Abstract
In the early stages of diabetic retinopathy (DR), diabetes-related hyperglycemia directly inhibits the AKT signaling pathway by increasing oxidative stress or inhibiting growth factor expression, which leads to retinal cell apoptosis, nerve proliferation and fundus microvascular disease. However, due to compensatory vascular hyperplasia in the late stage of DR, the vascular endothelial growth factor (VEGF)/phosphatidylinositol 3 kinase (PI3K)/AKT cascade is activated, resulting in opposite levels of AKT regulation compared with the early stage. Studies have shown that many factors, including insulin, insulin-like growth factor-1 (IGF-1), VEGF and others, can regulate the AKT pathway. Disruption of the insulin pathway decreases AKT activation. IGF-1 downregulation decreases the activation of AKT in DR, which abrogates the neuroprotective effect, upregulates VEGF expression and thus induces neovascularization. Although inhibiting VEGF is the main treatment for neovascularization in DR, excessive inhibition may lead to apoptosis in inner retinal neurons. AKT pathway substrates, including mammalian target of rapamycin (mTOR), forkhead box O (FOXO), glycogen synthase kinase-3 (GSK-3)/nuclear factor erythroid 2-related factor 2 (Nrf2), and nuclear factor kappa-B (NF-κB), are a research focus. mTOR inhibitors can delay or prevent retinal microangiopathy, whereas low mTOR activity can decrease retinal protein synthesis. Inactivated AKT fails to inhibit FOXO and thus causes apoptosis. The GSK-3/Nrf2 cascade regulates oxidation and inflammation in DR. NF-κB is activated in diabetic retinas and is involved in inflammation and apoptosis. Many pathways or vital activities, such as the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and mitogen-activated protein kinase (MAPK) signaling pathways, interact with the AKT pathway to influence DR development. Numerous regulatory methods can simultaneously impact the AKT pathway and other pathways, and it is essential to consider both the connections and interactions between these pathways. In this review, we summarize changes in the AKT signaling pathway in DR and targeted drugs based on these potential sites.
Similar content being viewed by others
Facts
-
DR is associated with alterations in the AKT pathway, which vary among different retinal cells at different stages of the disease.
-
During the progression of DR, the AKT signaling pathway plays an important role in various retinal cells, including retinal neurons, retinal ganglion cells (RGCs), retinal pericytes (RPCs), retinal capillary vascular endothelial cells (RCVECs), and retinal pigment epithelium cells (RPECs), regulating functions such as proliferation, apoptosis, inflammation, angiogenesis, and protein synthesis.
-
The regulation of the AKT pathway plays a significant role in the occurrence and progression of DR.
Open questions
-
How do the changes in the levels of AKT and its upstream/downstream molecules at different stages of DR impact the disease progression and the cellular activities involved?
-
What is the molecular mechanism of AKT’s role in the process of retinal cytopathic disease in DR, and what are the potential regulatory means and factors?
-
Could AKT signaling be a major target for the future treatment of DR?
Introduction
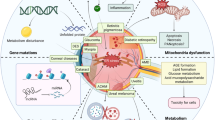
Diabetic retinopathy (DR) is the most common and serious ocular complication of diabetes mellitus and damages various structures of the eyeball (Fig. 1). The clinical signs of DR are divided into mild nonproliferative DR (NPDR) and proliferative DR(PDR) [1].
Located in the innermost layer of the eye wall, the retina consists of the retinal pigment epithelium (RPE) and nerve layer, which includes rod cells (R), cone cells (C), bipolar cells (B), horizontal cells (H), amacrine cells (A), interplexiform cells (Ip), ganglion cells (G) and Müller cells (M). The choroid is adjacent to the retina and rich in blood vessels that provide oxygen and nutrients to the outer retina. During PDR, neovascularization breaks through the choroid-retina barrier, easily leading to further diseases such as retinal detachment or glaucoma. Vascular endothelial cells and pericytes, as components of capillaries, play an important role in angiogenesis.
The blood retinal barrier (BRB) is an important structure in the retina consisting with internal components from tight junctions between retinal capillary endothelial cells and external component from tight junctions between retinal pigment epithelium cells (RPECs) [2]. The breakdown of the inner BRB is a hallmark of many degenerative retinal diseases, including DR, and can lead to changes in vascular permeability [3]. In NPDR, large quantities of studies observed BRB damage via retinal pericyte (RPC) loss, retinal capillary vascular endothelial cell (RCVEC) apoptosis and capillary basement membrane thickening. And these symptoms are accompanied by retinal microaneurysm, intraretinal hemorrhage, exudates of abnormal blood vessels and retinal neuron apoptosis. Proliferative diabetic retinopathy (PDR) is characterized by abnormal neovascularization.
The serine/threonine kinase AKT (also known as protein kinase B), which was discovered in 1987, plays an important role in regulating a variety of cellular functions, including metabolism, growth, proliferation, survival, transcription, and protein synthesis, and has become the focus of attention in the medical community [4]. Phosphatidylinositol 3 kinase (PI3K), which was discovered in 1985, is the most important activator of intracellular AKT-mediated signal transduction cascades [5, 6]. Recent discoveries and mechanistic studies on upstream regulators and downstream substrates of the AKT pathway were described in a previous review [7].
Many studies have shown that AKT signaling is attenuated in NPDR. Rats with diabetes induced by a high-fat diet exhibit low levels of AKT and p-AKT in retinal tissues, and this low expression is accompanied by the induction of ROS and decreased phosphorylated mammalian target of rapamycin (mTOR) and nitric oxide synthase, which synthesize NO and affect vascular permeability [8, 9]. Clinical studies on diabetics and nondiabetics examined the changes and the anti-apoptotic effects of AKT in retinal ganglion cells (RGCs) using immunohistochemical techniques, and the expression of the mitochondrial proteins cytochrome c, apoptosis-inducing factor (AIF) and Bad was found to be upregulated in the diabetic retina [10]. Gene Ontology and pathway analyses have shown significant enrichment of the PI3K/AKT signaling pathway in the DR rat retina [11, 12].
However, the AKT pathway is not always inhibited during DR, particularly in RCVECs during neovascularization in PDR. During the early stage of diabetic retinopathy (NPDR), the expression of AKT and p-AKT in the retina exhibited a decrease at 8 and 12 weeks after diabetes induction, but displayed an increase at 4 weeks in a study using streptozotocin-induced diabetic rats [13]. This emphasizes the dynamic changes in AKT signaling during the progression of DR. Additionally, other studies have shown that phosphoenolpyruvate carboxykinase 1 expression is upregulated and AKT is overactivated in the proliferative retinal tissues of PDR patients [14, 15]. The overexpression of phosphoenolpyruvate carboxykinase 1-induced heterotrimeric G proteins promotes vascular endothelial growth factor (VEGF)/VEGFR2-induced endocytosis and downstream activation of AKT/mTOR and mitogen-activated protein kinase (MAPK) [14]. Proteomics analysis of the aqueous humor of PDR patients revealed enrichment of the AKT signaling pathway [16].
Although there is currently no model that can fully mimic the complete pathophysiology of neuronal and vascular changes in both NPDR and PDR, each model recapitulates many of the disease phenotypes [17]. Studies on these DR models have revealed changes and mechanisms of the AKT pathway in DR progression from various aspects. However, the main problem is that there is no retrospective summary of the changes and regulation of AKT and its related factors in the different stages of DR as well as in different retinal cells. Thus, in this review, we summarize the changes and roles of the AKT pathway in DR and review research on select regulatory molecules and drugs that affect the upstream and downstream AKT pathway in DR. We also discuss the crosstalk between the AKT pathway and other pathways involving Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling and MAPK signaling.
