Abstract
DNA-encoded chemical libraries (DELs) have become a powerful technology platform in drug discovery. Dual-pharmacophore DELs display two sets of small molecules at the termini of DNA duplexes, thereby enabling the identification of synergistic binders against biological targets, and have been successfully applied in fragment-based ligand discovery and affinity maturation of known ligands. However, dual-pharmacophore DELs identify separate binders that require subsequent linking to obtain the full ligands, which is often challenging. Here we report a protein-templated DEL selection approach that can identify full ligand/inhibitor structures from DNA-encoded dynamic libraries (DEDLs) without the need for subsequent fragment linking. Our approach is based on dynamic DNA hybridization and target-templated in situ ligand synthesis, and it incorporates and encodes the linker structures in the library, along with the building blocks, to be sampled by the target protein. To demonstrate the performance of this method, 4.35-million- and 3.00-million-member DEDLs with different library architectures were prepared, and hit selection was achieved against four therapeutically relevant target proteins.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article, the associated Source Data files, Supplementary Information figures and Extended Data figures. All data are also available at figshare (https://doi.org/10.6084/m9.figshare.24297505). All the published tools and packages used for data analysis are provided with the paper. The Protein Data Bank references can be accessed at https://www.rcsb.org/ under accession codes 1ZPA (HIV-1 PR), 6VW1 (S-protein/ACE2 complex) and 7NIO (SARS-CoV-2 NSP13 helicase). Source data are provided with this paper.
Code availability
The custom Python scripts for sequencing data analysis have been made freely available for download at GitHub (https://github.com/GAOYingHKU/DEL_data_analysis; https://github.com/GAOYingHKU/DEL_data_analysis/tree/main/additional_script).
References
Brenner, S. & Lerner, R. A. Encoded combinatorial chemistry. Proc. Natl Acad. Sci. USA 89, 5381–5383 (1992).
Needels, M. C. et al. Generation and screening of an oligonucleotide-encoded synthetic peptide library. Proc. Natl Acad. Sci. USA 90, 10700–10704 (1993).
Huang, Y., Li, Y. & Li, X. Strategies for developing DNA-encoded libraries beyond binding assays. Nat. Chem. 14, 129–140 (2022).
Satz, A. L., Kuai, L. & Peng, X. Selections and screenings of DNA-encoded chemical libraries against enzyme and cellular targets. Bioorg. Med. Chem. Lett. 39, 127851 (2021).
Kodadek, T., Paciaroni, N. G., Balzarini, M. & Dickson, P. Beyond protein binding: recent advances in screening DNA-encoded libraries. Chem. Commun. 55, 13330–13341 (2019).
Fitzgerald, P. R. & Paegel, B. M. DNA-encoded chemistry: drug discovery from a few good reactions. Chem. Rev. 121, 7155–7177 (2021).
Götte, K., Chines, S. & Brunschweiger, A. Reaction development for DNA-encoded library technology: from evolution to revolution? Tetrahedron Lett. 61, 151889 (2020).
Patel, S., Badir, S. O. & Molander, G. A. Developments in photoredox-mediated alkylation for DNA-encoded libraries. Trends Chem. 3, 161–175 (2021).
Dickson, P. & Kodadek, T. Chemical composition of DNA-encoded libraries, past present and future. Org. Biomol. Chem. 17, 4676–4688 (2019).
Conole, D., J, H. H. & M, J. W. The maturation of DNA encoded libraries: opportunities for new users. Future Med. Chem. 13, 173–191 (2021).
Flood, D. T., Kingston, C., Vantourout, J. C., Dawson, P. E. & Baran, P. S. DNA encoded libraries: a visitor’s guide. Isr. J. Chem. 60, 268–280 (2020).
Lenci, E., Baldini, L. & Trabocchi, A. Diversity-oriented synthesis as a tool to expand the chemical space of DNA-encoded libraries. Bioorg. Med. Chem. 41, 116218 (2021).
Fair, R. J., Walsh, R. T. & Hupp, C. D. The expanding reaction toolkit for DNA-encoded libraries. Bioorg. Med. Chem. Lett. 51, 128339 (2021).
Gerry, C. J. & Schreiber, S. L. Recent achievements and current trajectories of diversity-oriented synthesis. Curr. Opin. Chem. Biol. 56, 1–9 (2020).
