Abstract
Nonalcoholic fatty liver disease (NAFLD), including its more severe manifestation, nonalcoholic steatohepatitis (NASH), has a global prevalence of 20–25% and is a major public health problem. Its incidence is increasing in parallel to the rise in obesity, diabetes and metabolic syndrome. Progression from NASH to NASH-related hepatocellular carcinoma (HCC) (~2% of cases per year) is influenced by many factors, including the tissue and immune microenvironment, germline mutations in PNPLA3, and the microbiome. NASH-HCC has unique molecular and immune traits compared with other aetiologies of HCC and is equally prevalent in men and women. Comorbidities associated with NASH, such as obesity and diabetes mellitus, can prevent the implementation of potentially curative therapies in certain patients; nonetheless, outcomes are similar in patients who receive treatment. NASH-HCC at the early to intermediate stages is managed with surgery and locoregional therapies, whereas advanced HCC is treated with systemic therapies, including anti-angiogenic therapies and immune-checkpoint inhibitors. In this Review, we present the latest knowledge of the pathogenic mechanisms and clinical management of NASH-HCC. We discuss data highlighting the controversy over varying responses to immune-checkpoint inhibitors according to underlying aetiology and suggest that the future of NASH-HCC management lies in improved surveillance, targeted combination therapies to overcome immune evasion, and identifying biomarkers to recognize treatment responders.
Key points
-
Nonalcoholic steatohepatitis (NASH)-related hepatocellular carcinoma (HCC) is a major public health problem, the incidence of which is increasing in parallel to the rise in obesity, diabetes and metabolic syndrome.
-
Progression from NASH to NASH-HCC occurs at an approximate rate of 2% per year and is influenced by many factors, including the tissue and immune microenvironment, germline mutations in PNPLA3, and the microbiome.
-
HCC surveillance in these at-risk patients with NASH-related cirrhosis is associated with earlier detection and improved survival.
-
NASH-HCC in the early to intermediate stages is managed with surgery and locoregional therapies; however, comorbidities associated with NASH, such as obesity and type 2 diabetes mellitus, can complicate the implementation of potentially curative therapies.
-
Advanced NASH-HCC is treated with systemic therapies, including anti-angiogenic therapies and immune-checkpoint inhibitors. Distinct microenvironmental factors could be limiting the response of NASH-HCC to immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7, 6 (2021). This review provides a comprehensive up-to-date overview of the epidemiology, pathogenesis and management of HCC.
Anstee, Q. M., Reeves, H. L., Kotsiliti, E., Govaere, O. & Heikenwalder, M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16, 411–428 (2019).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357 (2018).
Huang, D. Q., El-Serag, H. B. & Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 18, 223–238 (2021). This manuscript thoroughly analyses the global epidemiology, projections and risk factors for NAFLD-related HCC.
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease — meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904 (2018).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Lazarus, J. V. et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 19, 60–78 (2022).
Loomba, R., Lim, J. K., Patton, H. & El-Serag, H. B. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 158, 1822–1830 (2020).
Gawrieh, S. et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: a United States multicentre study. Aliment. Pharmacol. Ther. 50, 809–821 (2019).
Ioannou, G. N. Epidemiology and risk-stratification of NAFLD-associated HCC. J. Hepatol. 75, 1476–1484 (2021). This review covers the risk factors associated with NAFLD-HCC, and how the identification of at-risk patients is needed for effective surveillance strategies.
Pinyol, R. et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J. Hepatol. 75, 865–878 (2021).
Pfister, D. et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592, 450–456 (2021). This study demonstrates the existence of a CD8+PD1+ subset of pro-tumorigenic cells in NASH that favour the development of HCC and hamper response to ICIs.
Liu, Y. L. et al. Carriage of the PNPLA3 rs738409 C>g polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 61, 75–81 (2014). This study demonstrates that the PNPLA3 rs738409 C>G polymorphism is associated with greater risk of progressive steatohepatitis, fibrosis and HCC development.
Mittal, S. et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 14, 124–131.e1 (2016).
Stine, J. G. et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment. Pharmacol. Ther. 48, 696–703 (2018).
Kanwal, F. et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 155, 1828–1837.e2 (2018).
Piscaglia, F. et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 63, 827–838 (2016).