AKT-associated retinal destruction and neovascularization in DR
The downregulation of AKT in diabetes leads to cell damages in various cell types, such as cell apoptosis, oxidative stress, and cell cycle impairment in RPCs, cell apoptosis in RCVECs and retinal neurons [18,19,20]. AKT has been shown to protect these cells from apoptosis by affecting the activity of several transcription factors involved in the regulation of cell survival. However, diabetes and its associated oxidative/inflammatory stress promote an imbalance in the AKT pathway in the retina, leading to significant apoptosis in retinal cells, impaired mitochondrial function, and microvascular degeneration [21,22,23]. Advanced glycation end product (AGE)-modified substrates, which play important roles in the pathogenesis of diabetes, lead to RPC dysfunction and death by reducing AKT signaling and can induce endothelial to mesenchymal transition [24, 25]. Additionally, AGE receptors are associated with a variety of pathways, such as hypoxia inducible factor-1 (HIF-1), nuclear factor kappa-B (NF-κB), AKT and MAPK, that initiate and maintain an unfavorable proinflammatory state [26]. Moreover, hyperglycemia induces fibronectin, type IV collagen, and laminin expression in human RPECs through the PI3K/AKT signaling pathway, contributing to the formation of fibrotic membranes during DR development [27].
Nonetheless, based on the literature, we hypothesized that AKT is upregulated in RPECs and RCVECs during PDR [16]. The experiment confirmed that treatment with high glucose and AGE increases activation of AKT in cultured human retinal endothelial cells in vitro [28]. Due to hyperlipidemia and microcirculatory disorders, diabetes can cause tissue hypoxia as a result of decreased oxygen carrying and dispersal capacity of blood, as well as metabolic disorders leading to insufficient oxygen utilization [29, 30]. Thus, we hypothesize that retinal hypoxia could induce compensatory vascular hyperplasia, which explains the upregulation of VEGF expression and the activation of the AKT and mTOR pathways in PDR. The induction mechanism of angiogenesis has been examined, and studies have shown that under hypoxic conditions caused by diabetes, RPECs can trigger the HIF-1α pathway to release VEGF and other angiogenic factors [31,32,33]. The VEGF/VEGFR2-induced PI3K/AKT/endothelial nitric oxide synthase (eNOS) pathway then leads to the production of NO, causing physiological angiogenesis in RCVECs [34]. Certain proteins that promote angiogenesis can be directly or indirectly activated by VEGF/AKT through the intracellular pathway to promote neovascularization [35].
Inhibitors and activators of the PI3K/AKT signaling pathway are utilized in the treatment of diabetic retinopathy. Some commonly used inhibitors include LY294002, wortmannin, and GDC-0941 which suppress the activity of AKT by targeting its upstream molecules [36,37,38]. On the other hand, activators such as insulin and IGF-1 stimulate the PI3K/AKT pathway leading to a cascade of downstream effects including enhanced glucose uptake and angiogenesis [39, 40]. Additionally, AGE inhibitors such as aminoguanidine are also being studied for their potential ability to modulate the PI3K/AKT pathway in diabetic retinopathy [41].
Further research has uncovered three distinct AKT isoforms: AKT1, AKT2, and AKT3 [42]. While they exhibit an impressive 80% similarity, these homologs display interconnected roles under physiological and pathological conditions, signifying that their functions are not merely redundant. Recent findings suggest that AKT1 and AKT2 mutually regulate each other in RPECs derived from human DR tissues and diabetic mice [43]. A decrease in AKT2 within RPECs triggers a compensatory rise in AKT1, subsequently attenuating DR. Overexpression of AKT1 in RPECs counteracts the retinal abnormalities induced by diabetes, whereas the functional loss of AKT1 in RPECs accelerates retinal vascular damage in diabetic mice [43]. Notably, in RPEC cells from human DR eyes and diabetic mice, there’s an enhanced AKT2 activity, which correlates with epithelial–mesenchymal transition and cellular migration [44]. Moreover, transcriptomic analyses in RPCs reveal a marked reduction in AKT3 levels, playing a pivotal role in RPC functional deficits and BRB alterations [45]. The intricacies of these mechanisms beckon further investigation.
Upstream regulators of PI3K/AKT signaling in DR
Insulin
The receptor tyrosine kinase family, which is one of the most important upstream activators of the AKT pathway, includes 20 subfamilies [46]. Among these subfamilies, the insulin receptor family, which is downstream of insulin, can phosphorylate insulin receptor substrate (IRS) and thus activate the PI3K/AKT pathway [47]. Excess hexosamine caused by diabetes blocks the AKT-mediated neuroprotective effect of insulin, inducing apoptosis in retinal neurons [48]. Diabetes progressively impairs the constitutive retinal insulin receptor signaling pathway and decreases the AKT cascade, resulting in various pathologies, such as neuronal cell death and increased vascular permeability [49].
Mechanistically, insulin induces mRNA expression of haem oxygenase-1 (HO-1, an antioxidant and cell protector) through the IRS-1/PI3K/AKT pathway, which has anti-apoptotic properties [50]. The IRS-2 levels are reduced in streptozocin-induced diabetic rats and are responsible for the reduced activity of the retinal IRS/PI3K/AKT pathway [49]. Moreover, researchers compared the results with healthy controls and found that DR mouse RCVECs and RPCs exhibit decreased miR-126 expression, resulting in increased IRS-1 expression and decreased miR-7a cooperation with IRS-2 [51, 52]. Using mimics to induce overexpression inhibits the PI3K/AKT cascade and thereby reduces cell viability and invasion to suppress angiogenesis in PDR.
IGF-1
Insulin-like growth factor-1 (IGF-1) is a neurotrophin that has been implicated in the pathogenesis of diabetic neurological diseases [53, 54]. In diabetic patients, low expression of IGF-1, particularly in the vitreous humor, has been observed [55]. Low IGF expression may play an important role in the development of new blood vessels in diabetic patients.
Functional studies on RPECs and neuronal cells have shown that PI3K/AKT and MAPK are activated by IGF-1 for cell protection, and blocking the PI3K/AKT pathway eliminates the protective effect of IGF-1, whereas blocking the MAPK pathway is ineffective [56]. In the early stages of DR, the somatostatin levels decline with degenerative changes in photoreceptor cells because somatostatin acts as one of the most important neuroprotective factors in the retina by enhancing IGF-1-mediated AKT phosphorylation [57]. Interestingly, IGF-1 can work synergistically with dopamine to reduce angiogenesis in PDR by downregulating VEGF expression [58].
Studies have shown that therapies targeting the IGF-I signaling pathway can prevent and reverse the development of DR by regulating the AKT pathway. IGF-I treatment can prevent retinal cell death in diabetic rats and potentially help prevent neuronal cell loss in the retina in patients with diabetes [59, 60]. In mouse retinal cells, IGF-1 signaling is upregulated by silencing protein tyrosine phosphatase 1B to prevent nerve degeneration due to its AKT-regulating effect in vitro [61]. The quinic acid derivative KZ-41 functions as a survival factor for RCVECs because its inhibitory effect on caspase-3 activation is dependent on IGF-1/IRS-1/PI3K/AKT signal transduction [62]. Additionally, in RCVECs, treatment with an anti-rat integrin (IL)-associated protein antibody can attenuate aberrant IGF-1 signaling, leading to AKT activation and VEGF synthesis inhibition and thereby preventing or reversing the progression of DR [63].
Studies on IGF-1 at the RNA level offer some insight into potential treatment targets. IGF-1 is a direct target of miR-142-5p and/or miR-18b, and the downregulation of these two microRNAs in the retinal tissues of DR rats and human RCVECs stimulated with hyperglycemia leads to VEGF activation by affecting the IGF-1 and AKT signaling pathways [64, 65].
