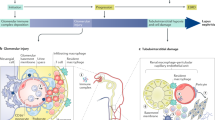
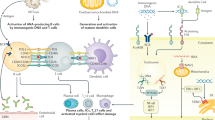
Abstract
Kidney involvement in patients with systemic lupus erythematosus — lupus nephritis (LN) — is one of the most important and common clinical manifestations of this disease and occurs in 40–60% of patients. Current treatment regimens achieve a complete kidney response in only a minority of affected individuals, and 10–15% of patients with LN develop kidney failure, with its attendant morbidity and considerable prognostic implications. Moreover, the medications most often used to treat LN — corticosteroids in combination with immunosuppressive or cytotoxic drugs — are associated with substantial side effects. Advances in proteomics, flow cytometry and RNA sequencing have led to important new insights into immune cells, molecules and mechanistic pathways that are instrumental in the pathogenesis of LN. These insights, together with a renewed focus on the study of human LN kidney tissue, suggest new therapeutic targets that are already being tested in lupus animal models and early-phase clinical trials and, as such, are hoped to eventually lead to meaningful improvements in the care of patients with systemic lupus erythematosus-associated kidney disease.
Key points
-
Understanding of the cellular and molecular mechanisms underlying lupus nephritis (LN) is progressing rapidly, facilitated by new tools for advanced ‘omic’ analyses.
-
Increasingly fine immune cell profiling based on patterns of expression of cell surface markers and genes is defining T cell and macrophage subsets that are important in disease mechanisms.
-
Chromatin release and/or its ineffective clearance stimulate adaptive and innate immunity in LN.
-
Resident kidney cells, including podocytes, mesangial cells and tubular epithelial cells, participate actively in the pathology of LN.
-
Closer attention to technical aspects in trial design is driving successes in clinical trials in systemic lupus erythematosus and stimulating much additional interest in the rational design and application of novel LN therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Parikh, S. V., Almaani, S., Brodsky, S. & Rovin, B. H. Update on lupus nephritis: core curriculum 2020. Am. J. Kidney Dis. 76, 265–281 (2020).
Hocaoglu, M. et al. Incidence, prevalence, and mortality of lupus nephritis: a population-based study over four decades — The Lupus Midwest Network (LUMEN). Arthritis Rheumatol. 75, 567–573 (2022).
Davidson, A. What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol. 12, 143–153 (2016).
Lech, M. & Anders, H. J. The pathogenesis of lupus nephritis. J. Am. Soc. Nephrol. 24, 1357–1366 (2013).
Mohan, C. & Putterman, C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 11, 329–341 (2015).
Wright, R. D., Dimou, P., Northey, S. J. & Beresford, M. W. Mesangial cells are key contributors to the fibrotic damage seen in the lupus nephritis glomerulus. J. Inflamm. 16, 22 (2019).
Kitching, A. R. & Hickey, M. J. Immune cell behaviour and dynamics in the kidney — insights from in vivo imaging. Nat. Rev. Nephrol. 18, 22–37 (2022).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Der, E. et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 20, 915–927 (2019).
Fava, A. et al. Integrated urine proteomics and renal single-cell genomics identify an IFN-γ response gradient in lupus nephritis. JCI Insight 5, e138345 (2020).
Chen, Z. et al. A single-cell survey of the human glomerulonephritis. J. Cell Mol. Med. 25, 4684–4695 (2021).
Der, E. et al. Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight 2, e93009 (2017).
Vanarsa, K. et al. Quantitative planar array screen of 1000 proteins uncovers novel urinary protein biomarkers of lupus nephritis. Ann. Rheum. Dis. 79, 1349–1361 (2020).
Stanley, S. et al. Comprehensive aptamer-based screening identifies a spectrum of urinary biomarkers of lupus nephritis across ethnicities. Nat. Commun. 11, 2197 (2020).
Stanley, S. et al. Identification of low-abundance urinary biomarkers in lupus nephritis using electrochemiluminescence immunoassays. Arthritis Rheumatol. 71, 744–755 (2019).
Fava, A. et al. Urine proteomics and renal single‐cell transcriptomics implicate interleukin‐16 in lupus nephritis. Arthritis Rheumatol. 74, 829–839 (2022).
Bertolo, M. et al. Deep phenotyping of urinary leukocytes by mass cytometry reveals a leukocyte signature for early and non-invasive prediction of response to treatment in active lupus nephritis. Front. Immunol. 11, 256 (2020).
Dhingra, S., Qureshi, R., Abdellatif, A., Gaber, L. W. & Truong, L. D. Tubulointerstitial nephritis in systemic lupus erythematosus: innocent bystander or ominous presage. Histol. Histopathol. 29, 553–565 (2014).
Clark, M. R., Trotter, K. & Chang, A. The pathogenesis and therapeutic implications of tubulointerstitial inflammation in human lupus nephritis. Semin. Nephrol. 35, 455–464 (2015).
Obrișcă B, J. R. et al. Histological predictors of renal outcome in lupus nephritis: the importance of tubulointerstitial lesions and scoring of glomerular lesions. Lupus 27, 1455–1463 (2018).
Tilstra, J. S. et al. Kidney-infiltrating T cells in murine lupus nephritis are metabolically and functionally exhausted. J. Clin. Invest. 128, 4884–4897 (2018).
Winchester, R. et al. Immunologic characteristics of intrarenal T cells: trafficking of expanded CD8+ T cell β-chain clonotypes in progressive lupus nephritis. Arthritis Rheum. 64, 1589–1600 (2012).
Linke, A., Tiegs, G. & Neumann, K. Pathogenic T-cell responses in immune-mediated glomerulonephritis. Cells 11, 1625 (2022).
Smita, S., Chikina, M., Shlomchik, M. J. & Tilstra, J. S. Heterogeneity and clonality of kidney-infiltrating T cells in murine lupus nephritis. JCI Insight 7, e156048 (2022).
Chen, P. M. & Tsokos, G. C. The role of CD8+ T-cell systemic lupus erythematosus pathogenesis: an update. Curr. Opin. Rheumatol. 33, 586–591 (2021).