Vogel, A., Meyer, T., Sapisochin, G., Salem, R. & Saborowski, A. Hepatocellular carcinoma. Lancet 400, 1345–1362 (2022).
Puigvehí, M. et al. Liver transplant for hepatocellular carcinoma in the United States: evolving trends over the last three decades. Am. J. Transplant. 20, 220–230 (2020).
Llovet, J. M. et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19, 151–172 (2022). Comprehensive overview of the immune microenvironment role according to aetiologies, mechanisms of response and role of immunotherapies for the treatment of HCC.
Haber, P. K. et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020). Gastroenterology 161, 879–898 (2021). Meta-analysis of RCTs of therapies in HCC from 2002 to 2020, including all relevant studies leading to a paradigm change in the management of HCC.
Kelley, R. K. & Greten, T. F. Hepatocellular carcinoma — origins and outcomes. N. Engl. J. Med. 385, 280–282 (2021).
Dyson, J. et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 60, 110–117 (2014).
Patkar, S., Parray, A., Mahendra, B., Kurunkar, S. & Goel, M. Performance of Hong Kong liver cancer staging system in patients of hepatocellular carcinoma treated with surgical resection: an Indian validation study. J. Surg. Oncol. 120, 1119–1125 (2019).
Mittal, S. et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin. Gastroenterol. Hepatol. 13, 594–601.e1 (2015).
Aljumah, A. A. et al. Clinical presentation, risk factors, and treatment modalities of hepatocellular carcinoma: a single tertiary care center experience. Gastroenterol. Res. Pract. 2016, 1989045 (2016).
Ganslmayer, M. et al. A large cohort of patients with hepatocellular carcinoma in a single European centre: aetiology and prognosis now and in a historical cohort. Swiss Med. Wkly 144, w13900 (2014).
Park, J. W. et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 35, 2155–2166 (2015).
White, D. L., Kanwal, F. & El-Serag, H. B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 10, 1342–1359.e2 (2012).
Balakrishnan, M. & El-Serag, H. B. Editorial: NAFLD-related hepatocellular carcinoma — increasing or not? With or without cirrhosis? Aliment. Pharmacol. Ther. 47, 437–438 (2018).
Orci, L. A. et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin. Gastroenterol. Hepatol. 20, 283–292.e10 (2022).
Natarajan, Y. et al. Risk of cirrhosis and hepatocellular cancer in patients with NAFLD and normal liver enzymes. Hepatology 72, 1242–1252 (2020).
Yang, J. D. et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology 71, 907–916 (2020).
Alexander, M. et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: Real-world study of 18 million patients in four European cohorts. BMC Med. 17, 95 (2019).
Chen, Y., Wang, X., Wang, J., Yan, Z. & Luo, J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur. J. Cancer 48, 2137–2145 (2012).
Abdel-Rahman, O. et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J. Evid. Based Med. 10, 245–254 (2017).
Ioannou, G. N., Green, P., Kerr, K. F. & Berry, K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J. Hepatol. 71, 523–533 (2019). Study developing models available as web-based tools for estimating HCC risk in patients with NAFLD-cirrhosis or alcohol-associated liver disease with cirrhosis.
Younes, R. et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J. Hepatol. 75, 786–794 (2021).
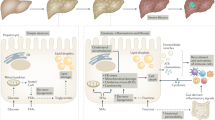
Marra, F. & Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 68, 280–295 (2018).
Fuchs, A. et al. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology 161, 968–981.e12 (2021).
Parthasarathy, G., Revelo, X. & Malhi, H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol. Commun. 4, 478–492 (2020).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018). Overview of the pathogenic and clinical features of NAFLD, preclinical models of NAFLD and emerging targets for drug development.
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Daugherity, E. K. et al. The DNA damage checkpoint protein ATM promotes hepatocellular apoptosis and fibrosis in a mouse model of non-alcoholic fatty liver disease. Cell Cycle 11, 1918–1928 (2012).
Ng, S. W. K. et al. Convergent somatic mutations in metabolism genes in chronic liver disease. Nature 598, 473–478 (2021).
Zhu, C. et al. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci. Transl Med. 10, eaat0344 (2018).
Michelotti, G. A. et al. Smoothened is a master regulator of adult liver repair. J. Clin. Invest. 123, 2380–2394 (2013).