Kolmel, D. K. et al. Employing photocatalysis for the design and preparation of DNA-encoded libraries: a case study. Chem. Rec. 21, 616–630 (2021).
Anderson, M. J. et al. in DNA-Encoded Libraries (eds Brunschweiger, A. & Young, D. W.) 65–121 (Springer, 2022).
Shi, Y., Wu, Y., Yu, J., Zhang, W. & Zhuang, C. DNA-encoded libraries (DELs): a review of on-DNA chemistries and their output. RSC Adv. 11, 2359–2376 (2021).
Luk, K.-C. & Satz, A. L. in A Handbook for DNA‐Encoded Chemistry (ed. Goodnow, R. A.) 67–98 (Wiley, 2014).
Song, M. & Hwang, G. T. DNA-encoded library screening as a core platform technology in drug discovery. Its synthetic method development and applications in DEL synthesis. J. Med. Chem. 63, 6578–6599 (2020).
Kunig, V. B. K., Potowski, M., Klika Skopic, M. & Brunschweiger, A. Scanning protein surfaces with DNA-encoded libraries. ChemMedChem 16, 1048–1062 (2021).
Huang, Y. & Li, X. Recent advances on the selection methods of DNA-encoded libraries. ChemBioChem 22, 2384–2397 (2021).
Satz, A. L. et al. DNA-encoded chemical libraries. Nat. Rev. Methods Primers 2, 3 (2022).
Ottl, J., Leder, L., Schaefer, J. V. & Dumelin, C. E. Encoded library technologies as integrated lead finding platforms for drug discovery. Molecules 24, 1629 (2019).
Plais, L. & Scheuermann, J. Macrocyclic DNA-encoded chemical libraries: a historical perspective. RSC Chem. Biol. 3, 7–17 (2022).
Gironda-Martínez, A., Donckele, E. J., Samain, F. & Neri, D. DNA-Encoded chemical libraries: a comprehensive review with succesful stories and future challenges. ACS Pharmacol. Trans. Sci. 4, 1265–1279 (2021).
Yuen, L. H. & Franzini, R. M. Achievements, challenges and opportunities in DNA-encoded library research: an academic point of view. ChemBioChem 18, 829–836 (2017).
Neri, D. & Lerner, R. A. DNA-encoded chemical libraries: a selection system based on endowing organic compounds with amplifiable information. Annu. Rev. Biochem. 87, 479–502 (2018).
Goodnow, R. A. Jr., Dumelin, C. E. & Keefe, A. D. DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 16, 131–147 (2017).
Kunig, V., Potowski, M., Gohla, A. & Brunschweiger, A. DNA-encoded libraries—an efficient small molecule discovery technology for the biomedical sciences. Biol. Chem. 399, 691–710 (2018).
Huang, Y., Savych, O., Moroz, Y., Chen, Y. Y. & Goodnow, R. A. DNA-encoded library chemistry: amplification of chemical reaction diversity for the exploration of chemical space. Aldrichim Acta 52, 75–87 (2019).
Reddavide, F. V., Thompson, M., Mannocci, L. & Zhang, Y. X. DNA-encoded fragment libraries: dynamic assembly, single-molecule detection and high-throughput hit validation. Aldrichim Acta 52, 63–74 (2019).
Goodnow, R. A. & Davie, C. P. DNA-encoded library technology: a brief guide to its evolution and impact on drug discovery. Ann. Rep. Med. Chem. 50, 1–15 (2017).
Dockerill, M. & Winssinger, N. DNA-encoded libraries: towards harnessing their full power with Darwinian evolution. Angew. Chem. Int. Ed. 62, e202215542 (2023).
Peterson, A. A. & Liu, D. R. Small-molecule discovery through DNA-encoded libraries. Nat. Rev. Drug Discov. 22, 699–722 (2023).
Clark, M. A. et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol. 5, 647–654 (2009).
Melkko, S., Scheuermann, J., Dumelin, C. E. & Neri, D. Encoded self-assembling chemical libraries. Nat. Biotechnol. 22, 568–574 (2004).
Wichert, M. et al. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat. Chem. 7, 241–249 (2015).