Couzi, L. et al. Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum. 56, 2362–2370 (2007).
Zhang, T. et al. Association between tubulointerstitial CD8+ T cells and renal prognosis in lupus nephritis. Int. Immunopharmacol. 99, 107877 (2021).
Wiechmann, A. et al. CD107a+ (LAMP-1) cytotoxic CD8+ T-cells in lupus nephritis patients. Front. Med. 8, 556776 (2021).
Li, L. et al. Targeting tissue-resident memory CD8+ T cells in the kidney is a potential therapeutic strategy to ameliorate podocyte injury and glomerulosclerosis. Mol. Ther. 30, 2746–2759 (2022).
Zhou, M. et al. JAK/STAT signaling controls the fate of CD8+CD103+ tissue-resident memory T cell in lupus nephritis. J. Autoimmun. 109, 102424 (2020).
Zhong, H. et al. TGF-beta-Induced CD8+CD103+ regulatory T cells show potent therapeutic effect on chronic graft-versus-host disease lupus by suppressing B cells. Front. Immunol. 9, 35 (2018).
Wang, H., Lan, L., Chen, J., Xiao, L. & Han, F. Peripheral blood T-cell subset and its clinical significance in lupus nephritis patients. Lupus Sci. Med 9, e000717 (2022).
Okamoto, A., Fujio, K., Tsuno, N. H., Takahashi, K. & Yamamoto, K. Kidney-infiltrating CD4+ T-cell clones promote nephritis in lupus-prone mice. Kidney Int. 82, 969–979 (2012).
Masutani, K. et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 44, 2097–2106 (2001).
Mesquita, D. Jr et al. CD4+ T helper cells and regulatory T cells in active lupus nephritis: an imbalance towards a predominant Th1 response? Clin. Exp. Immunol. 191, 50–59 (2018).
Fakhfakh, R. et al. Th17 and Th1 cells in systemic lupus erythematosus with focus on lupus nephritis. Immunol. Res. 70, 644–653 (2022).
Paquissi, F. C. & Abensur, H. The Th17/IL-17 Axis and kidney diseases, with focus on lupus nephritis. Front. Med. 8, 654912 (2021).
Chen, D. Y. et al. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis. Lupus 21, 1385–1396 (2012).
Shenoy, S. et al. Effect of induction therapy on circulating T-helper 17 and T-regulatory cells in active proliferative lupus nephritis. Int. J. Rheum. Dis. 21, 1040–1048 (2018).
Koga, T., Ichinose, K. & Tsokos, G. C. T cells and IL-17 in lupus nephritis. Clin. Immunol. 185, 95–99 (2017).
Pisitkun, P. et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity 37, 1104–1115 (2012).
Schmidt, T. et al. Function of the Th17/interleukin-17A immune response in murine lupus nephritis. Arthritis Rheumatol. 67, 475–487 (2015).
Li, Y., Tang, D., Yin, L. & Dai, Y. New insights for regulatory T cell in lupus nephritis. Autoimmun. Rev. 21, 103134 (2022).
Rose, A. et al. IL-2 Therapy diminishes renal inflammation and the activity of kidney-infiltrating CD4+ T cells in murine lupus nephritis. Cells 8, 1234 (2019).
Xie, J. H. et al. Mouse IL-2/CD25 fusion protein induces regulatory T cell expansion and immune suppression in preclinical models of systemic lupus erythematosus. J. Immunol. 207, 34–43 (2021).
Yan, J. J. et al. IL-2/anti-IL-2 complexes ameliorate lupus nephritis by expansion of CD4+CD25+Foxp3+ regulatory T cells. Kidney Int. 91, 603–615 (2017).
Donadei, C. et al. Erythropoietin inhibits SGK1-dependent TH17 induction and TH17-dependent kidney disease. JCI Insight 5, e127428 (2019).
Gensous, N., Schmitt, N., Richez, C., Ueno, H. & Blanco, P. T follicular helper cells, interleukin-21 and systemic lupus erythematosus. Rheumatology 56, 516–523 (2017).
Liarski, V. M. et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci. Transl. Med. 6, 230ra246 (2014).
Sitrin, J. et al. The Ox40/Ox40 ligand pathway promotes pathogenic Th cell responses, plasmablast accumulation, and lupus nephritis in NZB/W F1 mice. J. Immunol. 199, 1238–1249 (2017).
Cortini, A. et al. B cell OX40L supports T follicular helper cell development and contributes to SLE pathogenesis. Ann. Rheum. Dis. 76, 2095–2103 (2017).
Mountz, J. D., Hsu, H. C. & Ballesteros-Tato, A. Dysregulation of T follicular helper cells in lupus. J. Immunol. 202, 1649–1658 (2019).
Zhang X, L. E. et al. Circulating CXCR5+CD4+ helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 24, 909–917 (2015).
Choi, J. Y. et al. Disruption of pathogenic cellular networks by IL-21 blockade leads to disease amelioration in murine lupus. J. Immunol. 198, 2578–2588 (2017).
Miao, M. et al. Therapeutic potential of targeting Tfr/Tfh cell balance by low-dose-IL-2 in active SLE: a post hoc analysis from a double-blind RCT study. Arthritis Res. Ther. 23, 167 (2021).
Xu, B. et al. The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clin. Immunol. 183, 46–53 (2017).
Kumar, P. et al. Restoration of follicular T regulatory/helper cell balance by OX40L-JAG1 cotreatment suppresses lupus nephritis in NZBWF1/j mice. J. Immunol. 208, 2467–2481 (2022).
Liang, K. et al. Sustained low-dose interleukin-2 therapy alleviates pathogenic humoral immunity via elevating the Tfr/Tfh ratio in lupus. Clin. Transl. Immunol. 10, e1293 (2021).
Rubtsova, K. et al. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J. Clin. Invest. 127, 1392–1404 (2017).