Zhu, C., Tabas, I., Schwabe, R. F. & Pajvani, U. B. Maladaptive regeneration — the reawakening of developmental pathways in NASH and fibrosis. Nat. Rev. Gastroenterol. Hepatol. 18, 131–142 (2021).
Dewhurst, M. R. et al. Loss of hepatocyte cell division leads to liver inflammation and fibrosis. PLoS Genet. 16, e1009084 (2020).
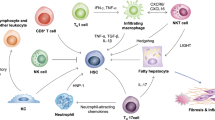
Huby, T. & Gautier, E. L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 22, 429–443 (2022).
Kazankov, K. et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159 (2019).
Sanyal, A. J. et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 385, 1559–1569 (2021). Prospective multicentre study of patients with NAFLD demonstrating that bridging fibrosis and cirrhosis is associated with increased risks of liver-related complications and death.
Xiong, X. et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol. Cell 75, 644–660.e5 (2019).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Kitade, M. et al. Neovascularization and oxidative stress in the progression of non-alcoholic steatohepatitis. Mol. Med. Rep. 1, 543–548 (2008).
Coulon, S. et al. Role of vascular endothelial growth factor in the pathophysiology of nonalcoholic steatohepatitis in two rodent models. Hepatology 57, 1793–1805 (2013).
Lefere, S. et al. Angiopoietin-2 promotes pathological angiogenesis and is a therapeutic target in murine nonalcoholic fatty liver disease. Hepatology 69, 1087–1104 (2019).
Ibrahim, S. H. Sinusoidal endotheliopathy in nonalcoholic steatohepatitis: therapeutic implications. Am. J. Physiol. Gastrointest. Liver Physiol. 321, G67–G74 (2021).
Piersma, B., Hayward, M. K. & Weaver, V. M. Fibrosis and cancer: a strained relationship. Biochim. Biophys. Acta Rev. Cancer 1873, 188356 (2020).
Zhang, D. Y. & Friedman, S. L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 56, 769–775 (2012).
Dunham, R. M. et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J. Immunol. 190, 2009–2016 (2013).
Lei, H., Reinke, P., Volk, H. D., Lv, Y. & Wu, R. Mechanisms of immune tolerance in liver transplantation-crosstalk between alloreactive T cells and liver cells with therapeutic prospects. Front. Immunol. 10, 2667 (2019).
Van Der Meer, A. J. et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. J. Am. Med. Assoc. 308, 2584–2593 (2012).
Kamp, W. M., Sellers, C. M., Stein, S., Lim, J. K. & Kim, H. S. Impact of direct acting antivirals on survival in patients with chronic hepatitis C and hepatocellular carcinoma. Sci. Rep. 9, 17081 (2019).
Colombo, M. & Lleo, A. The impact of antiviral therapy on hepatocellular carcinoma epidemiology. Hepatic Oncol. 5, HEP03 (2018).
Kwak, M. et al. Bariatric surgery is associated with reduction in non-alcoholic steatohepatitis and hepatocellular carcinoma: a propensity matched analysis. Am. J. Surg. 219, 504–507 (2020).
Vuppalanchi, R., Noureddin, M., Alkhouri, N. & Sanyal, A. J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 18, 373–392 (2021).
Konerman, M. A., Jones, J. C. & Harrison, S. A. Pharmacotherapy for NASH: current and emerging. J. Hepatol. 68, 362–375 (2018).
Aggarwal, P., Noureddin, M., Harrison, S., Jeannin, S. & Alkhouri, N. Nonalcoholic steatohepatitis (NASH) cirrhosis: a snapshot of therapeutic agents in clinical development and the optimal design for clinical trials. Expert Opin. Investig. Drugs 31, 163–172 (2022).
Rowe, I. A., Wong, V. W. S. & Loomba, R. Treatment candidacy for pharmacologic therapies for NASH. Clin. Gastroenterol. Hepatol. 20, 1209–1217 (2022).
Montironi, C. et al. Inflamed and non-inflamed classes of HCC: a revised immunogenomic classification. Gut 72, 129–140 (2023).
Sutti, S. & Albano, E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 17, 81–92 (2020).
Wolf, M. J. et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 26, 549–564 (2014).
Shalapour, S. et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017).
Ma, C. et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257 (2016).
Brown, Z. J. et al. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4+ T cell apoptosis promoting HCC development. Cell Death Dis. 9, 620 (2018).