In addition, IGF-1 binding protein-3 (IGFBP-3) protects the BRB integrity, and this response is independent of IGF-1 and calcium but requires PI3K/AKT activation, suggesting a novel protective effect of IGFBP-3 on the retina [66]. miR-15b and miR-16 can increase the IGFBP-3 levels by reducing the tumor necrosis factor-α (TNF-α) and suppressor of cytokine signaling 3 signaling pathways, which increase RCVEC apoptosis by modulating IRS-1 and protect against apoptosis induced by hyperglycemia in retinal ECs [67, 68]. Therefore, we conclude that the upregulation of IGFBP-3 can have a positive effect on DR and is inextricably linked to the AKT pathway.
VEGF
Growth factors (mainly VEGF) are upregulated during hypoxia and are important stimulators of retinal neovascularization in PDR [69]. Tissue ischemia or hypoxia can lead to the production of a transcription factor named as hypoxia-inducible factor 1 (HIF-1), which binds to the VEGF gene promoter and initiates the transcription process [70]. The binding of the VEGF receptor to its ligands activates downstream signaling cascades, including the PI3K/AKT, MAPK and other signaling pathways and thereby controls RCVEC survival, proliferation and vascular formation [71].
Inhibiting VEGF and AKT has become one of the main treatments for vascular abnormalities in DR. To date, a range of pharmacological antibodies that target VEGF and prevent neovascularization and PDR progression have been designed [72,73,74,75]. New small molecules, such as JP-153 and nanomaterial gold nanoparticles, have been shown to exert therapeutic effects by affecting VEGF to inhibit AKT activation [76, 77]. Herbal or biological extracts, such as erianin, the plant proteolytic enzyme papain, tangeretin, blueberry anthocyanins, plantaginis semen, arctiin, and sanguinarine, can affect VEGF/PI3K/AKT-induced angiogenesis [78,79,80,81,82,83,84]. Quercetin, which is an effective polyphenol, can reduce diabetes and diabetic complications. Quercetin can not only inactivate NF-κB signaling by inhibiting MAPK and AKT to inhibit VEGF-induced excessive inflammation and angiogenesis of retinal photoreceptor cells but also increase the level of diabetic retinal neurotrophic factors, thus preventing retinal neurodegeneration caused by oxidation [85,86,87].
Studies on biomolecules and mechanisms provide new ideas for the treatment of DR through the VEGF/AKT pathway. Some factors or drugs that inhibit angiogenesis and damage to the BRB by inhibiting VEGF/AKT and MAPK include the endothelial growth factor receptor decoy KH902, the IL-linked kinase inhibitor QLT0267, Wnt inhibitory 1, tocotrienol, and ephrinA2/ephrinA1, which regulate vascular development during embryogenesis [88,89,90,91,92,93]. A similar protective effect of the AKT pathway was achieved by inhibiting N-methyl D-aspartate receptor, β2-glycoprotein I, and erythropoietin expression [94,95,96]. Aldose reductase inhibition can inhibit the VEGF/PI3K/AKT pathway, which has been demonstrated as an effective solution for addressing the accumulation of sorbitol and fructose in the retina of diabetic animals [97, 98]. The overexpression of the conserved endoplasmic reticulum protein Nogo-B promotes angiogenesis in PDR via the VEGF/PI3K/AKT pathway in an autocrine manner [99]. Silencing Nogo-B improves the integrity of the BRB in DR by increasing the p-AKT level and decreasing the p-ERK1/2 level [100].
VEGF can act as a stimulator for the PI3K/AKT pathway [7], while the expression of VEGF in endothelial cells is mediated by the PI3K/AKT signaling cascade [101]. It’s important to note that although the AKT-mediated pathway is one way of upregulating VEGF expression, it is not the sole mechanism [102, 103]. In recent years, research on microRNAs has developed rapidly. miR-21 and miR-19a can target and activate PTEN, which negatively mediates the PI3K/AKT/VEGF pathway, and inhibiting these two microRNAs can treat DR [104, 105]. Silencing miR-183 and miR-20b-5p can inhibit DR by regulating the VEGF/PI3K/AKT signaling pathway by upregulating BTG1 and inactivating the THBS1 gene, respectively [106, 107]. The upregulation of miR-199a-3p, miR-7, miR-20b and miR-126 can alleviate the increase in cell proliferation, migration and angiogenesis caused by hyperglycemia by reducing VEGF or blocking the VEGF/PI3K/AKT pathway [108,109,110,111,112]. In addition, lncRNA HEIH is highly expressed in the serum of patients with DR and may promote DR by sponging miR-939-targeted VEGF expression and regulating the activation of the PI3K/AKT pathway [113].
Although the current studies on VEGF inhibitors have shown potent antiangiogenic effects, other studies have shown adverse effects of excessive inhibition of this factor, which are related to cell death induced by AKT inhibition [114]. After intravitreal anti-VEGF treatment of PDR, anti-VEGF crunch syndrome may develop, which can lead to sudden blindness in severe cases [115,116,117]. In addition, due to the suppressive mechanism of VEGF stimulation, AKT phosphorylation, and NO production, low doses of the drug simvastatin are beneficial in preventing pathological neovascularization, whereas high doses are harmful for increasing neovascularization in the retina due to RCVEC apoptosis [118]. Additionally, inhibiting VEGF may cause neuronal apoptosis in the retina [119]. VEGF is a positive regulator of brain-derived neurotrophic factor production in DR that mediates Müller cell viability and neuroprotection by activating AKT signaling [120]. VEGF inhibition significantly increases RGC apoptosis and neuronal apoptosis in the inner nuclear layer in DR [121]. Studies targeting different VEGF family proteins have shown that VEGF-A acts directly on RGCs to promote their survival [122]. VEGF-B inhibits hyperglycemia-induced retinal apoptosis [123]. Therefore, the study of VEGF needs to be more in-depth and comprehensive, and the dose of VEGF inhibitors should be determined carefully.
Other growth factors
The expression levels of other growth factors are altered due to DR, and these factors can be targets for treatment. The growth differentiation factor 11 (produced in RPECs in retina) treatment of DR is related to the activation of TGF-β/Smad2 and PI3K/AKT/forkhead box O 1 (FOXO1) and the inhibition of the NF-κB pathway to improve pathological changes in mouse RCVECs [124]. Increased levels of hepatocyte growth factor in the retina in diabetes can activate the AKT signaling pathway, prevent RPC apoptosis caused by TNF-α, and protect the BRB [125]. Normal vitreous bodies promote angiogenesis by activating the epidermal growth factor receptor signaling pathway and AKT cascades [126, 127]. Nerve growth factor resists oxidation and inhibits apoptosis by regulating the PI3K/AKT and MAPK signaling pathways, thus protecting RGCs from damage [128]. Stem cell factor can increase NO production in ECs through the PI3K/AKT signaling pathway, suggesting that anti-SCF is a potential treatment [129].
Therefore, given the dual roles of the AKT pathway in angiogenesis and cell survival, the study of VEGF needs to be more in-depth and comprehensive, and the dose should be decided carefully. In vitro or animal experiments should be accompanied by clinical evaluations.
Substrates of PI3K/AKT signaling in DR
mTOR
mTOR is an atypical serine/threonine protein kinase that is a member of the PIKK protein family. There are two different complexes (mTORC1 and mTORC2) in the cell, and these complexes can regulate cell signaling pathways by phosphorylating downstream proteins. PI3K/AKT/mTOR is an important signaling pathway associated with oxidative stress-induced DR. Compared to that in muscle and liver, there is a unique set of reactions in the mTORC1 and mTORC2 pathways during diabetes-induced retinal protein conversion: perturbations of mTORC1 rather than mTORC2 in the retina may be associated with reduced protein synthesis under diabetic conditions [130]. Diabetes reduces phosphatidic acid in the retina, leading to decreased mTOR signaling and increased neuronal cell and RPC death [131, 132]. Decreased retinal protein synthesis is associated with decreased mTORC2 activity in DR, but there are no obvious changes in mTORC1 [130].