Liu, Y. et al. T-bet+CD11c+ B cells are critical for antichromatin immunoglobulin G production in the development of lupus. Arthritis Res. Ther. 19, 225 (2017).
Wang, S. et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 9, 1758 (2018).
Ramskold, D. et al. B cell alterations during BAFF inhibition with belimumab in SLE. EBioMedicine 40, 517–527 (2019).
Zhang, W. et al. Excessive CD11c+Tbet+ B cells promote aberrant TFH differentiation and affinity-based germinal center selection in lupus. Proc. Natl Acad. Sci. USA 116, 18550–18560 (2019).
Du, S. W., Arkatkar, T., Jacobs, H. M., Rawlings, D. J. & Jackson, S. W. Generation of functional murine CD11c+ age-associated B cells in the absence of B cell T-bet expression. Eur. J. Immunol. 49, 170–178 (2019).
Karnell, J. L. et al. Role of CD11c+ T-bet+ B cells in human health and disease. Cell Immunol. 321, 40–45 (2017).
You, X. et al. Double negative B cell is associated with renal impairment in systemic lupus erythematosus and acts as a marker for nephritis remission. Front. Med. 7, 85 (2020).
Espeli, M. et al. Local renal autoantibody production in lupus nephritis. J. Am. Soc. Nephrol. 22, 296–305 (2011).
Kinloch, A. J. et al. Vimentin is a dominant target of in situ humoral immunity in human lupus tubulointerstitial nephritis. Arthritis Rheumatol. 66, 3359–3370 (2014).
Abraham, R. et al. Specific in situ inflammatory states associate with progression to renal failure in lupus nephritis. J. Clin. Invest 132, e155350 (2022).
Becker, M., Gnirck, A. C. & Turner, J. E. Innate lymphoid cells in renal inflammation. Front. Immunol. 11, 72 (2020).
Park, Y. W. et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 60, 1753–1763 (2009).
Hudspeth, K. et al. Natural killer cell expression of Ki67 is associated with elevated serum IL-15, disease activity and nephritis in systemic lupus erythematosus. Clin. Exp. Immunol. 196, 226–236 (2019).
Scheffschick, A., Fuchs, S., Malmstrom, V., Gunnarsson, I. & Brauner, H. Kidney infiltrating NK cells and NK-like T-cells in lupus nephritis: presence, localization, and the effect of immunosuppressive treatment. Clin. Exp. Immunol. 207, 199–204 (2022).
Spada, R. et al. NKG2D ligand overexpression in lupus nephritis correlates with increased NK cell activity and differentiation in kidneys but not in the periphery. J. Leukoc. Biol. 97, 583–598 (2015).
Duster, M. et al. T cell-derived IFN-γ downregulates protective group 2 innate lymphoid cells in murine lupus erythematosus. Eur. J. Immunol. 48, 1364–1375 (2018).
Jourde-Chiche, N. et al. Modular transcriptional repertoire analyses identify a blood neutrophil signature as a candidate biomarker for lupus nephritis. Rheumatology 56, 477–487 (2017).
Banchereau, R. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 165, 551–565 (2016).
Nishi, H. & Mayadas, T. N. Neutrophils in lupus nephritis. Curr. Opin. Rheumatol. 31, 193–200 (2019).
Nishi, H. et al. Neutrophil FcγRIIA promotes IgG-mediated glomerular neutrophil capture via Abl/Src kinases. J. Clin. Invest. 127, 3810–3826 (2017).
Skopelja-Gardner, S. et al. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc. Natl Acad. Sci. USA 118, e2019097118 (2021).
Fousert, E., Toes, R. & Desai, J. Neutrophil extracellular traps (NETs) take the central stage in driving autoimmune responses. Cells 9, 915 (2020).
van der Linden, M. et al. Neutrophil extracellular trap release is associated with antinuclear antibodies in systemic lupus erythematosus and anti-phospholipid syndrome. Rheumatology 57, 1228–1234 (2018).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA 107, 9813–9818 (2010).
Tay, S. H., Celhar, T. & Fairhurst, A. M. Low-density neutrophils in systemic lupus erythematosus. Arthritis Rheumatol. 72, 1587–1595 (2020).
Moore, S. et al. Neutrophil extracellular traps identify patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J. Rheumatol. 47, 190875 (2019).
Frangou, E. et al. REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann. Rheum. Dis. 78, 238–248 (2019).
Fortner, K. A. et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci. Med. 7, e000387 (2020).
Goel, R. R. & Kaplan, M. J. Deadliest catch: neutrophil extracellular traps in autoimmunity. Curr. Opin. Rheumatol. 32, 64–70 (2020).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Campbell, A. M., Kashgarian, M. & Shlomchik, M. J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 4, 157ra141 (2012).
Kienhofer, D. et al. Experimental lupus is aggravated in mouse strains with impaired induction of neutrophil extracellular traps. JCI Insight 2, e92920 (2017).
Gordon, R. A. et al. Lupus and proliferative nephritis are PAD4 independent in murine models. JCI Insight 2, e92926 (2017).
Gordon, R. A. et al. Murine lupus is neutrophil elastase-independent in the MRL.Faslpr model. PLoS One 15, e0226396 (2020).
Maria, N. I. & Davidson, A. Renal macrophages and dendritic cells in SLE nephritis. Curr. Rheumatol. Rep. 19, 81 (2017).
Mysore, V. et al. Monocytes transition to macrophages within the inflamed vasculature via monocyte CCR2 and endothelial TNFR2. J. Exp. Med. 219, e20210562 (2022).
Maria, N. I. & Davidson, A. Protecting the kidney in systemic lupus erythematosus: from diagnosis to therapy. Nat. Rev. Rheumatol. 16, 255–267 (2020).
Sahu, R., Bethunaickan, R., Singh, S. & Davidson, A. Structure and function of renal macrophages and dendritic cells from lupus-prone mice. Arthritis Rheumatol. 66, 1596–1607 (2014).
Stamatiades, E. G. et al. Immune monitoring of trans-endothelial transport by kidney-resident macrophages. Cell 166, 991–1003 (2016).