Heinrich, B. et al. Steatohepatitis impairs T-cell-directed immunotherapies against liver tumors in mice. Gastroenterology 160, 331–345.e6 (2021). This study reveals how steatohepatitis reduces CD4+ T cell and effector memory cell infiltration into liver tumours, which reduces the anti-tumour activity of immunotherapeutic agents.
Malehmir, M. et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 25, 641–655 (2019).
Deczkowska, A. et al. XCR1+ type 1 conventional dendritic cells drive liver pathology in non-alcoholic steatohepatitis. Nat. Med. 27, 1043–1054 (2021).
Ou, R. et al. Neutrophil depletion improves diet-induced non-alcoholic fatty liver disease in mice. Endocrine 57, 72–82 (2017).
Zhang, H., van der Windt, D. J., Ren, J., Tsung, A. & Huang, H. The role of neutrophil extracellular traps in nonalcoholic steatohepatitis-associated hepatocellular carcinoma. J. Immunol. 202 (Suppl. 1), 135.2 (2019).
Leslie, J. et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut 71, 2093–2106 (2022).
Ramadori, P., Kam, S. & Heikenwalder, M. T cells: friends and foes in NASH pathogenesis and hepatocarcinogenesis. Hepatology 75, 1038–1049 (2022).
McVey, J. C. et al. NAFLD indirectly impairs antigen-specific CD8+ T cell immunity against liver cancer in mice. iScience 25, 103847 (2022).
Cappuyns, S. et al. Pretreatment immune cell composition and checkpoint ligand expression define the response to immunotherapy in advanced HCC: a study using single-cell sequencing. Clin. Cancer Res. 28 (Suppl. 17), PR03 (2022).
Wabitsch, S. et al. Metformin treatment rescues CD8+ T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J. Hepatol. 77, 748–760 (2022).
Hoechst, B. et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology 135, 234–243 (2008).
Dudek, M. et al. Auto-aggressive CXCR6+CD8 T cells cause liver immune pathology in NASH. Nature 592, 444–449 (2021).
Ma, C. et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Aron-Wisnewsky, J. et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 17, 279–297 (2020).
Jadhav, K. & Cohen, T. S. Can you trust your gut? Implicating a disrupted intestinal microbiome in the progression of NAFLD/NASH. Front. Endocrinol. 11, 592157 (2020).
Mouries, J. et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 71, 1216–1228 (2019).
Zhu, L. et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57, 601–609 (2013).
Boursier, J. et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63, 764–775 (2016).
Jian, J. et al. Rifaximin ameliorates non-alcoholic steatohepatitis in mice through regulating gut microbiome-related bile acids. Front. Pharmacol. 13, 841132 (2022).
Gangarapu, V. et al. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 27, 840–845 (2015).
Madrid, A. M., Hurtado, C., Venegas, M., Cumsille, F. & Defilippi, C. Long-term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am. J. Gastroenterol. 96, 1251–1255 (2001).
Zucman-Rossi, J., Villanueva, A., Nault, J. C. & Llovet, J. M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149, 1226–1239.e4 (2015). Review describing the landscape of genetic alterations and mutations in HCC and their potential as biomarkers as well as the molecular classification of HCC.
Anstee, Q. M. et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 73, 505–515 (2020).
Liu, Y. L. et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 5, 4309 (2014).
Donati, B. et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 7, 4492 (2017).
Teo, K. et al. rs641738C>T near MBOAT7 is associated with liver fat, ALT and fibrosis in NAFLD: a meta-analysis. J. Hepatol. 74, 20–30 (2021).
Tan, H. L. et al. Association of glucokinase regulatory gene polymorphisms with risk and severity of non-alcoholic fatty liver disease: an interaction study with adiponutrin gene. J. Gastroenterol. 49, 1056–1064 (2014).
Kawaguchi, T. et al. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS ONE 13, e0185490 (2018).
BasuRay, S., Smagris, E., Cohen, J. C. & Hobbs, H. H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 66, 1111–1124 (2017).
BasuRay, S., Wang, Y., Smagris, E., Cohen, J. C. & Hobbs, H. H. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc. Natl Acad. Sci. USA 116, 9521–9526 (2019).
Kozlitina, J. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46, 352–356 (2014).
Prill, S. et al. The TM6SF2 E167K genetic variant induces lipid biosynthesis and reduces apolipoprotein B secretion in human hepatic 3D spheroids. Sci. Rep. 9, 11585 (2019).