Scheuermann, J. & Neri, D. Dual-pharmacophore DNA-encoded chemical libraries. Curr. Opin. Chem. Biol. 26, 99–103 (2015).
Dal Corso, A., Catalano, M., Schmid, A., Scheuermann, J. & Neri, D. Affinity enhancement of protein ligands by reversible covalent modification of neighboring lysine residues. Angew. Chem. Int. Ed. 57, 17178–17182 (2018).
Zimmermann, G. et al. A specific and covalent JNK-1 ligand selected from an encoded self-assembling chemical library. Chem. Eur. J. 23, 8152–8155 (2017).
Catalano, M. et al. Discovery, affinity maturation and multimerization of small molecule ligands against human tyrosinase and tyrosinase-related protein 1. RSC Med. Chem. 12, 363–369 (2020).
Oehler, S., Plais, L., Bassi, G., Neri, D. & Scheuermann, J. Modular assembly and encoding strategies for dual-display DNA-encoded chemical libraries. Chem. Commun. 57, 12289–12292 (2021).
Plais, L. et al. Universal encoding of next generation DNA-encoded chemical libraries. Chem. Sci. 13, 967–974 (2022).
Kuai, L., O'Keeffe, T. & Arico-Muendel, C. Randomness in DNA encoded library selection data can be modeled for more reliable enrichment calculation. SLAS Discov 23, 405–416 (2018).
Satz, A. L., Hochstrasser, R. & Petersen, A. C. Analysis of current DNA encoded library screening data indicates higher false negative rates for numerically larger libraries. ACS Comb. Sci. 19, 234–238 (2017).
Satz, A. L. Simulated screens of DNA encoded libraries: the potential influence of chemical synthesis fidelity on interpretation of structure-activity relationships. ACS Comb. Sci. 18, 415–424 (2016).
Pianowski, Z. L. & Winssinger, N. Nucleic acid encoding to program self-assembly in chemical biology. Chem. Soc. Rev. 37, 1330–1336 (2008).
Zambaldo, C., Barluenga, S. & Winssinger, N. PNA-encoded chemical libraries. Curr. Opin. Chem. Biol. 26, 8–15 (2015).
Daguer, J. P. et al. DNA display of fragment pairs as a tool for the discovery of novel biologically active small molecules. Chem. Sci. 6, 739–744 (2015).
Daguer, J. P., Ciobanu, M., Alvarez, S., Barluenga, S. & Winssinger, N. DNA-templated combinatorial assembly of small molecule fragments amenable to selection/amplification cycles. Chem. Sci. 2, 625–632 (2011).
Ciobanu, M. et al. Selection of a synthetic glycan oligomer from a library of DNA-templated fragments against DC-SIGN and inhibition of HIV gp120 binding to dendritic cells. Chem. Commun. 47, 9321–9323 (2011).
Vummidi, B. R. et al. A mating mechanism to generate diversity for the Darwinian selection of DNA-encoded synthetic molecules. Nat. Chem. 14, 141–152 (2022).
Diezmann, F. & Seitz, O. DNA-guided display of proteins and protein ligands for the interrogation of biology. Chem. Soc. Rev. 40, 5789–5801 (2011).
Winssinger, N. Nucleic acid-programmed assemblies: translating instruction into function in chemical biology. Chimia 67, 340–348 (2013).
Barluenga, S. et al. Novel PTP1B inhibitors identified by DNA display of fragment pairs. Bioorg. Med. Chem. Lett. 26, 1080–1085 (2016).
Eberhard, H., Diezmann, F. & Seitz, O. DNA as a molecular ruler: interrogation of a tandem SH2 domain with self-assembled, bivalent DNA-peptide complexes. Angew. Chem. Int. Ed. 50, 4146–4150 (2011).
Yeldell, S. B. & Seitz, O. Nucleic acid constructs for the interrogation of multivalent protein interactions. Chem. Soc. Rev. 49, 6848–6865 (2020).
Bandlow, V. et al. Spatial screening of hemagglutinin on Influenza A virus particles: sialyl-LacNAc displays on DNA and PEG scaffolds reveal the requirements for bivalency enhanced interactions with weak monovalent binders. J. Am. Chem. Soc. 139, 16389–16397 (2017).