Richoz, N. et al. Distinct pathogenic roles for resident and monocyte-derived macrophages in lupus nephritis. JCI Insight 7, e159751 (2022).
Barrera Garcia, A. et al. Infiltrating CD16+ are associated with a reduction in peripheral CD14+CD16++ monocytes and severe forms of lupus nephritis. Autoimmune Dis. 2016, 9324315 (2016).
Nakatani, K. et al. Fractalkine expression and CD16+ monocyte accumulation in glomerular lesions: association with their severity and diversity in lupus models. Am. J. Physiol. Renal Physiol. 299, F207–F216 (2010).
Yoshimoto, S. et al. Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis. Am. J. Kidney Dis. 50, 47–58 (2007).
Olaru, F. et al. Intracapillary immune complexes recruit and activate slan-expressing CD16+ monocytes in human lupus nephritis. JCI Insight 3, e96492 (2018).
Davidson, A. Renal mononuclear phagocytes in lupus nephritis. ACR Open Rheumatol. 3, 442–450 (2021).
Schiffer L, B. R. et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J. Immunol. 180, 1938–1947 (2008).
Bethunaickan, R. et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J. Immunol. 186, 4994–5003 (2011).
Kuriakose, J. et al. Patrolling monocytes promote the pathogenesis of early lupus-like glomerulonephritis. J. Clin. Invest. 129, 2251–2265 (2019).
Dias, C. B. et al. Role of renal expression of CD68 in the long-term prognosis of proliferative lupus nephritis. J. Nephrol. 30, 87–94 (2017).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Tang, P. M., Nikolic-Paterson, D. J. & Lan, H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158 (2019).
Orme, J. M. C. Macrophage subpopulations in systemic lupus erythematosus. Discov. Med. 13, 151–158 (2012).
Iwata, Y. et al. Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J. Immunol. 188, 4568–4580 (2012).
Kwant, L. E. et al. Macrophages in lupus nephritis: exploring a potential new therapeutic avenue. Autoimmun. Rev. 21, 103211 (2022).
Olmes, G. et al. CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res. Ther. 18, 90 (2016).
Kishimoto, D. et al. Dysregulated heme oxygenase-1low M2-like macrophages augment lupus nephritis via Bach1 induced by type I interferons. Arthritis Res. Ther. 20, 64 (2018).
Zhang, T. et al. Association of urine sCD163 with proliferative lupus nephritis, fibrinoid necrosis, cellular crescents and intrarenal M2 macrophages. Front. Immunol. 11, 671 (2020).
Mejia-Vilet, J. M. et al. Urinary soluble CD163: a novel noninvasive biomarker of activity for lupus nephritis. J. Am. Soc. Nephrol. 31, 1335–1347 (2020).
Sciascia, S. et al. Renal fibrosis in lupus nephritis. Int J. Mol. Sci. 23, 14317 (2022).
Chalmers, S. A. et al. Macrophage depletion ameliorates nephritis induced by pathogenic antibodies. J. Autoimmun. 57, 42–52 (2015).
Chalmers, S. A. et al. CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus. Clin. Immunol. 185, 100–108 (2017).
Chalmers, S. A., Garcia, S. J., Reynolds, J. A., Herlitz, L. & Putterman, C. NF-κB signaling in myeloid cells mediates the pathogenesis of immune-mediated nephritis. J. Autoimmun. 98, 33–43 (2019).
Hu, L. et al. Interleukin-22 from type 3 innate lymphoid cells aggravates lupus nephritis by promoting macrophage infiltration in lupus-prone mice. Front. Immunol. 12, 584414 (2021).
Arazi, A. et al. Immune cell heterogeneity in lupus nephritis kidneys and its relation to histopathological features [abstract]. Arthritis Rheumatol. 74 (suppl. 9), abstr 640 (2022).
Hoover, P. et al. Differentiation of injury-associated macrophages in lupus kidneys is conserved in humans and lupus mouse models [abstract]. Arthritis Rheumatol. 74, (suppl. 9), abstr 1666 (2022).
Kurts, C., Ginhoux, F. & Panzer, U. Kidney dendritic cells: fundamental biology and functional roles in health and disease. Nat. Rev. Nephrol. 16, 391–407 (2020).
Wardowska, A., Komorniczak, M., Bullo-Piontecka, B., Debska-Slizien, M. A. & Pikula, M. Transcriptomic and epigenetic alterations in dendritic cells correspond with chronic kidney disease in lupus nephritis. Front. Immunol. 10, 2026 (2019).
Tucci, M. et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 58, 251–262 (2008).
Liao, X. et al. Renal-infiltrating CD11c+ cells are pathogenic in murine lupus nephritis through promoting CD4+ T cell responses. Clin. Exp. Immunol. 190, 187–200 (2017).
Castellano, G. et al. Infiltrating dendritic cells contribute to local synthesis of C1q in murine and human lupus nephritis. Mol. Immunol. 47, 2129–2137 (2010).
Parikh, S. V. et al. A novel inflammatory dendritic cell that is abundant and contiguous to T cells in the kidneys of patients with lupus nephritis. Front. Immunol. 12, 621039 (2021).
Chang, A. et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J. Immunol. 186, 1849–1860 (2011).
Dorraji, E. S. et al. Kidney tertiary lymphoid structures in lupus nephritis develop into large interconnected networks and resemble lymph nodes in gene signature. Am. J. Pathol. 190, 2203–2225 (2020).
Jamaly, S., Rakaee, M., Abdi, R., Tsokos, G. C. & Fenton, K. A. Interplay of immune and kidney resident cells in the formation of tertiary lymphoid structures in lupus nephritis. Autoimmun. Rev. 20, 102980 (2021).
Steinmetz, O. M. et al. Analysis and classification of B-cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 74, 448–457 (2008).
Yoshikawa, T., Lee, Y. H., Sato, Y. & Yanagita, M. Tertiary lymphoid tissues in kidney diseases: a perspective for the pediatric nephrologist. Pediatr. Nephrol. 38, 1399–1409 (2022).