Therefore, activation of the PI3K/AKT/mTOR signaling pathway can play a protective role. Leukocyte cell-derived chemotaxin 2 (an antiangiogenic factor) increases the level of endothelial tight junction proteins by activating the Tie2/AKT/mTOR signaling pathway and can improve BRB damage associated with diabetes [133]. Curcumin inhibits hyperglycemia-induced inflammatory damage in human RPECs through the ROS-PI3K/AKT/mTOR signaling pathway [134]. Mangiferin and adiponectin treatment inhibits autophagy by promoting activation of the PI3K/AKT/mTOR cascade [135, 136].
However, it is possible that conventional mTOR inhibitors, such as rapamycin, may modulate HIF-1α-mediated downstream activation of growth factors, such as transcriptional regulation of retinal VEGF, to help prevent BRB damage and retinal microangiopathy. These agents are expected to effectively manage disease progression in both NPDR and PDR. In NPDR, mTOR inhibitors inhibit HIF-1α, VEGF, leakage, and disruption of the BRB. These inhibitors inhibit NF-κB as well as downstream inflammatory cytokines, chemokines, and adherent molecules [137]. In PDR, mTOR inhibitors inhibit several growth factors that play key roles in inducing pathological angiogenesis, such as IGF-1, VEGF, etc. [137].
Studies of signaling pathways have revealed the mechanism of the mTOR pathway. The mTOR kinase inhibitor INK128 inhibits the migration of cultured RPCs [138]. The mTORC1 inhibitor REDD1 can play a role in diabetes-induced VEGF expression [139]. Trimetazidine (a metabolic regulator) protects the diabetic retina by inhibiting the PI3K/AKT/mTOR pathway and restoring autophagy in retina [140]. The overexpression of the lncRNA MEG3 inhibits the endothelial to mesenchymal transition by inhibiting PI3K/AKT/mTOR signaling in DR rats and cell models [141]. Finally, miR-7 mimics can reduce the mTOR levels through the PI3K/AKT/mTOR signaling pathway and thereby reduce hyperglycemia-induced damage in RPECs, which indicates that it is a potential therapeutic strategy for the prevention and treatment of DR [142].
In conclusion, the biological processes involving the mTOR pathway are complex and diverse. Its mechanism still needs to be investigated, ad more biological and clinical tests are needed to understand its regulation.
FOXO
The proapoptotic transcription factor FOXO is a downstream target of AKT. Activated AKT inhibits the function of FOXO by phosphorylating FOXO and promoting cell survival, growth and proliferation [143]. Inhibiting AKT with high glucose activates FOXO, which plays an important role in RPC apoptosis and enhances RCVEC apoptosis and loss [144, 145]. TNF-α and AGEs induce RPC apoptosis by activating the transcription factor FOXO1 [144]. Additionally, inhibiting the upregulation of FOXO6 inhibits hyperglycemia-induced oxidative stress and apoptosis in RPECs, which is mediated by the downregulation of FOXO6 to activate the AKT/nuclear factor erythroid 2-related factor 2 (Nrf2) pathway [146]. Thus, inhibiting FOXO overactivation can prevent and ameliorate DR.
Nonetheless, the transcription factor FOXO plays an important role in vascular hyperplasia in PDR [147]. The activation of FOXO can prevent and improve vascular protection. Epigallocatechin-3 gallate is a polyphenol compound in green tea that inhibits the PI3K/AKT and MAPK pathways, synergistically enhances the antiangiogenic effect of EGCG by activating FOXO transcription factors and is a safe and effective vasoprotective agent for ischemic retinopathy [148,149,150].
GSK-3/Nrf2
AKT can phosphorylate glycogen synthase kinase-3 (GSK-3) to keep it inactive and act as an upstream kinase of Nrf2 to promote the expression of Nrf2 [151, 152]. In the early stage of DR, due to the decrease in AKT phosphorylation, the activity of GSK-3 is abnormally increased, and the expression of Nrf2 is inhibited, which is not conducive to the transcription of Nrf2-initiated antioxidant enzymes and the intracellular antioxidant effect [153, 154].
Since GSK-3 is a key kinase associated with neuronal apoptosis in early retinopathy and contributes to endothelial dysfunction and BRB leakage in DR, the use of the GSK-3 inhibitor lithium chloride reduces apoptosis in retinal neurons and protects the retinal integrity [155, 156]. Increasing the survival factor sulfiredoxin-1 and senescence marker protein 30 or inhibiting serine/threonine protein kinase 25 can activate the AKT/GSK-3β/Nrf2 pathway and protect RGCs from hyperglycemia-induced oxidative damage [157,158,159]. The antioxidant and anti-inflammatory agents pterostilbene, ginsenoside Rg1, sauchinone, lutein, and astaxanthin act on the AKT/GSK-3β/Nrf2 pathway to reduce apoptosis [160,161,162,163,164]. Additionally, as a novel nanomaterial, tetrahedral framework nucleic acids have good biocompatibility and can prevent retinal ischemia‒reperfusion damage caused by oxidative stress by activating the AKT/Nrf2 pathway [165].
NF-κB
Usually, the NF-κB pathway is activated after the phosphorylation of inhibitory IκB kinase. AKT is the enzyme that phosphorylates lkB kinase. Therefore, AKT is the upstream/regulator controlling NF-κB activation. AKT1 can directly activate NF-κB through a series of intermediate proteins such as IKK (IκB kinase). When AKT1 is activated, it can phosphorylate IKK, leading to the phosphorylation and degradation of IκB, releasing NF-κB to enter the cell nucleus and initiate the transcription of target genes [166, 167]. There is relatively less information about the direct regulation of NF-κB by AKT2 and AKT3, but considering the potential functional overlap among AKT isoforms, they may also be involved in the activation of NF-κB [43]. However, recent studies have shown that AKT2 interacts with AKT1 to exert its effects through the GSK-3/NF-κB axis. However, it has been shown that the events necessary for NF-κB activation (e.g., IκB degradation, nuclear translocation, and increased NF-κB DNA binding) all occur before the increase in AKT phosphorylation [168]. This finding suggests that AKT is a downstream target of NF-κB. Studies have shown that NF-κB is not activated in the normal retina but is activated in the diabetic retina, and the higher the blood glucose and the longer the time, the higher the activity of NF-κB [169]. NF-κB plays an important role in the inflammatory response and participates in the transcriptional regulation of many inflammatory factor genes, such as binding to inflammatory factors and regulating their expression, thereby increasing the speed of apoptosis and new blood vessel proliferation [170].
There have been many studies on the regulation of the NF-κB and AKT pathways. Melatonin is a potent antioxidant that protects various retinal cells from oxidative damage and is an effective activator of AKT in Müller cells, protecting the retina from damage during DR [171]. Melatonin maintains the integrity of the BRB by inhibiting the PI3K/AKT/STAT3/NF-κB signaling pathway and the production of proinflammatory cytokines and proteins, including IL-1β, TNF-α and inducible nitric oxide synthase (iNOS), through the NF-κB pathway [172, 173]. For new targets of retinal disease, anti-CD146 therapy combined with anti-VEGF therapy enhances the damage induced by hypoxia-induced angiogenesis in vitro and in vivo because under hypoxic conditions, CD146 is involved in the activation of the NF-κB, extracellular regulated protein kinase (ERK), and AKT signaling pathways [174]. Preclinical evidence suggests that crocin has cytoprotective, antioxidant, anti-inflammatory, and blood flow-enhancing effects on retinal tissue by activating PI3K/AKT and inhibiting the NF-κB signaling pathway [175, 176]. Additionally, the upregulation of lncRNA SNHG16 promotes diabetes-related human RCVEC dysfunction by activating the NF-κB and PI3K/AKT pathways and thus promoting the proliferation, migration and angiogenesis of RCVECs [177].