Saarbach, J., Sabale, P. M. & Winssinger, N. Peptide nucleic acid (PNA) and its applications in chemical biology, diagnostics and therapeutics. Curr. Opin. Chem. Biol. 52, 112–124 (2019).
Spinelli, N., Defrancq, E. & Morvan, F. Glycoclusters on oligonucleotide and PNA scaffolds: synthesis and applications. Chem. Soc. Rev. 42, 4557–4573 (2013).
Morvan, F., Vidal, S., Souteyrand, E., Chevolot, Y. & Vasseur, J. J. DNA glycoclusters and DNA-based carbohydrate microarrays: from design to applications. RSC Adv. 2, 12043–12068 (2012).
Bigatti, M. et al. Impact of a central scaffold on the binding affinity of fragment pairs isolated from DNA-encoded self-assembling chemical libraries. ChemMedChem 12, 1748–1752 (2017).
Chung, S., Parker, J. B., Bianchet, M., Amzel, L. M. & Stivers, J. T. Impact of linker strain and flexibility in the design of a fragment-based inhibitor. Nat. Chem. Biol. 5, 407–413 (2009).
Deng, Y. et al. Selection of DNA-encoded dynamic chemical libraries for direct inhibitor discovery. Angew. Chem. Int. Ed. 59, 14965–14972 (2020).
Cui, M. et al. Trio-pharmacophore DNA-encoded chemical library for simultaneous selection of fragments and linkers. Nat. Commun. 14, 1481 (2023).
Shi, B., Zhou, Y. & Li, X. Y. Recent advances in DNA-encoded dynamic libraries. RSC Chem. Biol. 3, 407–419 (2022).
Erlanson, D. A., Fesik, S. W., Hubbard, R. E., Jahnke, W. & Jhoti, H. Twenty years on: the impact of fragments on drug discovery. Nat. Rev. Drug Discov. 15, 605–619 (2016).
Rees, D. C., Congreve, M., Murray, C. W. & Carr, R. Fragment-based lead discovery. Nat. Rev. Drug Discov. 3, 660–672 (2004).
Abendroth, F. et al. DNA-controlled bivalent presentation of ligands for the estrogen receptor. Angew. Chem. Int. Ed. 50, 8592–8596 (2011).
Scheuermann, J. et al. DNA-encoded chemical libraries for the discovery of MMP-3 inhibitors. Bioconjug. Chem. 19, 778–785 (2008).
Zhou, Y. et al. DNA-encoded dynamic chemical library and its applications in ligand discovery. J. Am. Chem. Soc. 140, 15859–15867 (2018).
Zhou, Y., Peng, J., Shen, W. & Li, X. Psoralen as an interstrand DNA crosslinker in the selection of DNA-encoded dynamic chemical library. Biochem. Biophys. Res. Commun. 533, 215–222 (2020).
Bosc, D. et al. Kinetic target-guided synthesis: reaching the age of maturity. J. Med. Chem. 63, 3817–3833 (2020).
Bosc, D., Jakhlal, J., Deprez, B. & Deprez-Poulain, R. Kinetic target-guided synthesis in drug discovery and chemical biology: a comprehensive facts and figures survey. Future Med. Chem. 8, 381–404 (2016).
Hu, X. D. & Manetsch, R. Kinetic target-guided synthesis. Chem. Soc. Rev. 39, 1316–1324 (2010).
Green, N. M. Thermodynamics of the binding of biotin and some analogues by avidin. Biochem. J. 101, 774–780 (1966).
Hirsch, J. D. et al. Easily reversible desthiobiotin binding to streptavidin, avidin and other biotin-binding proteins: uses for protein labeling, detection and isolation. Anal. Biochem. 308, 343–357 (2002).
Li, G. et al. Design, preparation and selection of DNA-encoded dynamic libraries. Chem. Sci. 6, 7097–7104 (2015).
Huang, Y. et al. Selection of DNA-encoded chemical libraries against endogenous membrane proteins on live cells. Nat. Chem. 13, 77–88 (2021).
Kibbe, W. A. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 35, W43–W46 (2007).
Hamblett, K. J. et al. A streptavidin-biotin binding system that minimizes blocking by endogenous biotin. Bioconjug. Chem. 13, 588–598 (2002).