Kang, S. et al. BAFF induces tertiary lymphoid structures and positions T cells within the glomeruli during lupus nephritis. J. Immunol. 198, 2602–2611 (2017).
Dorraji, S. E. et al. Mesenchymal stem cells and T cells in the formation of tertiary lymphoid structures in lupus nephritis. Sci. Rep. 8, 7861 (2018).
Bhargava, R., Li, H. & Tsokos, G. C. Pathogenesis of lupus nephritis: the contribution of immune and kidney resident cells. Curr. Opin. Rheumatol. 35, 107–116 (2023).
Bhargava, R. & Tsokos, G. C. The immune podocyte. Curr. Opin. Rheumatol. 31, 167–174 (2019).
Sakhi, H. et al. Podocyte injury in lupus nephritis. J. Clin. Med. 8, 1340 (2019).
Wright, R. D. & Beresford, M. W. Podocytes contribute, and respond, to the inflammatory environment in lupus nephritis. Am. J. Physiol. Renal Physiol. 315, F1683–F1694 (2018).
Goldwich, A. et al. Podocytes are nonhematopoietic professional antigen-presenting cells. J. Am. Soc. Nephrol. 24, 906–916 (2013).
Wang, Y., Yu, F., Song, D., Wang, S. X. & Zhao, M. H. Podocyte involvement in lupus nephritis based on the 2003 ISN/RPS system: a large cohort study from a single centre. Rheumatology 53, 1235–1244 (2014).
Rezende, G. M. et al. Podocyte injury in pure membranous and proliferative lupus nephritis: distinct underlying mechanisms of proteinuria? Lupus 23, 255–262 (2014).
Bruschi, M. et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo (2): planted antigens. J. Am. Soc. Nephrol. 26, 1905–1924 (2015).
Fu, R. et al. Podocyte activation of NLRP3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol. 69, 1636–1646 (2017).
Maeda, K. et al. CaMK4 compromises podocyte function in autoimmune and nonautoimmune kidney disease. J. Clin. Invest. 128, 3445–3459 (2018).
Nowling, T. K. Mesangial cells in lupus nephritis. Curr. Rheumatol. Rep. 23, 83 (2022).
Yu, H. et al. Mesangial cells exhibit features of antigen-presenting cells and activate CD4+ T cell responses. J. Immunol. Res. 2019, 2121849 (2019).
Han, X. et al. MicroRNA-130b ameliorates murine lupus nephritis through targeting the type I interferon pathway on renal mesangial cells. Arthritis Rheumatol. 68, 2232–2243 (2016).
Ichinose, K. et al. Cutting edge: calcium/calmodulin-dependent protein kinase type IV is essential for mesangial cell proliferation and lupus nephritis. J. Immunol. 187, 5500–5504 (2011).
Ferretti, A. P., Bhargava, R., Dahan, S., Tsokos, M. G. & Tsokos, G. C. Calcium/calmodulin kinase IV controls the function of both T cells and kidney resident cells. Front. Immunol. 9, 2113 (2018).
Hong, S., Healy, H. & Kassianos, A. J. The emerging role of renal tubular epithelial cells in the immunological pathophysiology of lupus nephritis. Front. Immunol. 11, 578952 (2020).
Breda PC, W. T. et al. Renal proximal tubular epithelial cells exert immunomodulatory function by driving inflammatory CD4+ T cell responses. Am. J. Physiol. Renal Physiol. 317, F77–F89 (2019).
Linke, A. et al. Antigen cross-presentation by murine proximal tubular epithelial cells induces cytotoxic and inflammatory CD8+ T cells. Cells 11, 1510 (2022).
Li H, T. M. et al. IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J. Clin. Invest. 131, e142428 (2021).
Chasset, F. & Arnaud, L. Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun. Rev. 17, 44–52 (2018).
Mustelin, T., Lood, C. & Giltiay, N. V. Sources of pathogenic nucleic acids in systemic lupus erythematosus. Front. Immunol. 10, 1028 (2019).
Arriens C, R. Q. et al. Increased risk of progression to lupus nephritis for lupus patients with elevated interferon signature [abstract]. Arthritis Rheumatol. 71 abstr 1914 (2019).
Crow, M. K., Olferiev, M. & Kirou, K. A. Targeting of type I interferon in systemic autoimmune diseases. Transl. Res. 165, 296–305 (2015).
Iwamoto, T. et al. High systemic type I interferon activity is associated with active class III/IV lupus nephritis. J. Rheumatol. 49, 388–397 (2022).
Zickert, A. et al. Interferon (IFN)-λ is a potential mediator in lupus nephritis. Lupus Sci. Med. 3, e000170 (2016).
Oke, V. et al. High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Res. Ther. 21, 107 (2019).
Oke, V. et al. IFN-λ1 with Th17 axis cytokines and IFN-α define different subsets in systemic lupus erythematosus (SLE). Arthritis Res. Ther. 19, 139 (2017).
Psarras, A. et al. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nat. Commun. 11, 6149 (2020).
Lindau, D. et al. TLR9 independent interferon α production by neutrophils on NETosis in response to circulating chromatin, a key lupus autoantigen. Ann. Rheum. Dis. 73, 2199–2207 (2014).
Fairhurst, A. M. et al. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J. Immunol. 183, 6831–6838 (2009).
Castellano, G. et al. Local synthesis of interferon-α in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res. Ther. 17, 72 (2015).
Kalunian, K. C. et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 75, 196–202 (2016).
Khamashta, M. et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 1909–1916 (2016).
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020).
Houssiau, F. A. et al. IFN-α kinoid in systemic lupus erythematosus: results from a phase IIb, randomised, placebo-controlled study. Ann. Rheum. Dis. 79, 347–355 (2020).
Chaichian, Y., Wallace, D. J. & Weisman, M. H. A promising approach to targeting type 1 IFN in systemic lupus erythematosus. J. Clin. Invest. 129, 958–961 (2019).
Furie, R. et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J. Clin. Invest. 129, 1359–1371 (2019).
