48, 1876–1882 (2021).
Sangle, S. R. et al. Relapsing polychondritis — a single centre study in the United Kingdom. Autoimmun. Rev. 22, 103352 (2023).
Petitdemange, A. et al. Treatment of relapsing polychondritis: a systematic review. Clin. Exp. Rheumatol. 40, 81–85 (2022).
Yoshida, T., Nishimura, K., Murabe, H. & Yokota, T. Dapsone-induced methaemoglobinaemia in relapsing polychondritis. BMJ Case Rep. 15, e252431 (2022).
Moulis, G. et al. Efficacy and safety of biologics in relapsing polychondritis: a French national multicentre study. Ann. Rheum. Dis. 77, 1172–1178 (2018).
Handa, H., Ooka, S., Shimizu, J., Suzuki, N. & Mineshita, M. Evaluation of airway involvement and treatment in patients with relapsing polychondritis. Sci. Rep. 13, 8307 (2023).
Heiblig, M. et al. Ruxolitinib is more effective than other JAK inhibitors to treat VEXAS syndrome: a retrospective multicenter study. Blood 140, 927–931 (2022).
Boyadzhieva, Z., Ruffer, N., Burmester, G., Pankow, A. & Krusche, M. Effectiveness and safety of JAK inhibitors in autoinflammatory diseases: a systematic review. Front. Med. 9, 930071 (2022).
Boyadzhieva, Z., Ruffer, N., Kötter, I. & Krusche, M. How to treat VEXAS syndrome: a systematic review on effectiveness and safety of current treatment strategies. Rheumatology 62, 3518–3525 (2023).
Kirino, Y. et al. Tocilizumab in VEXAS relapsing polychondritis: a single-center pilot study in Japan. Ann. Rheum. Dis. 80, 1501–1502 (2021).
Goyal, A. et al. Tocilizumab for treatment of cutaneous and systemic manifestations of vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome without myelodysplastic syndrome. JAAD Case Rep. 23, 15–19 (2022).
Comont, T. et al. Azacitidine for patients with vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic syndrome (VEXAS) and myelodysplastic syndrome: data from the French VEXAS registry. Br. J. Haematol. 196, 969–974 (2022).
Xie, C., Shah, N., Shah, P. L. & Sandhu, G. Laryngotracheal reconstruction for relapsing polychondritis: case report and review of the literature. J. Laryngol. Otol. 127, 932–935 (2013).
Lee, J. W. et al. Auricular reconstruction for bilateral auricular deformity caused by relapsing polychondritis. JAMA Otolaryngol. Head Neck Surg. 149, 94–95 (2023).
Khayyal, M. T. et al. Radiation exposure and the effect of piroxicam and diclofenac on mediator release from isolated guinea-pig lung. Arch. Int. Pharmacodyn. Ther. 298, 247–263 (1989).
Dib, C. et al. Surgical treatment of the cardiac manifestations of relapsing polychondritis: overview of 33 patients identified through literature review and the Mayo Clinic records. Mayo Clin. Proc. 81, 772–776 (2006).
Arashi, K. et al. Bilateral deafness due to relapsing polychondritis with semicircular canal calcification treated with cochlear implantation: a case report. Ear Nose Throat J. https://doi.org/10.1177/01455613231215173 (2023).
Peng, Y., Ni, N. & Jiang, Z. Collapse crisis of tracheomalacia caused by undiagnosed relapsing polychondritis during general anesthesia: a case report. Anaesthesiologie 72, 36–38 (2023).
Ferrada, M. A. et al. Patient perception of disease-related symptoms and complications in relapsing polychondritis. Arthritis Care Res. 70, 1124–1131 (2018).
Acknowledgements
The authors wish to thank K. Baumgaertner for her invaluable assistance in the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.A. and P.M. researched data for the article and wrote the article. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
G.M. has received research grants from Amgen, Grifols, Novartis and Sanofi, and is on the advisory board for Amgen, Argenx, Grifols, Novartis, Sanofi and Sobi. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks David D’Cruz, who co-reviewed with Kathryn Biddle, Jun Shimizu and Manuel Ugarte-Gil for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mertz, P., Costedoat-Chalumeau, N., Ferrada, M.A. et al. Relapsing polychondritis: clinical updates and new differential diagnoses. Nat Rev Rheumatol (2024). https://doi.org/10.1038/s41584-024-01113-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41584-024-01113-9