Mancina, R. M. et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology 150, 1219–1230.e6 (2016).
Perez-Martinez, P. et al. Glucokinase regulatory protein genetic variant interacts with omega-3 PUFA to influence insulin resistance and inflammation in metabolic syndrome. PLoS ONE 6, e20555 (2011).
Bianco, C. et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J. Hepatol. 74, 775–782 (2021).
Hoshida, Y. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 69, 7385–7392 (2009).
Chiang, D. Y. et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 68, 6779–6788 (2008).
Seki, S. et al. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 37, 56–62 (2002).
Tanaka, S. et al. Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J. Gastroenterol. 48, 1249–1258 (2013).
Jühling, F. et al. Targeting clinical epigenetic reprogramming for chemoprevention of metabolic and viral hepatocellular carcinoma. Gut 70, 157–169 (2021).
Chang, M.-H. et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N. Engl. J. Med. 336, 1855–1859 (1997).
Papatheodoridis, G. V., Chan, H. L. Y., Hansen, B. E., Janssen, H. L. A. & Lampertico, P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J. Hepatol. 62, 956–967 (2015).
Kanwal, F. et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 153, 996–1005.e1 (2017).
Zhou, Y. Y. et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci. Rep. 6, 33743 (2016).
Singh, S., Singh, P. P., Singh, A. G., Murad, M. H. & Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 144, 323–332 (2013).
Kennedy, O. J. et al. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open 7, e013739 (2017).
Simon, T. G. et al. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N. Engl. J. Med. 382, 1018–1028 (2020).
Zheng, L. et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin. Cancer Res. 19, 5372–5380 (2013).
Buzzai, M. et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 67, 6745–6752 (2007).
Forslund, K. et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266 (2015).
Bugianesi, E. et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 100, 1082–1090 (2005).
Lee, H. & Ko, G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 80, 5935–5943 (2014).
Zhang, X. et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 60, 2008–2016 (2014).
Cao, Z. et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 71, 2286–2297 (2011).
Roudier, E., Mistafa, O. & Stenius, U. Statins induce mammalian target of rapamycin (mTOR)-mediated inhibition of Akt signaling and sensitize p53-deficient cells to cytostatic drugs. Mol. Cancer Ther. 5, 2706–2715 (2006).
Relja, B. et al. Simvastatin modulates the adhesion and growth of hepatocellular carcinoma cells via decrease of integrin expression and ROCK. Int. J. Oncol. 38, 879–885 (2011).
Yang, P. M. et al. Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer Res. 70, 7699–7709 (2010).
Sutter, A. P. et al. Cell cycle arrest and apoptosis induction in hepatocellular carcinoma cells by HMG-CoA reductase inhibitors. Synergistic antiproliferative action with ligands of the peripheral benzodiazepine receptor. J. Hepatol. 43, 808–816 (2005).
Kah, J. et al. Selective induction of apoptosis by HMG-CoA reductase inhibitors in hepatoma cells and dependence on p53 expression. Oncol. Rep. 28, 1077–1083 (2012).
Tanaka, T. et al. Inhibitory effects of chlorogenic acid, reserpine, polyprenoic acid (E-5166), or coffee on hepatocarcinogenesis in rats and hamsters. Basic Life Sci. 52, 429–440 (1990).
Cavin, C., Holzhäuser, D., Constable, A., Huggett, A. C. & Schilter, B. The coffee-specific diterpenes cafestol and kahweol protect against aflatoxin B1-induced genotoxicity through a dual mechanism. Carcinogenesis 19, 1369–1375 (1998).
Cavin, C. et al. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem. Toxicol. 40, 1155–1163 (2002).
Majer, B. J. et al. Coffee diterpenes prevent the genotoxic effects of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and N-nitrosodimethylamine in a human derived liver cell line (HepG2). Food Chem. Toxicol. 43, 433–441 (2005).
Huxley, R. et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch. Intern. Med. 169, 2053–2063 (2009).
El-Serag, H. B., Hampel, H. & Javadi, F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 4, 369–380 (2006).
Polesel, J. et al. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann. Oncol. 20, 353–357 (2009).
Galle, P. R. et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Kramer, J. R. et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology 75, 1420–1428 (2022).