Chyuan, I. T., Tzeng, H. T. & Chen, J. Y. Signaling pathways of type I and type III interferons and targeted therapies in systemic lupus erythematosus. Cells 8, 963 (2019).
Tanaka, Y. & Tummala, R. Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials. Mod. Rheumatol. 31, 1–12 (2021).
Mullard, A. FDA approves AstraZeneca’s anifrolumab for lupus. Nat. Rev. Drug Discov. 20, 658 (2021).
Jayne, D. et al. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann. Rheum. Dis. 81, 496–506 (2022).
Barrat, F. J., Elkon, K. B. & Fitzgerald, K. A. Importance of nucleic acid recognition in inflammation and autoimmunity. Annu. Rev. Med. 67, 323–336 (2016).
Lorenz, G., Lech, M. & Anders, H. J. Toll-like receptor activation in the pathogenesis of lupus nephritis. Clin. Immunol. 185, 86–94 (2017).
Rubtsova K, M. P. & Rubtsov, A. V. TLR7, IFNγ, and T-bet: their roles in the development of ABCs in female-biased autoimmunity. Cell Immunol. 294, 80–83 (2015).
Gong, L. et al. Activation of toll-like receptor-7 exacerbates lupus nephritis by modulating regulatory T cells. Am. J. Nephrol. 40, 325–344 (2014).
Wirth, J. R., Molano, I., Ruiz, P., Coutermarsh-Ott, S. & Cunningham, M. A. TLR7 agonism accelerates disease and causes a fatal myeloproliferative disorder in NZM 2410 lupus mice. Front. Immunol. 10, 3054 (2019).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022).
Wang, T. et al. High TLR7 expression drives the expansion of CD19+CD24hiCD38hi transitional B cells and autoantibody production in SLE patients. Front. Immunol. 10, 1243 (2019).
Souyris M, C. C. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 3, eaap8855 (2018).
Fairhurst, A. M. et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur. J. Immunol. 38, 1971–1978 (2008).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Gies, V. et al. Impaired TLR9 responses in B cells from patients with systemic lupus erythematosus. JCI Insight 3, e96795 (2018).
Sharma, S., Fitzgerald, K. A., Cancro, M. P. & Marshak-Rothstein, A. Nucleic acid-sensing receptors: rheostats of autoimmunity and autoinflammation. J. Immunol. 195, 3507–3512 (2015).
Jackson, S. W. et al. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 192, 4525–4532 (2014).
Bossaller, L. et al. TLR9 deficiency leads to accelerated renal disease and myeloid lineage abnormalities in pristane-induced murine lupus. J. Immunol. 197, 1044–1053 (2016).
Rubtsov AV, R. K., Kappler, J. W. & Marrack, P. TLR7 drives accumulation of ABCs and autoantibody production in autoimmune-prone mice. Immunol. Res. 55, 210–216 (2013).
Chauhan, S. K., Singh, V. V., Rai, R., Rai, M. & Rai, G. Distinct autoantibody profiles in systemic lupus erythematosus patients are selectively associated with TLR7 and TLR9 upregulation. J. Clin. Immunol. 33, 954–964 (2013).
Lyn-Cook, B. D. et al. Increased expression of Toll-like receptors (TLRs) 7 and 9 and other cytokines in systemic lupus erythematosus (SLE) patients: ethnic differences and potential new targets for therapeutic drugs. Mol. Immunol. 61, 38–43 (2014).
Crow, M. K. Autoimmunity: interferon α or β: which is the culprit in autoimmune disease? Nat. Rev. Rheumatol. 12, 439–440 (2016).
Buskiewicz IA, M. T. et al. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci. Signal. 9, ra115 (2016).
Wang, J. et al. Association of abnormal elevations in IFIT3 with overactive cyclic GMP-AMP synthase/stimulator of interferon genes signaling in human systemic lupus erythematosus monocytes. Arthritis Rheumatol. 70, 2036–2045 (2018).
Shao, W. H. et al. Prion-like aggregation of mitochondrial antiviral signaling protein in lupus patients is associated with increased levels of type I interferon. Arthritis Rheumatol. 68, 2697–2707 (2016).
Sun, W. et al. Antiviral adaptor MAVS promotes murine lupus with a B cell autonomous role. Front. Immunol. 10, 2452 (2019).
An, J. et al. Expression of cyclic GMP-AMP synthase in patients with systemic lupus erythematosus. Arthritis Rheumatol. 69, 800–807 (2017).
Konig, N. et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 76, 468–472 (2017).
Jeremiah, N. et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Invest. 124, 5516–5520 (2014).
Decout, A., Katz, J. D., Venkatraman, S. & Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Klarquist, J. et al. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J. Immunol. 193, 6124–6134 (2014).
Sharma, S. et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc. Natl Acad. Sci. USA 112, E710–E717 (2015).
Motwani, M. et al. cGAS-STING Pathway does not promote autoimmunity in murine models of SLE. Front. Immunol. 12, 605930 (2021).
Skopelja-Gardner, S., An, J. & Elkon, K. B. Role of the cGAS-STING pathway in systemic and organ-specific diseases. Nat. Rev. Nephrol. 18, 558–572 (2022).
Zang, N. et al. cGAS-STING activation contributes to podocyte injury in diabetic kidney disease. iScience 25, 105145 (2022).
Yiu, W. H., Lin, M. & Tang, S. C. Toll-like receptor activation: from renal inflammation to fibrosis. Kidney Int. Suppl. 4, 20–25 (2014).
Mohan C, A. S., Stanik, V. & Datta, S. K. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 177, 1367–1381 (1993).
Lovgren, T., Eloranta, M. L., Bave, U., Alm, G. V. & Ronnblom, L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004).
Vorobjeva, N. V. & Chernyak, B. V. NETosis: molecular mechanisms, role in physiology and pathology. Biochemistry 85, 1178–1190 (2020).