Crosstalk between AKT signaling and other signaling pathways in DR
JAK/STAT signaling pathway
Apart from the AKT/PI3K pathway, the phosphorylation of JAK2 leads to the initiation of intracellular signaling and the activation of STAT and NF-κB [178]. The JAK/STAT pathway can rapidly transduce signals from the membrane to the nucleus and is widely involved in various physiological and pathological processes, such as cell proliferation, differentiation, apoptosis, inflammation, and tumors [179]. There is a certain activating relationship between the JAK/STAT and AKT pathways, and some drugs exert protective effects through the coregulation of the JAK/STAT and AKT pathways.
Under hyperglycemia stimulation, p-STAT is significantly enhanced, and STAT3 activation increases apoptosis in diabetic RPCs through the TNF-ɑ/AKT/p70S6 kinase signaling pathway [180]. Genipin protects RPEC functional activity, the inflammatory response, and mitochondrial damage by promoting AKT signaling and regulating the expression of the miR-4429/JAK2 signaling axis [181].
In addition, erythropoietin treatment can protect the barrier function of RPECs, induce axonal regeneration and maintain homeostasis in rat retinal neurons. Low-dose erythropoietin has antioxidant effects on organs affected by diabetes and reduces oxidative and nitriding stress in tissues, as well as AGEs in the retina, preventing NPDR microvascular damage in the diabetic retina [182]. These outcomes depend on the activation and joint actions of the JAK2/STAT pathway, MAPK pathway and PI3K/AKT pathway [183,184,185].
MAPK signaling pathway
The MAPK signaling pathway is present in most cells in all organisms. This important signal transduction pathway in eukaryotic cells can stimulate the transduction of cell surface signaling to cells and their nuclei and is closely related to cell proliferation, survival, differentiation, apoptosis and other physiological processes [186]. This pathway is composed of three main families: ERKs, Jun kinases and p38MAPKs. Many regulatory factors can affect the MAPK pathway while simultaneously affecting the PI3K/AKT pathway, and the connection and interaction between them cannot be ignored.
Apelin expression is upregulated in RPCs under hyperglycaemic conditions in vitro [187]. Apelin induces proliferation, migration and the expression of cytoskeletal and tightly linked proteins in human RPCs by activating the expression of PI3K/AKT and MAPK signaling pathway proteins, such as AKT and ERK phosphorylation, and promotes retinal vascular permeability in the early stage of DR [188,189,190]. Systemic injection of an apelin receptor agonist prevents N-methyl-D-aspartate-induced retinal neuronal cell loss [190].
Pituitary adenylate cyclase-activated polypeptide and vasoactive intestinal peptide play beneficial roles in retinal injury associated with diabetic macular edema progression caused by hyperglycaemic injury and can promote neuronal survival in early experimental DR. Their actions are mediated by activation of the PI3K/AKT and mammalian ERK/MAPK kinase signaling pathways [191]. Pituitary adenylate cyclase-activated polypeptide treatment reduces the levels of oxidative stress-induced markers of apoptosis, including HIF-1, multiple heat shock proteins and TNF-α-associated apoptosis-induced ligands; the PI3K/AKT cascade and MAPK cascade are elevated to inhibit apoptosis, resulting in decreased levels of proapoptotic p-p38 MAPK, c-Jun kinases, and activated caspase [192, 193].
Maspin is a potential target protein for the prevention and treatment of PDR. Maspin reduces the mRNA and protein levels of hyperglycemia-induced HIF-1α and VEGF in a dose-dependent manner. In addition, an increase in hyperglycemia-induced p-PI3K and p-AKT is inhibited by maspin [194]. Inhibiting miR-21-5p inhibits hyperglycaemic induction of human retinal EC proliferation and angiogenesis, which may depend in part on the regulation of the PI3K/AKT and MAPK pathways by its target protein maspin [195].
Pigment epithelial-derived factor derivatives induce p-ERK1/2 and p78 to promote p-AKT in Müller glial cells, which are suppressed under diabetic conditions [196]. Pigment epithelial-derived factors inhibit glycosylation-induced retinal vascular permeability [197]. Pigment epithelial-derived factors induce apoptosis by activating PI3K/AKT to downregulate RPC in hyperglycemia [198].
The local administration of glucagon-like peptide-1 (GLP-1) can restore the expression of GLP-1R and reduce the levels of p-AKT and p-ERK1/2 in DR [199]. In NPDR, this peptide can reduce NF-κB, the inflammasome and proinflammatory factors to play an anti-inflammatory role, and it can promote cell survival by increasing anti-apoptotic proteins and activating the GLP-1R/AKT/GSK-3β signaling pathway [200]. Liraglutide (a glucagon-like peptide-1 analog) attenuates AGE-induced RPC migration and retinal neurodegeneration, significantly attenuates the migration of RPCs, and reverses age-induced changes in p-AKT levels [201, 202].
Conclusion and perspective
Although many studies on DR have involved signaling pathways, few articles have summarized the signaling pathways related to AKT in the different stages of DR. Our review addresses this unresolved issue by summarizing the manifestations of the AKT pathway in different stages of DR as well as potential targets and therapies related to this pathway (Tables 1 and 2). We found that the AKT pathway is inhibited in early DR and that this inhibition is accompanied by retinal cell apoptosis and structural destruction (Fig. 2). However, during the PDR phase, the AKT signaling pathway is overactivated in retinal cells and retinal capillary ECs (Fig. 3). We suspect that the main reason for this difference in AKT signaling between these two stages is that hypoxia leads to vascular compensatory hyperplasia, which is regulated by a series of signaling factors.
The early stage of DR, that is, non-PDR, may lead to BRB injury caused by RPC loss, EC apoptosis, capillary basement membrane thickening, retinal microaneurysms, internal retinal bleeding, exudate of abnormal blood vessels, and retinal neuron apoptosis. At this stage, AKT signaling is downregulated due to changes in upstream regulators of AKT signaling, such as insulin and IGF-1, and thus, downstream substrates of AKT exert a concentrated influence on the activity of several transcription factors related to the regulation of cellular protein synthesis, inflammation, apoptosis and other vital activities. Medical or experimental regulation of the disease is mainly aimed at promoting cell survival and reducing cell inflammation and apoptosis, which would result in curbing the retinopathy caused by diabetes. Common and recent activators and inhibitors of different signaling molecular targets in the AKT pathway in the early stage of DR are detailed in Table 2. In addition, many microRNAs also serve as targets for regulating the AKT pathway. GDF11 growth differentiation factor 11, HGF hepatocyte growth factor, EGF epidermal growth factor, NGF nerve growth factor, SCF stem cell factor, TSC1/2 tuberous sclerosis complex 1/2.
The most significant feature of PDR is pathological neovascularization, which is accompanied by further inflammation and cell apoptosis. Tissue hypoxia or ischemia induces compensatory angiogenesis, which can be regulated by the AKT pathway. At this stage, AKT is upregulated compared to normal levels, contrary to the early stage of DR. Various kinds of antiangiogenic factors are common regulatory methods and research directions for PDR. Among them, VEGF and mTOR are the most frequently used regulatory sites. The research progress relate dot commonly used and latest activators and inhibitors of different signaling molecular targets in the AKT pathway during the PDR period is detailed in Table 2. In addition, many microRNAs also serve as targets for regulating the AKT pathway. However, some antiangiogenic factors, such as VEGF inhibitors, can also lead to increased apoptosis and further deterioration of DR, which should be considered. In addition to the AKT pathway, the interactions between different signaling pathways, such as the JAK/STAT and MAPK pathways discussed in this paper, can also affect each other with the AKT pathway, altering the pathologic process of DR, which suggests that clinical drug selection should not focus on a single pathway. IFN interferon, GF growth factor, ROS reactive oxygen species.