Chen, Q. et al. Exploring the lower limit of individual DNA-encoded library molecules in selection. SLAS Discov. 25, 523–529 (2020).
Sannino, A. et al. Quantitative assessment of affinity selection performance by using DNA-encoded chemical libraries. ChemBioChem 20, 955–962 (2019).
Reddavide, F. V. et al. Second generation DNA-encoded dynamic combinatorial chemical libraries. Chem. Commun. 55, 3753–3756 (2019).
Kohl, N. E. et al. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl Acad. Sci. USA 85, 4686–4690 (1988).
Ghosh, A. K., Osswald, H. L. & Prato, G. Recent progress in the development of HIV-1 protease inhibitors for the treatment of HIV/AIDS. J. Med. Chem. 59, 5172–5208 (2016).
Whiting, M. et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. 45, 1435–1439 (2006).
Brik, A. et al. Rapid diversity-oriented synthesis in microtiter plates for in situ screening of HIV protease inhibitors. ChemBioChem 4, 1246–1248 (2003).
Cheeseman, J. D., Corbett, A. D., Gleason, J. L. & Kazlauskas, R. J. Receptor-assisted combinatorial chemistry: thermodynamics and kinetics in drug discovery. Chem. Eur. J. 11, 1708–1716 (2005).
Brik, A. et al. 1,2,3-triazole as a peptide surrogate in the rapid synthesis of HIV-1 protease inhibitors. ChemBioChem 6, 1167–1169 (2005).
Bassi, G. et al. A single-stranded DNA-encoded chemical library based on a stereoisomeric scaffold enables ligand discovery by modular assembly of building blocks. Adv. Sci. 7, 2001970 (2020).
Gironda-Martinez, A., Neri, D., Samain, F. & Donckele, E. J. DNA-compatible diazo-transfer reaction in aqueous media suitable for DNA-encoded chemical library synthesis. Org. Lett. 21, 9555–9558 (2019).
Wrapp, D. et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263 (2020).
Walls, A. C. et al. Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281–292 (2020).
Kleiner, R. E., Dumelin, C. E., Tiu, G. C., Sakurai, K. & Liu, D. R. In vitro selection of a DNA-templated small-molecule library reveals a class of macrocyclic kinase inhibitors. J. Am. Chem. Soc. 132, 11779–11791 (2010).
Lossouarn, A., Renard, P.-Y. & Sabot, C. Tailored bioorthogonal and bioconjugate chemistry: a source of inspiration for developing kinetic target-guided synthesis strategies. Bioconjug. Chem. 32, 63–72 (2021).
Jaegle, M. et al. Protein-templated fragment ligations-from molecular recognition to drug discovery. Angew. Chem. Int. Ed. 56, 7358–7378 (2017).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective ‘ligation’ of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Zhang, L. et al. Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc. 127, 15998–15999 (2005).
Johansson, J. R., Beke-Somfai, T., Said Stålsmeden, A. & Kann, N. Ruthenium-catalyzed azide alkyne cycloaddition reaction: scope, mechanism and applications. Chem. Rev. 116, 14726–14768 (2016).
Boren, B. C. et al. Ruthenium-catalyzed azide-alkyne cycloaddition: scope and mechanism. J. Am. Chem. Soc. 130, 8923–8930 (2008).
Ferrini, S. et al. Ruthenium-catalyzed synthesis of 5-amino-1,2,3-triazole-4-carboxylates for triazole-based scaffolds: beyond the Dimroth rearrangement. J. Org. Chem. 80, 2562–2572 (2015).
Rasmussen, L. K., Boren, B. C. & Fokin, V. V. Ruthenium-catalyzed cycloaddition of aryl azides and alkynes. Org. Lett. 9, 5337–5339 (2007).
Montoya, A. L. et al. Combining pharmacophore models derived from DNA-encoded chemical libraries with structure-based exploration to predict Tankyrase 1 inhibitors. Eur. J. Med. Chem. 246, 114980 (2022).
Arena, B. J. Deactivation of ruthenium catalysts in continuous glucose hydrogenation. Appl. Catal. A 87, 219–229 (1992).
V’kovski, P., Kratzel, A., Steiner, S., Stalder, H. & Thiel, V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19, 155–170 (2021).