Sorensen, O. E. & Borregaard, N. Neutrophil extracellular traps — the dark side of neutrophils. J. Clin. Invest. 126, 1612–1620 (2016).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Soni, C. & Reizis, B. Self-DNA at the epicenter of SLE: immunogenic forms, regulation, and effects. Front. Immunol. 10, 1601 (2019).
Wigerblad, G. & Kaplan, M. J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-022-00787-0 (2022).
Georgakis, S. et al. NETs decorated with bioactive IL-33 infiltrate inflamed tissues and induce IFN-α production in patients with SLE. JCI Insight 6, e147671 (2021).
Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107–125 (2021).
Li, P. et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 22, 1107–1117 (2021).
Alli, A. A. et al. Kidney tubular epithelial cell ferroptosis links glomerular injury to tubulointerstitial pathology in lupus nephritis. Clin. Immunol. 248, 109213 (2022).
Linge, P., Fortin, P. R., Lood, C., Bengtsson, A. A. & Boilard, E. The non-haemostatic role of platelets in systemic lupus erythematosus. Nat. Rev. Rheumatol. 14, 195–213 (2018).
Melki I, A. I. et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci. Transl. Med. 13, eaav5928 (2021).
Caielli, S. et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 184, 4464–4479 e4419 (2021).
Frangou, E., Vassilopoulos, D., Boletis, J. & Boumpas, D. T. An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): implications for the pathogenesis and treatment. Autoimmun. Rev. 18, 751–760 (2019).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Wang, H., Li, T., Chen, S., Gu, Y. & Ye, S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 67, 3190–3200 (2015).
Blanco, L. P. et al. Improved mitochondrial metabolism and reduced inflammation following attenuation of murine lupus with coenzyme Q10 analog idebenone. Arthritis Rheumatol. 72, 454–464 (2020).
Kim, J. et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366, 1531–1536 (2019).
Fortin, P. R. et al. Distinct subtypes of microparticle-containing immune complexes are associated with disease activity, damage, and carotid intima-media thickness in systemic lupus erythematosus. J. Rheumatol. 43, 2019–2025 (2016).
Lopez, P., Rodriguez-Carrio, J., Martinez-Zapico, A., Caminal-Montero, L. & Suarez, A. Circulating microparticle subpopulations in systemic lupus erythematosus are affected by disease activity. Int. J. Cardiol. 236, 138–144 (2017).
Dieker, J. et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. 68, 462–472 (2016).
Mobarrez, F. et al. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci. Rep. 6, 36025 (2016).
Nielsen, C. T., Ostergaard, O., Johnsen, C., Jacobsen, S. & Heegaard, N. H. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 63, 3067–3077 (2011).
Rother, N., Pieterse, E., Lubbers, J., Hilbrands, L. & van der Vlag, J. Acetylated histones in apoptotic microparticles drive the formation of neutrophil extracellular traps in active lupus nephritis. Front. Immunol. 8, 1136 (2017).
Arneth, B. Systemic lupus erythematosus and DNA degradation and elimination defects. Front. Immunol. 10, 1697 (2019).
Leffler, J. et al. Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus. Arthritis Res. Ther. 15, R84 (2013).
Monteith, A. J. et al. Defects in lysosomal maturation facilitate the activation of innate sensors in systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 113, E2142–E2151 (2016).
Zhou, Y. et al. Cathepsin K deficiency ameliorates systemic lupus erythematosus-like manifestations in Faslpr mice. J. Immunol. 198, 1846–1854 (2017).
Bonam, S. R., Wang, F. & Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 18, 923–948 (2019).
Sisirak, V. et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166, 88–101 (2016).
Soni, C. et al. Plasmacytoid dendritic cells and type I interferon promote extrafollicular B cell responses to extracellular self-DNA. Immunity 52, 1022–1038.e1027 (2020).
Wang, X. & Xia, Y. Anti-double stranded DNA antibodies: origin, pathogenicity, and targeted therapies. Front. Immunol. 10, 1667 (2019).
Pisetsky, D. S., Garza Reyna, A., Belina, M. E. & Spencer, D. M. The interaction of anti-DNA antibodies with DNA: evidence for unconventional binding mechanisms. Int. J. Mol. Sci. 23, 5227 (2022).
Rekvig, O. P. The anti-DNA antibodies: their specificities for unique DNA structures and their unresolved clinical impact — a system criticism and a hypothesis. Front. Immunol. 12, 808008 (2021).
Pisetsky, D. S. & Lipsky, P. E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 16, 565–579 (2020).
Putterman, C. New approaches to the renal pathogenicity of anti-DNA antibodies in systemic lupus erythematosus. Autoimmun. Rev. 3, 7–11 (2004).
Xia, Y., Janda, A., Eryilmaz, E., Casadevall, A. & Putterman, C. The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol. Immunol. 56, 28–37 (2013).
Xia, Y., Eryilmaz, E., Zhang, Q., Cowburn, D. & Putterman, C. Anti-DNA antibody mediated catalysis is isotype dependent. Mol. Immunol. 69, 33–43 (2016).
Deocharan B, Q. X., Beger, E. & Putterman, C. Antigenic triggers and molecular targets for anti-double-stranded DNA antibodies. Lupus 11, 865–871 (2002).
Shang, X. et al. Anti-dsDNA, anti-nucleosome, anti-C1q, and anti-histone antibodies as markers of active lupus nephritis and systemic lupus erythematosus disease activity. Immun. Inflamm. Dis. 9, 407–418 (2021).
Choi, M. Y., FitzPatrick, R. D., Buhler, K., Mahler, M. & Fritzler, M. J. A review and meta-analysis of anti-ribosomal P autoantibodies in systemic lupus erythematosus. Autoimmun. Rev. 19, 102463 (2020).
Dumestre-Perard, C., Clavarino, G., Colliard, S., Cesbron, J. Y. & Thielens, N. M. Antibodies targeting circulating protective molecules in lupus nephritis: interest as serological biomarkers. Autoimmun. Rev. 17, 890–899 (2018).
Lewis, M. J. et al. Autoantibodies targeting TLR and SMAD pathways define new subgroups in systemic lupus erythematosus. J. Autoimmun. 91, 1–12 (2018).