Methods for regulating the AKT pathway, including its activation and inhibition, such as routine insulin therapy, IGF-1 therapy and the very popular growth factor therapy (particularly VEGF), have received considerable attention. An increasing number of potential targets, drugs and bioderived health products have been gradually discovered and used in clinical research. Furthermore, substrate studies targeting the AKT pathway provide other ideas for the treatment of AKT-related DR.
However, there remain many limitations to the study of signaling factors, which may become the directions of future research. For example, the response of PDR patients to VEGF treatment is heterogeneous, suggesting that the pathological basis of this condition is multifactorial in nature [203]. The application of VEGF inhibitors clearly needs more clinical evaluation. A similar situation exists with mTOR [204].
The interaction between different signaling pathways in organisms should not be ignored, although the study of these interactions is far from sufficient. Crosstalk was proposed in the 1980s to explain the unlikely pharmacological effects of drugs [204]. Due to the complex and delicate interactions between signaling pathways in organisms, the study of signaling pathways and drug development faces great challenges.
Overall, we are aware that despite many research achievements on AKT-related DR, more in-depth and comprehensive studies on the pathway mechanism are still lacking due to the complexity of the related signaling pathways. More effective drugs targeting AKT signaling are still needed to treat DR at the source, and more clinical evaluation should be conducted.
Data availability
We searched the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) using the keywords “diabetic retinopathy AND AKT”, and mainly focused on the recent studies and reviews (published between 2002 and 2022). Additional studies were discovered by consulting the reference lists of the selected articles. Our figures were edited with Adobe Illustrator CC 2018 software (Adobe, San Jose, CA).
References
Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1816.
Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21:S3–9.
Rudraraju M, Narayanan SP, Somanath PR. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol Res. 2020;161:105115.
Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis Int J Program Cell Death. 2004;9:667–76.
Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239–42.
Guo H, German P, Bai S, Barnes S, Guo W, Qi X, et al. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics. 2015;42:343–53.
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405.
Marçal AC, Leonelli M, Fiamoncini J, Deschamps FC, Rodrigues MA, Curi R, et al. Diet-induced obesity impairs AKT signalling in the retina and causes retinal degeneration. Cell Biochem Funct. 2013;31:65–74.
Zeng J, Zhao H, Chen B. DJ-1/PARK7 inhibits high glucose-induced oxidative stress to prevent retinal pericyte apoptosis via the PI3K/AKT/mTOR signaling pathway. Exp Eye Res. 2019;189:107830.
Abu El-Asrar AM, Dralands L, Missotten L, Geboes K. Expression of antiapoptotic and proapoptotic molecules in diabetic retinas. Eye. 2007;21:238–45.
Xing X, Jiang Y, Wang H, Zhang Y, Niu T, Qu Y, et al. Identification of novel differentially expressed genes in retinas of STZ-induced long-term diabetic rats through RNA sequencing. Mol Genet Genom Med. 2020;8:e1115.
Kumari N, Karmakar A, Chakrabarti S, Ganesan SK. Integrative computational approach revealed crucial genes associated with different stages of diabetic retinopathy. Front Genet. 2020;11:576442.
Li YJ, Hui YN, Yan F, Du ZJ. Up-regulation of integrin-linked kinase in the streptozotocin-induced diabetic rat retina. Graefes Arch Clin Exp Ophthalmol. 2007;245:1523–32.
Sun J, Huang W, Yang SF, Zhang XP, Yu Q, Zhang ZQ, et al. Gαi1 and Gαi3mediate VEGF-induced VEGFR2 endocytosis, signaling and angiogenesis. Theranostics. 2018;8:4695–709.
Yao J, Wu XY, Yu Q, Yang SF, Yuan J, Zhang ZQ, et al. The requirement of phosphoenolpyruvate carboxykinase 1 for angiogenesis in vitro and in vivo. Sci Adv. 2022;8:eabn6928.
Xiao H, Xin W, Sun LM, Li SS, Zhang T, Ding XY. Comprehensive proteomic profiling of aqueous humor proteins in proliferative diabetic retinopathy. Transl Vis Sci Technol 2021;10:3.
Olivares AM, Althoff K, Chen GF, Wu S, Morrisson MA, DeAngelis MM, et al. Animal models of diabetic retinopathy. Curr Diab Rep. 2017;17:93.
Orike N, Middleton G, Borthwick E, Buchman V, Cowen T, Davies AM. Role of PI 3-kinase, Akt and Bcl-2-related proteins in sustaining the survival of neurotrophic factor-independent adult sympathetic neurons. J Cell Biol. 2001;154:995–1005.
Weishaupt JH, Rohde G, Pölking E, Siren AL, Ehrenreich H, Bähr M. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–22.
Nakazawa T, Shimura M, Tomita H, Akiyama H, Yoshioka Y, Kudou H, et al. Intrinsic activation of PI3K/Akt signaling pathway and its neuroprotective effect against retinal injury. Curr Eye Res. 2003;26:55–63.
Mohamed IN, Soliman SA, Alhusban A, Matragoon S, Pillai BA, Elmarkaby AA, et al. Diabetes exacerbates retinal oxidative stress, inflammation, and microvascular degeneration in spontaneously hypertensive rats. Mol Vis. 2012;18:1457–66.
Guo C, Zhang Z, Zhang P, Makita J, Kawada H, Blessing K, et al. Novel transgenic mouse models develop retinal changes associated with early diabetic retinopathy similar to those observed in rats with diabetes mellitus. Exp Eye Res. 2014;119:77–87.
Zhong Q, Kowluru RA. Diabetic retinopathy and damage to mitochondrial structure and transport machinery. Invest Ophthalmol Vis Sci. 2011;52:8739–46.
Stitt AW, Hughes SJ, Canning P, Lynch O, Cox O, Frizzell N, et al. Substrates modified by advanced glycation end-products cause dysfunction and death in retinal pericytes by reducing survival signals mediated by platelet-derived growth factor. Diabetologia. 2004;47:1735–46.
Ma J, Liu T, Dong X. Advanced glycation end products of bovine serum albumin-induced endothelial-to-mesenchymal transition in cultured human and monkey endothelial cells via protein kinase B signaling cascades. Mol Vis. 2010;16:2669–79.
Serveaux-Dancer M, Jabaudon M, Creveaux I, Belville C, Blondonnet R, Gross C, et al. Pathological implications of receptor for advanced glycation end-product (AGER) gene polymorphism. Dis Markers. 2019;2019:2067353.
Qin D, Zhang GM, Xu X, Wang LY. The PI3K/Akt signaling pathway mediates the high glucose-induced expression of extracellular matrix molecules in human retinal pigment epithelial cells. J Diabetes Res. 2015;2015:920280.
Bharadwaj AS, Appukuttan B, Wilmarth PA, Pan Y, Stempel AJ, Chipps TJ, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retinal Eye Res. 2013;32:102–80.
Isoe T, Makino Y, Mizumoto K, Sakagami H, Fujita Y, Honjo J, et al. High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int. 2010;78:48–59.
Dodd MS, Sousa Fialho MDL, Montes Aparicio CN, Kerr M, Timm KN, Griffin JL, et al. Fatty acids prevent hypoxia-inducible factor-1α signaling through decreased succinate in diabetes. JACC Basic Transl Sci. 2018;3:485–98.
Otrock ZK, Hatoum HA, Awada AH, Ishak RS, Shamseddine AI. Hypoxia-inducible factor in cancer angiogenesis: structure, regulation and clinical perspectives. Crit Rev Oncol Hematol. 2009;70:93–102.
Zhang P, Zhang X, Hao X, Wang Y, Hui Y, Wang H, et al. Rac1 activates HIF-1 in retinal pigment epithelium cells under hypoxia. Graefes Arch Clin Exp Ophthalmol. 2009;247:633–9.
Zhang P, Wang Y, Hui Y, Hu D, Wang H, Zhou J, et al. Inhibition of VEGF expression by targeting HIF-1 alpha with small interference RNA in human RPE cells. Ophthalmologica. 2007;221:411–7.