Marchesini, G. et al. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Chitturi, S. et al. The Asia–Pacific working party on non-alcoholic fatty liver disease guidelines 2017 — part 2: management and special groups. J. Gastroenterol. Hepatol. 33, 86–98 (2018).
Baumeister, S. E. et al. Association between physical activity and risk of hepatobiliary cancers: a multinational cohort study. J. Hepatol. 70, 885–892 (2019).
Promrat, K. et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51, 121–129 (2010).
Lange, N. F., Radu, P. & Dufour, J. F. Prevention of NAFLD-associated HCC: role of lifestyle and chemoprevention. J. Hepatol. 75, 1217–1227 (2021).
Marrero, J. A. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68, 723–750 (2018).
Singal, A. G. et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a meta-analysis. J. Hepatol. 77, 128–139 (2022). Meta-analysis demonstrating that HCC surveillance is associated with improved early detection and survival in patients with cirrhosis.
Lo, S. T., Gane, E., Bartlett, A. & Orr, D. Clinical features and survival of non-alcoholic fatty liver disease-related hepatocellular carcinoma. Hepatol. Int. 10, S437 (2016).
Aby, E., Phan, J., Truong, E., Grotts, J. & Saab, S. Inadequate hepatocellular carcinoma screening in patients with nonalcoholic steatohepatitis cirrhosis. J. Clin. Gastroenterol. 53, 142–146 (2019).
Singal, A. G. et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev. Res. 5, 1124–1130 (2012).
Wolf, E., Rich, N. E., Marrero, J. A., Parikh, N. D. & Singal, A. G. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology 73, 713–725 (2021).
Chong, N. et al. Association between ultrasound quality and test performance for HCC surveillance in patients with cirrhosis: a retrospective cohort study. Aliment. Pharmacol. Ther. 55, 683–690 (2022).
Schoenberger, H. et al. Dynamic changes in ultrasound quality for hepatocellular carcinoma screening in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 20, 1561–1569.e4 (2022).
Tzartzeva, K. et al. Surveillance imaging and α-fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 154, 1706–1718.e1 (2018).
Singal, A. G., Lok, A. S., Feng, Z., Kanwal, F. & Parikh, N. D. Conceptual model for the hepatocellular carcinoma screening continuum: current status and research agenda. Clin. Gastroenterol. Hepatol. 20, 9–18 (2022).
Llovet, J. M., Brú, C. & Bruix, J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 19, 329–337 (1999). This paper describes the BCLC classification widely used in American and European guidelines for the management of HCC.
Llovet, J. M. et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 3, 386–401 (2022).
Koh, Y. X. et al. Liver resection for nonalcoholic fatty liver disease-associated hepatocellular carcinoma. J. Am. Coll. Surg. 229, 467–478.e1 (2019).
Foerster, F., Gairing, S. J., Müller, L. & Galle, P. R. NAFLD-driven HCC: safety and efficacy of current and emerging treatment options. J. Hepatol. 76, 446–457 (2022).
Wong, C. R., Njei, B., Nguyen, M. H., Nguyen, A. & Lim, J. K. Survival after treatment with curative intent for hepatocellular carcinoma among patients with vs without non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 46, 1061–1069 (2017).
Tan, D. J. H. et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 23, 521–530 (2022). Meta-analysis revealing that NAFLD-HCC is associated with lower rates of surveillance and cirrhosis compared with HCC due to other causes.
Petrelli, F. et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw. Open 4, e213520 (2021).
Wang, Y. G. et al. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS ONE 9, e95485 (2014).
Su, C. W. et al. Impact of steatosis on prognosis of patients with early-stage hepatocellular carcinoma after hepatic resection. Ann. Surg. Oncol. 22, 2253–2261 (2015).
Molinari, M. et al. Hepatic resection for hepatocellular carcinoma in nonalcoholic fatty liver disease: a systematic review and meta-analysis of 7226 patients. Ann. Surg. 2, e065 (2021).
Chin, K. M. et al. Outcomes after curative therapy for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: a meta-analysis and review of current literature. HPB 23, 1164–1174 (2021).
Wong, R. J. et al. Improved survival outcomes in patients with non-alcoholic steatohepatitis and alcoholic liver disease following liver transplantation: an analysis of 2002–2012 United Network for Organ Sharing data. Clin. Transplant. 28, 713–721 (2014).