Newman, J. A. et al. Structure, mechanism and crystallographic fragment screening of the SARS-CoV-2 NSP13 helicase. Nat. Commun. 12, 4848 (2021).
Keum, Y.-S. & Jeong, Y.-J. Development of chemical inhibitors of the SARS coronavirus: viral helicase as a potential target. Biochem. Pharmacol. 84, 1351–1358 (2012).
Jia, Z. et al. Delicate structural coordination of the Severe Acute Respiratory Syndrome coronavirus Nsp13 upon ATP hydrolysis. Nucleic Acids Res. 47, 6538–6550 (2019).
Shu, T. et al. SARS-coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 35, 321–329 (2020).
Yang, N. et al. Bismuth complexes inhibit the SARS coronavirus. Angew. Chem. Int. Ed. 46, 6464–6468 (2007).
Upadhaya, S., Neftelinov, S. T., Hodge, J. & Campbell, J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat. Rev. Drug Discov. 21, 482–483 (2022).
Levy, D. E. & Darnell, J. E. STATs: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651–662 (2002).
Bunting, K. D. STAT5 signaling in normal and pathologic hematopoiesis. Front. Biosci. 12, 2807–2820 (2007).
Manaswiyoungkul, P. et al. Optimization of a high-throughput fluorescence polarization assay for STAT5B DNA binding domain-targeting inhibitors. J. Pharm. Biomed. Anal. 184, 113182 (2020).
Catalano, M. et al. Selective fragments for the CREBBP bromodomain identified from an encoded self-assembly chemical library. ChemMedChem 15, 1752–1756 (2020).
Melkko, S., Zhang, Y., Dumelin, C. E., Scheuermann, J. & Neri, D. Isolation of high-affinity trypsin inhibitors from a DNA-encoded chemical library. Angew. Chem. Int. Ed. 46, 4671–4674 (2007).
Riching, K. M., Caine, E. A., Urh, M. & Daniels, D. L. The importance of cellular degradation kinetics for understanding mechanisms in targeted protein degradation. Chem. Soc. Rev. 51, 6210–6221 (2022).
Boutureira, O. & Bernardes, G. J. Advances in chemical protein modification. Chem. Rev. 115, 2174–2195 (2015).
Zuily, L. et al. Copper induces protein aggregation, a toxic process compensated by molecular chaperones. mBio 13, e03251-21 (2022).
Ingle, A. P., Duran, N. & Rai, M. Bioactivity, mechanism of action and cytotoxicity of copper-based nanoparticles: a review. Appl. Microbiol. Biotechnol. 98, 1001–1009 (2014).
Gutteridge, J. M. & Wilkins, S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim. Biophys. Acta 759, 38–41 (1983).
Cervantes-Cervantes, M. P., Calderón-Salinas, J. V., Albores, A. & Muñoz-Sánchez, J. L. Copper increases the damage to DNA and proteins caused by reactive oxygen species. Biol. Trace Elem. Res. 103, 229–248 (2005).
Parker, C. G. & Pratt, M. R. Click chemistry in proteomic investigations. Cell 180, 605–632 (2020).
Pickens, C. J., Johnson, S. N., Pressnall, M. M., Leon, M. A. & Berkland, C. J. Practical considerations, challenges and limitations of bioconjugation via azide-alkyne cycloaddition. Bioconjug. Chem. 29, 686–701 (2018).
Darabedian, N. & Pratt, M. R. in Methods Enzymology Vol. 622. (ed. Shukla, A. K.) 293–307 (Academic, 2019).
Schneider, D. et al. Anionic surfactants enhance click reaction-mediated protein conjugation with ubiquitin. Bioorg. Med. Chem. 24, 995–1001 (2016).
Dang, C. V., Reddy, E. P., Shokat, K. M. & Soucek, L. Drugging the ‘undruggable’ cancer targets. Nat. Rev. Cancer 17, 502–508 (2017).
Dale, B. et al. Advancing targeted protein degradation for cancer therapy. Nat. Rev. Cancer 21, 638–654 (2021).
White, M. A., Lin, W. & Cheng, X. Discovery of COVID-19 inhibitors targeting the SARS-CoV-2 Nsp13 helicase. J. Phys. Chem. Lett. 11, 9144–9151 (2020).