Zhang Y, Liu NM, Wang Y, Youn JY, Cai H. Endothelial cell calpain as a critical modulator of angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1326–35.
Wu W, Xu H, Meng Z, Zhu J, Xiong S, Xia X, et al. Axl is essential for in-vitro angiogenesis induced by vitreous from patients with proliferative diabetic retinopathy. Front Med. 2021;8:787150.
Wu X, Pu L, Chen W, Zhao Q, Wu G, Li D, et al. LY294002 attenuates inflammatory response in endotoxin-induced uveitis by downregulating JAK3 and inactivating the PI3K/Akt signaling. Immunopharmacol Immunotoxicol. 2022;44:510–8.
Wang Y, Kuramitsu Y, Baron B, Kitagawa T, Tokuda K, Akada J, et al. PI3K inhibitor LY294002, as opposed to wortmannin, enhances AKT phosphorylation in gemcitabine-resistant pancreatic cancer cells. Int J Oncol. 2017;50:606–12.
Ehrhardt M, Craveiro RB, Holst MI, Pietsch T, Dilloo D. The PI3K inhibitor GDC-0941 displays promising in vitro and in vivo efficacy for targeted medulloblastoma therapy. Oncotarget. 2015;6:802–13.
Wu X, Reiter CE, Antonetti DA, Kimball SR, Jefferson LS, Gardner TW. Insulin promotes rat retinal neuronal cell survival in a p70S6K-dependent manner. J Biol Chem. 2004;279:9167–75.
Poulaki V, Joussen AM, Mitsiades N, Mitsiades CS, Iliaki EF, Adamis AP. Insulin-like growth factor-I plays a pathogenetic role in diabetic retinopathy. Am J Pathol. 2004;165:457–69.
Luo D, Fan Y, Xu X. The effects of aminoguanidine on retinopathy in STZ-induced diabetic rats. Bioorg Med Chem Lett. 2012;22:4386–90.
Yu H, Littlewood T, Bennett M. Akt isoforms in vascular disease. Vasc Pharmacol. 2015;71:57–64.
Liu H, Stepicheva NA, Ghosh S, Shang P, Chowdhury O, Daley RA, et al. Reducing Akt2 in retinal pigment epithelial cells causes a compensatory increase in Akt1 and attenuates diabetic retinopathy. Nat Commun. 2022;13:6045.
Daley R, Maddipatla V, Ghosh S, Chowdhury O, Hose S, Zigler JS Jr., et al. Aberrant Akt2 signaling in the RPE may contribute to retinal fibrosis process in diabetic retinopathy. Cell Death Discov. 2023;9:243.
Rangasamy S, Monickaraj F, Legendre C, Cabrera AP, Llaci L, Bilagody C, et al. Transcriptomics analysis of pericytes from retinas of diabetic animals reveals novel genes and molecular pathways relevant to blood-retinal barrier alterations in diabetic retinopathy. Exp Eye Res. 2020;195:108043.
Ségaliny AI, Tellez-Gabriel M, Heymann MF, Heymann D. Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J Bone Oncol. 2015;4:1–12.
Hancock ML, Meyer RC, Mistry M, Khetani RS, Wagschal A, Shin T, et al. Insulin receptor associates with promoters genome-wide and regulates gene expression. Cell. 2019;177:722–36.e22.
Nakamura M, Barber AJ, Antonetti DA, LaNoue KF, Robinson KA, Buse MG, et al. Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem. 2001;276:43748–55.
Reiter CE, Wu X, Sandirasegarane L, Nakamura M, Gilbert KA, Singh RS, et al. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–56.
Geraldes P, Yagi K, Ohshiro Y, He Z, Maeno Y, Yamamoto-Hiraoka J, et al. Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J Biol Chem. 2008;283:34327–36.
Fang S, Ma X, Guo S, Lu J. MicroRNA-126 inhibits cell viability and invasion in a diabetic retinopathy model via targeting IRS-1. Oncol Lett. 2017;14:4311–8.
Ji Z, Luo J, Su T, Chen C, Su Y. miR-7a targets insulin receptor substrate-2 gene and suppresses viability and invasion of cells in diabetic retinopathy mice via PI3K-Akt-VEGF pathway. Diabetes Metab Syndr Obes Targets Ther. 2021;14:719–28.
Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769–76.
Hyer SL, Sharp PS, Brooks RA, Burrin JM, Kohner EM. Serum IGF-1 concentration in diabetic retinopathy. Diabet Med J Br Diabet Assoc. 1988;5:356–60.
Meyer-Schwickerath R, Pfeiffer A, Blum WF, Freyberger H, Klein M, Lösche C, et al. Vitreous levels of the insulin-like growth factors I and II, and the insulin-like growth factor binding proteins 2 and 3, increase in neovascular eye disease. Studies in nondiabetic and diabetic subjects. J Clin Investig. 1993;92:2620–5.
Wang H, Liao S, Geng R, Zheng Y, Liao R, Yan F, et al. IGF-1 signaling via the PI3K/Akt pathway confers neuroprotection in human retinal pigment epithelial cells exposed to sodium nitroprusside insult. J Mol Neurosci. 2015;55:931–40.
Arroba AI, Mazzeo A, Cazzoni D, Beltramo E, Hernández C, Porta M, et al. Somatostatin protects photoreceptor cells against high glucose-induced apoptosis. Mol Vis. 2016;22:1522–31.
Upreti S, Sen S, Nag TC, Ghosh MP. Insulin like growth factor-1 works synergistically with dopamine to attenuate diabetic retinopathy by downregulating vascular endothelial growth factor. Biomed Pharmacother. 2022;149:112868.
Seigel GM, Lupien SB, Campbell LM, Ishii DN. Systemic IGF-I treatment inhibits cell death in diabetic rat retina. J Diabetes Complications. 2006;20:196–204.
Kummer A, Pulford BE, Ishii DN, Seigel GM. Des(1-3)IGF-1 treatment normalizes type 1 IGF receptor and phospho-Akt (Thr 308) immunoreactivity in predegenerative retina of diabetic rats. Int J Exp Diabesity Res. 2003;4:45–57.
Arroba AI, Valverde ÁM. Inhibition of protein tyrosine phosphatase 1B improves IGF-I receptor signaling and protects against inflammation-induced gliosis in the retina. Invest Ophthalmol Vis Sci.2015;56:8031–44.
He H, Weir RL, Toutounchian JJ, Pagadala J, Steinle JJ, Baudry J, et al. The quinic acid derivative KZ-41 prevents glucose-induced caspase-3 activation in retinal endothelial cells through an IGF-1 receptor dependent mechanism. PLoS ONE. 2017;12:e0180808.
Xi G, Wai C, Clemmons D. Inhibition of aberrant IGF-I signaling in diabetic male rat retina prevents and reverses changes of diabetic retinopathy. J Diabetes Res. 2019;2019:6456032.
Liu X, Li J, Li X. miR-142-5p regulates the progression of diabetic retinopathy by targeting IGF1. Int J Immunopathol Pharmacol. 2020;34:2058738420909041.
Wu JH, Wang YH, Wang W, Shen W, Sang YZ, Liu L, et al. MiR-18b suppresses high-glucose-induced proliferation in HRECs by targeting IGF-1/IGF1R signaling pathways. Int J Biochem Cell Biol. 2016;73:41–52.
Jarajapu YP, Cai J, Yan Y, Li Calzi S, Kielczewski JL, Hu P, et al. Protection of blood retinal barrier and systemic vasculature by insulin-like growth factor binding protein-3. PLoS ONE. 2012;7:e39398.
Jiang Y, Zhang Q, Soderland C, Steinle JJ. TNFα and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cell Signal. 2012;24:1086–92.