Haldar, D. et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J. Hepatol. 71, 313–322 (2019).
Kern, B. et al. High incidence of hepatocellular carcinoma and postoperative complications in patients with nonalcoholic steatohepatitis as a primary indication for deceased liver transplantation. Eur. J. Gastroenterol. Hepatol. 31, 205–210 (2019).
Llovet, J. M. et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 293–313 (2021). Comprehensive analysis of the use of locoregional therapies for early-stage and intermediate-stage HCC.
Salem, R. et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology 74, 2342–2352 (2021).
Garin, E. et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 6, 17–29 (2021).
Wahl, D. R. et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J. Clin. Oncol. 34, 452–459 (2016).
Salem, R. et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 151, 1155–1163.e2 (2016).
Young, S. et al. Transarterial chemoembolization of hepatocellular carcinoma: propensity score matching study comparing survival and complications in patients with nonalcoholic steatohepatitis versus other causes cirrhosis. Cardiovasc. Interv. Radiol. 43, 65–75 (2020).
Schotten, C. et al. NAFLD-associated comorbidities in advanced stage HCC do not alter the safety and efficacy of Yttrium-90 radioembolization. Liver Cancer 8, 491–504 (2019).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008). This study demonstrates survival benefit for systemic therapy (sorafenib) in advanced HCC compared with placebo.
Bruix, J. et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J. Hepatol. 57, 821–829 (2012).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Zhu, A. X. et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 16, 859–870 (2015).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020). Phase III trial demonstrating that combination therapy (atezolizumab plus bevacizumab) is superior in terms of survival benefit compared with sorafenib in advanced-stage HCC.
Abou-Alfa, G. K. et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence 1, EVIDoa2100070 (2022).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Yau, T. et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 23, 77–90 (2022).
Finn, R. S. et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 38, 193–202 (2020).
Qin, S. et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study. J. Clin. Oncol. 40, 383 (2022).
Hause, R. J., Pritchard, C. C., Shendure, J. & Salipante, S. J. Classification and characterization of microsatellite instability across 18 cancer types. Nat. Med. 22, 1342–1350 (2016).
Sangro, B. et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 73, 1460–1469 (2020).
Haber, P. K. et al. Molecular markers of response to anti-PD1 therapy in advanced hepatocellular carcinoma. J. Clin. Oncol. 39, 4100 (2021).
Neely, J. et al. Genomic and transcriptomic analyses related to the clinical efficacy of first-line nivolumab in advanced hepatocellular carcinoma from the phase 3 CheckMate 459 trial [abstract]. Cancer Res. 82 (Suppl. 12), 2145 (2022).
Zhu, A. X. et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 28, 1599–1611 (2022).
Kelley, R. K. et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 23, 995–1008 (2022).
Llovet, J. M., Montal, R., Sia, D. & Finn, R. S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 15, 599–616 (2018).
Greten, T. F. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J. Immunother. Cancer 9, e002794 (2021).
Beste, L. A., Green, P., Berry, K., Belperio, P. & Ioannou, G. N. Hepatitis C-related hepatocellular carcinoma incidence in the Veterans Health Administration after introduction of direct-acting antivirals. JAMA 324, 1003 (2020).
Ren, Z. et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 22, 977–990 (2021).
Cheng, A. L. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 (2009).
Cheng, A. L. et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III sorafenib Asia-Pacific trial. Eur. J. Cancer 48, 1452–1465 (2012).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 282–296 (2019).
Fassio, E. et al. Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann. Hepatol. 9, 66–69 (2010).
Yapali, S. & Tozun, N. Epidemiology and viral risk factors for hepatocellular carcinoma in the Eastern Mediterranean countries. Hepatoma Res. 4, 24 (2018).
Candia, J. et al. The genomic landscape of Mongolian hepatocellular carcinoma. Nat. Commun. 11, 4383 (2020).
Ikawa-Yoshida, A. et al. Hepatocellular carcinoma in a mouse model fed a choline-deficient, L-amino acid-defined, high-fat diet. Int. J. Exp. Pathol. 98, 221–233 (2017).
Asgharpour, A. et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 65, 579–588 (2016).
Zaki, M. Y. W. et al. Key features of the environment promoting liver cancer in the absence of cirrhosis. Sci. Rep. 11, 16727 (2021).
Fujii, M. et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med. Mol. Morphol. 46, 141–152 (2013).