Cantarelli, C., Leventhal, J. & Cravedi, P. Complement in lupus: biomarker, therapeutic target, or a little bit of both? Kidney Int. Rep. 6, 2031–2032 (2021).
Obrisca, B., Sorohan, B., Tuta, L. & Ismail, G. Advances in lupus nephritis pathogenesis: from bench to bedside. Int. J. Mol. Sci. 22, 3766 (2021).
Satyam, A., Hisada, R., Bhargava, R., Tsokos, M. G. & Tsokos, G. C. Intertwined pathways of complement activation command the pathogenesis of lupus nephritis. Transl. Res. 245, 18–29 (2022).
Putterman, C. et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci. Med. 1, e000056 (2014).
Wright, R. D., Bannerman, F., Beresford, M. W. & Oni, L. A systematic review of the role of eculizumab in systemic lupus erythematosus-associated thrombotic microangiopathy. BMC Nephrol. 21, 245 (2020).
Lee, A. Avacopan: first approval. Drugs 82, 79–85 (2021).
Li, N. L., Birmingham, D. J. & Rovin, B. H. Expanding the role of complement therapies: the case for lupus nephritis. J. Clin. Med. 10, 626 (2021).
Kostopoulou, M., Pitsigavdaki, S. & Bertsias, G. Lupus nephritis: improving treatment options. Drugs 82, 735–748 (2022).
Yap, D. Y. H. & Mok, C. C. Novel and emerging treatment strategies for lupus nephritis. Expert. Rev. Clin. Pharmacol. 15, 1283–1292 (2022).
Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT05138133 (2021).
Zhao, Z. et al. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J. Immunol. 179, 7949–7958 (2007).
Michaelson, J. S., Wisniacki, N., Burkly, L. C. & Putterman, C. Role of TWEAK in lupus nephritis: a bench-to-bedside review. J. Autoimmun. 39, 130–142 (2012).
Xia, Y. et al. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin. Immunol. 145, 108–121 (2012).
Xia, Y. et al. Deficiency of fibroblast growth factor-inducible 14 (Fn14) preserves the filtration barrier and ameliorates lupus nephritis. J. Am. Soc. Nephrol. 26, 1053–1070 (2015).
Dias, R., Hasparyk, U. G., Lopes, M. P., de Barros, J. & Simoes, E. S. A. C. Novel biomarkers for lupus nephritis in the “OMICS” era. Curr. Med. Chem. 28, 6011–6044 (2021).
Palazzo, L., Lindblom, J., Mohan, C. & Parodis, I. Current insights on biomarkers in lupus nephritis: a systematic review of the literature. J. Clin. Med. 11, 5759 (2022).
Bolouri, N. et al. Role of the innate and adaptive immune responses in the pathogenesis of systemic lupus erythematosus. Inflamm. Res. 71, 537–554 (2022).
Radin, M. et al. Prognostic and diagnostic values of novel serum and urine biomarkers in lupus nephritis: a systematic review. Am. J. Nephrol. 52, 559–571 (2021).
Ghafouri-Fard, S., Shahir, M., Taheri, M. & Salimi, A. A review on the role of chemokines in the pathogenesis of systemic lupus erythematosus. Cytokine 146, 155640 (2021).
Hayry, A. et al. Interleukin (IL) 16: a candidate urinary biomarker for proliferative lupus nephritis. Lupus Sci. Med. 9, e000744 (2022).
Niewold, T. B. et al. Proteome study of cutaneous lupus erythematosus (CLE) and dermatomyositis skin lesions reveals IL-16 is differentially upregulated in CLE. Arthritis Res. Ther. 23, 132 (2021).
Xie, S., Louis Sam Titus, A. S. C. & Mohan, C. Elevated expression of receptors for EGF, PDGF, transferrin and folate within murine and human lupus nephritis kidneys. Clin. Immunol. 246, 109188 (2023).
Lei, R. et al. A novel technology for home monitoring of lupus nephritis that tracks the pathogenic urine biomarker ALCAM. Front. Immunol. 13, 1044743 (2022).
Song, K., Liu, L., Zhang, X. & Chen, X. An update on genetic susceptibility in lupus nephritis. Clin. Immunol. 210, 108272 (2020).
Lorenzo-Vizcaya, A. & Isenberg, D. A. Clinical trials in systemic lupus erythematosus: the dilemma — why have phase III trials failed to confirm the promising results of phase II trials? Ann. Rheum. Dis. 82, 169–174 (2023).
Isenberg, D. A. & Merrill, J. T. Why, why, why de-lupus (does so badly in clinical trials). Expert. Rev. Clin. Immunol. 12, 95–98 (2016).
Hruskova, Z. & Tesar, V. Lessons learned from the failure of several recent trials with biologic treatment in systemic lupus erythematosus. Expert. Opin. Biol. Ther. 18, 989–996 (2018).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 64, 1215–1226 (2012).
Krustev, E., Clarke, A. E. & Barber, M. R. W. B cell depletion and inhibition in systemic lupus erythematosus. Expert. Rev. Clin. Immunol. 19, 55–70 (2023).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content and wrote, reviewed or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
C.P. is one of the holders of a patent describing the diagnostic use of urinary TWEAK in lupus nephritis (Method of treating lupus nephritis; Grant US-9730947-B2). C.M. and C.P. serve as scientific consultants to Equillium, which is developing anti-CD6 as a therapy for lupus nephritis. The other author declares no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks T. Chan, O. Steinmetz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohan, C., Zhang, T. & Putterman, C. Pathogenic cellular and molecular mediators in lupus nephritis. Nat Rev Nephrol 19, 491–508 (2023). https://doi.org/10.1038/s41581-023-00722-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00722-z
This article is cited by
-
LATS2 degradation promoted fibrosis damage and rescued by vitamin K3 in lupus nephritis
Arthritis Research & Therapy (2024)
-
The immunoregulatory roles of non-haematopoietic cells in the kidney
Nature Reviews Nephrology (2024)