Lim, C. T. et al. Identifying SARS-CoV-2 antiviral compounds by screening for small molecule inhibitors of Nsp3 papain-like protease. Biochem. J 478, 2517–2531 (2021).
Corona, A. et al. Natural compounds inhibit SARS-CoV-2 nsp13 unwinding and ATPase enzyme activities. ACS Pharmacol. Transl. Sci. 5, 226–239 (2022).
Shi, B., Deng, Y., Zhao, P. & Li, X. Selecting a DNA-encoded chemical library against non-immobilized proteins using a ‘Ligate-Cross-Link-Purify’ strategy. Bioconjug. Chem. 28, 2293–2301 (2017).
Zhou, Y., Shen, W., Peng, J., Deng, Y. & Li, X. Identification of isoform/domain-selective fragments from the selection of DNA-encoded dynamic library. Bioorg. Med. Chem. 45, 116328 (2021).
Monty, O. B. C., Simmons, N., Chamakuri, S., Matzuk, M. M. & Young, D. W. Solution-phase fmoc-based peptide synthesis for DNA-encoded chemical libraries: reaction conditions, protecting group strategies and pitfalls. ACS Comb. Sci. 22, 833–843 (2020).
Tse, B. N., Snyder, T. M., Shen, Y. H. & Liu, D. R. Translation of DNA into a library of 13,000 synthetic small-molecule macrocycles suitable for in vitro selection. J. Am. Chem. Soc. 130, 15611–15626 (2008).
Ewing, B. & Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8, 186–194 (1998).
Durbin, R. & Dear, S. Base qualities help sequencing software. Genome Res. 8, 161–162 (1998).
Acknowledgements
This work was supported by grants from the Shenzhen Bay Laboratory, Shenzhen, China (SZBL2020090501008), the Research Grants Council of Hong Kong SAR, China (AoE/P-705/16, 17301118, 17111319, 17303220, 17300321, 17318322, C7005-20G, C7016-22G and 2122-7S04), NSFC of China (21877093, 22222702 and 91953119), GuangDong Basic and Applied Basic Research Foundation (2023A1515010711), the Fundamental Research Funds for the Central Universities (2022CDJQY-001) and Beijing National Laboratory for Molecular Sciences (BNLMS202104). We acknowledge support from the ‘Laboratory for Synthetic Chemistry and Chemical Biology’ under the Health@InnoHK Program and State Key Laboratory of Synthetic Chemistry by Innovation and Technology Commission, Hong Kong SAR, China. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank Y. Jia of The University of Hong Kong for assistance with the physiochemical property analysis of the hit compounds.
Author information
Authors and Affiliations
Contributions
Y.Z., W.S., H.S., Y.C. and X.L. conceived and designed the experiments. Y.Z., W.S., J.P, Q.L., X.W., S.L., F.S.L., J.M.-L. and G.Z. performed the experiments. Y.Z., W.S., Y.G., G.L., Y.L. and X.L. analysed the data. Y.Z., W.S., H.S., Y.C. and X.L. co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The University of Hong Kong has filed two patent applications on the chemical structures of the inhibitors of S-protein (Chinese patent application no. 202310194549.8) and NSP13 helicase (US provisional patent application no. 63/494,329). X.L., H.S., W.S., Y.Z., X.W. and G.L. are the inventors. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Alexander Satz, Yixin Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
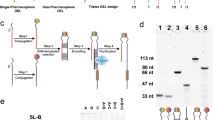
Extended Data Fig. 1 Model selections with streptavidin.
a. A model library consisting of DB-DNA and amino-DNA (1:9) was selected against streptavidin, CA-2, or without protein. The linker structures are the same as A-1/B-1. The products were gel-purified, amplified by relay primer bypass PCR, and decoded with Sanger sequencing. b-d. Sanger sequencing results. The colored peaks represent individual bases (green: A; blue: C; red: T; black: G). The bar graph data in the background represent sequencing quality score for each base, which is calculated based on the estimated error probability for the base call as previously described137,138.