Liang, J. Q. et al. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat. Commun. 9, 4490 (2018).
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Tsuchida, T. et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 69, 385–395 (2018).
Nakagawa, H. et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26, 331–343 (2014).
Gomes, A. L. et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell 30, 161–175 (2016).
Febbraio, M. A. et al. Preclinical models for studying NASH-driven HCC: how useful are they? Cell Metab. 29, 18–26 (2019).
Acknowledgements
The authors acknowledge U. Balaseviciute and J. Huguet Pradell (Translational Research in Hepatic Oncology, Liver Unit, Institut d’Investigacions Biomèdiques August Pi i Sunyer, Barcelona), and F. Castet (Gastrointestinal and Endocrine Tumour Unit, Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Barcelona) for their help in preparing the original versions of the figures for this manuscript.
Author information
Authors and Affiliations
Contributions
Introduction (J.M.L. and S.L.F.); Epidemiology (H.B.E.-S.); Pathogenesis (S.L.F.); Molecular features (T.F.G., M.H. and C.E.W.); Surveillance and prevention (A.G.S. and C.E.W.); Locoregional therapies (A.G.S.); Systemic therapies (J.M.L. and R.S.F.); Future prospects (J.M.L. and R.S.F.); Boxes (C.E.W., R.S.F. and T.F.G.); Figures (C.E.W.); and Tables (C.E.W. and A.G.S.).
Corresponding author
Ethics declarations
Competing interests
J.M.L. received research support from Bayer HealthCare Pharmaceuticals, Eisai Inc., Bristol Myers Squibb, Boehringer-Ingelheim and Ipsen and consulting fees from Merck, Eli Lilly, Eisai Inc., Bayer HealthCare Pharmaceuticals, Bristol Myers Squibb, Exelixis, Ipsen, Genentech, Roche, Glycotest, Nucleix, Mina Alpha Ltd. and AstraZeneca. A.G.S. has served as a consultant or on advisory boards for Bayer, Eisai, Genentech, BMS, Exelixis, AstraZeneca, Wako Diagnostics, Exact Sciences, Roche, Glycotest, GRAIL and TARGET PharmaSolutions. H.B.E.-S. received research support from Glycotest, Gilead Sciences, Merck Sharp & Dohme BV and AbbVie. R.S.F. reports consulting fees from AstraZeneca, Bayer, CStone, BMS, Eisai, Exilixis, Eli Lilly, Pfizer, Merck, Roche/Genentech and Hengrui. S.L.F. is a consultant to 89 Bio, Amgen, Axcella Health, Blade Therapeutics, Bristol Myers Squibb, Can-Fite Biopharma, Casma Therapeutics, ChemomAb, Escient Pharmaceuticals, Forbion, Galmed, Gordian Biotechnology, Glycotest, Glympse Bio, Insitro, Morphic Therapeutics, North Sea Therapeutics, Novartis, Ono Pharmaceuticals, Pfizer Pharmaceuticals, Scholar Rock and Surrozen and has stock options (all less than 1% of company value) in Blade Therapeutics, Escient, Galectin, Galmed, Genfit, Glympse, Hepgene, Lifemax, Metacrine, Morphic Therapeutics, Nimbus, North Sea Therapeutics, Scholar Rock and Surrozen. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Jean-Francois Dufour, James Harding and Frank Tacke for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Llovet, J.M., Willoughby, C.E., Singal, A.G. et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol 20, 487–503 (2023). https://doi.org/10.1038/s41575-023-00754-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00754-7
This article is cited by
-
Unraveling the underlying pathogenic factors driving nonalcoholic steatohepatitis and hepatocellular carcinoma: an in-depth analysis of prognostically relevant gene signatures in hepatocellular carcinoma
Journal of Translational Medicine (2024)
-
Impact of underlying liver disease on unresectable hepatocellular carcinoma treated with immune checkpoint inhibitors
BJC Reports (2024)
-
Cryptogenic non-cirrhotic HCC: Clinical, prognostic and immunologic aspects of an emerging HCC etiology
Scientific Reports (2024)
-
Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma
Nature Reviews Clinical Oncology (2024)
-
Physiologically Based Pharmacokinetic (PBPK) Model Predictions of Disease Mediated Changes in Drug Disposition in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)
Pharmaceutical Research (2024)