Extended Data Fig. 2 Model selections with HIV-1 PR.
a. The model library in Fig. 3e was selected against HIV-1 PR and the selection results were analyzed by Sanger sequencing. b. The same model library was subjected to the selection conditions without protein as a control experiment. The colored peaks represent individual bases (green: A; blue: C; red: T; black: G). The bar graph data in the background represent sequencing quality score for each base, which is calculated based on the estimated error probability for the base call as previously reported137,138.
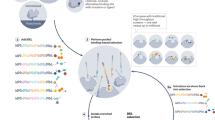
Extended Data Fig. 3 Two large DEDLs were prepared for the selection.
R1, R2: building blocks; L: linker. The number of the building blocks and linkers in each library are shown. In sub-library A, R1 is installed before L in DEDL-1 and after L in DEDL-2, both using amidation reactions. Both libraries share the same sub-library B containing the same set of R2, which was installed by direct coupling to the respective DNA via amidation reactions. The azide group was synthesized through on-DNA diazo-transfer reactions from the amino group. See the Supplementary Information for details on DNA sequences, building block structures, library synthesis, and characterization data (Supplementary Figs. 14–17).
Extended Data Fig. 4 Docking analysis of the selected S-protein inhibitors.
a. Crystal structure of the S-protein/ACE2 binding complex (PDB: 6VW1). The interface is highlighted in yellow and the residues involved are shown in sticks. b. S-10 (62-39-224, 1,4-isomer) is docked to S-protein and the detailed interactions are highlighted; the small molecule is shown in green and the amino acids are shown yellow. The hydrogen bonds are shown as dashed lines. c. Comparison of the binding sites of S-10, S-18 (the 1,5-isomer), and S-13 (62C-39-224), highlighted in yellow.
Extended Data Fig. 5 Characterization of the hit compounds from NPS13 selection.
a. Gel analysis of the selections. Top panel: after the selection; bottom panel: after relay primer bypass PCR; see complete gel images in Supplementary Figs. 46, 47. The gel analysis was performed three times with independent samples. All experiments were reproduced with similar results. b. NSP13 helicase ATPase activity assay of the compounds (200 µM); n = 3 independent experiments; data are presented as mean values ± s.d. c. Docking analysis using the crystal structure of NSP13 helicase (PDB: 7NIO). The ATP-binding site is enlarged; top panel: interaction map of 73-1-143; bottom panel: overlay of 73-1-143, ATP, and ADP; small molecules are shown as sticks and amino acids are shown yellow.
Extended Data Fig. 6 Selection against PD-L1 and STAT5b and characterization of the hit compounds.
a. 2D scatter plots of the selection results with PD-L1. x-axis: post-selection sequence count; y-axis: enrichment fold. b. Results of the time-resolved fluorescence energy transfer (TR-FRET) PD-L1/PD-1 binding assay. Compounds were tested at 1 mM. c. Inhibition titration curve of P-3 (219-16-170, 1,4-isomer) to determine the IC50 values. d. 2D scatter plots of the selection results with STAT5b. x-axis: post-selection sequence count; y-axis: enrichment fold. e. Fluorescence polarization assay to determine the inhibition activity against STAT5b/DNA binding; FAM: fluorescein. f. Results of the fluorescence polarization assay; the compounds were tested at 2 mM. Selection gel images are shown in Supplementary Figs. 46, 47. In b, c, and f, n = 3 independent experiments; data are presented as mean values ± s.d.
Supplementary information
Supplementary Information
Supplementary Figs. 1–47, Notes, detailed experimental procedure, additional discussion and other additional information related to this study.
Supplementary Table 1
Complete list of building blocks and DNA sequences of the libraries.
Supplementary Table 2
Source data for Supplementary figures.
Supplementary Data 1
Source data for library selections in Supplementary Figs. 13–34.
Supplementary Data 2
Source data for library selections in Supplementary Figs. 35–39.
Source data
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 3
Unprocessed gels.
Source Data Fig. 3
Library selection source data.
Source Data Fig. 4
Unprocessed gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Library selection source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Library selection source data.
Source Data Fig. 6
Library selection source data.
Source Data Extended Data Fig. 5
Unprocessed gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Library selection source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Shen, W., Gao, Y. et al. Protein-templated ligand discovery via the selection of DNA-encoded dynamic libraries. Nat. Chem. 16, 543–555 (2024). https://doi.org/10.1038/s41557-024-01442-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-024-01442-y