Ye EA, Steinle JJ. miR-15b/16 protects primary human retinal microvascular endothelial cells against hyperglycemia-induced increases in tumor necrosis factor alpha and suppressor of cytokine signaling 3. J Neuroinflammation. 2015;12:44.
Campochiaro PA, Aiello LP, Rosenfeld PJ. Anti-vascular endothelial growth factor agents in the treatment of retinal disease: from bench to bedside. Ophthalmology. 2016;123:S78–88.
Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–5.
Jin J, Yuan F, Shen MQ, Feng YF, He QL. Vascular endothelial growth factor regulates primate choroid-retinal endothelial cell proliferation and tube formation through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem. 2013;381:267–72.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25.
Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123:2376–85.
Huang C, Ji H, Han X. The effectiveness of conbercept combined with panretinal photocoagulation vs. panretinal photocoagulation in the treatment of diabetic retinopathy: a meta-analysis. J Ophthalmol. 2021;2021:5591719.
Toutounchian JJ, Pagadala J, Miller DD, Baudry J, Park F, Chaum E, et al. Novel small molecule JP-153 targets the Src-FAK-Paxillin signaling complex to inhibit VEGF-induced retinal angiogenesis. Mol Pharmacol. 2017;91:1–13.
Chan CM, Hsiao CY, Li HJ, Fang JY, Chang DC, Hung CF. The inhibitory effects of gold nanoparticles on VEGF-A-induced cell migration in choroid-retina endothelial cells. Int J Mol Sci. 2019;21:109.
Yu Z, Zhang T, Gong C, Sheng Y, Lu B, Zhou L, et al. Erianin inhibits high glucose-induced retinal angiogenesis via blocking ERK1/2-regulated HIF-1α-VEGF/VEGFR2 signaling pathway. Sci Rep. 2016;6:34306.
Mohr T, Desser L. Plant proteolytic enzyme papain abrogates angiogenic activation of human umbilical vein endothelial cells (HUVEC) in vitro. BMC Complement Altern Med. 2013;13:231.
Qin D, Jiang YR. Tangeretin inhibition of high-glucose-induced IL-1β, IL-6, TGF-β1, and VEGF expression in human RPE cells. J Diabetes Res. 2020;2020:9490642.
Huang W, Yan Z, Li D, Ma Y, Zhou J, Sui Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid Med Cell Longev. 2018;2018:1862462.
Tzeng TF, Liu WY, Liou SS, Hong TY, Liu IM. Antioxidant-rich extract from plantaginis semen ameliorates diabetic retinal injury in a streptozotocin-induced diabetic rat model. Nutrients. 2016;8:572.
Chen Y, Sun J, Zhang Z, Liu X, Wang Q, Yu Y. The potential effects and mechanisms of hispidulin in the treatment of diabetic retinopathy based on network pharmacology. BMC Complement Med Ther. 2022;22:141.
Basini G, Santini SE, Bussolati S, Grasselli F. Sanguinarine inhibits VEGF-induced Akt phosphorylation. Ann N Y Acad Sci. 2007;1095:371–6.
Lupo G, Cambria MT, Olivieri M, Rocco C, Caporarello N, Longo A, et al. Anti-angiogenic effect of quercetin and its 8-methyl pentamethyl ether derivative in human microvascular endothelial cells. J Cell Mol Med. 2019;23:6565–77.
Lee M, Yun S, Lee H, Yang J. Quercetin mitigates inflammatory responses induced by vascular endothelial growth factor in mouse retinal photoreceptor cells through suppression of nuclear factor kappa B. Int J Mol Sci. 2017;18:2497.
Ola MS, Ahmed MM, Shams S, Al-Rejaie SS. Neuroprotective effects of quercetin in diabetic rat retina. Saudi J Biol Sci. 2017;24:1186–94.
Huang J, Li X, Li M, Li S, Xiao W, Chen X, et al. Effects of intravitreal injection of KH902, a vascular endothelial growth factor receptor decoy, on the retinas of streptozotocin-induced diabetic rats. Diabetes Obes Metab. 2012;14:644–53.
Ojima T, Takagi H, Suzuma K, Oh H, Suzuma I, Ohashi H, et al. EphrinA1 inhibits vascular endothelial growth factor-induced intracellular signaling and suppresses retinal neovascularization and blood-retinal barrier breakdown. Am J Pathol. 2006;168:331–9.
Li Y, Zhang J, Yan H. Integrin-linked kinase inhibition attenuates permeability of the streptozotocin-induced diabetic rat retina. Cell Biochem Biophys. 2013;67:1467–72.
Tan W, Xu H, Chen B, Duan T, Liu K, Zou J. Wnt inhibitory 1 ameliorates neovascularization and attenuates photoreceptor injury in an oxygen-induced retinopathy mouse model. BioFactors. 2022;48:683–98.
Miyazawa T, Shibata A, Nakagawa K, Tsuzuki T. Anti-angiogenic function of tocotrienol. Asia Pac J Clin Nutr. 2008;17:253–6.
Nakagawa K, Shibata A, Yamashita S, Tsuzuki T, Kariya J, Oikawa S, et al. In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J Nutr. 2007;137:1938–43.
Cervantes-Villagrana AR, Garcia-Román J, González-Espinosa C, Lamas M. Pharmacological inhibition of N-methyl d-aspartate receptor promotes secretion of vascular endothelial growth factor in müller cells: effects of hyperglycemia and hypoxia. Curr Eye Res. 2010;35:733–41.
Wang QQ, Zhou SJ, Meng ZX, Wang J, Chen R, Lv L, et al. Domain I-IV of β2-glycoprotein I inhibits advanced glycation end product-induced angiogenesis by down-regulating vascular endothelial growth factor 2 signaling. Mol Med Rep. 2015;11:2167–72.
Zhang C, Xie H, Yang Q, Yang Y, Li W, Tian H, et al. Erythropoietin protects outer blood-retinal barrier in experimental diabetic retinopathy by up-regulating ZO-1 and occludin. Clin Exp Ophthalmol. 2019;47:1182–97.
Rajala RV. Phospho-site-specific antibody microarray to study the state of protein phosphorylation in the retina. J Proteom Bioinforma. 2008;1:242.
Yadav UC, Srivastava SK, Ramana KV. Prevention of VEGF-induced growth and tube formation in human retinal endothelial cells by aldose reductase inhibition. J Diabetes Complications. 2012;26:369–77.
Zhang Y, Wang L, Zhang Y, Wang M, Sun Q, Xia F, et al. Nogo-B promotes angiogenesis in proliferative diabetic retinopathy via VEGF/PI3K/Akt pathway in an autocrine manner. Cell Physiol Biochem. 2017;43:1742–54.
Yang Q, Zhang C, Xie H, Tang L, Liu D, Qiu Q, et al. Silencing Nogo-B improves the integrity of blood-retinal barrier in diabetic retinopathy via regulating Src, PI3K/Akt and ERK pathways. Biochem Biophys Res Commun. 2021;581:96–102.
Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–53.
Zhang D, Lv FL, Wang GH. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci. 2018;22:5071–6.
Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol. 2019;30:187–200.
Lu JM, Zhang ZZ, Ma X, Fang SF, Qin XH. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Exp Eye Res. 2020;190:107886.
Zhang XL, Liu Z. MiR-19a inhibitor improves diabetic retinopathy in rats through PTEN/Akt/P-Akt signaling pathway. J Biol Regul Homeost Agents. 2020;34:509–15.
Zhang ZZ, Qin XH, Zhang J. MicroRNA-183 inhibition exerts suppressive effects on diabetic retinopathy by inactivating BTG1-mediated PI3K/Akt/VEGF signaling pathway. Am J Physiol Endocrinol Metab. 2019;316:E1050–60.
Ma Y, Dong C, Chen X, Zhu R, Wang J. Silencing of miR-20b-5p exerts inhibitory effect on diabetic retinopathy via inactivation of THBS1 gene induced VEGF/Akt/PI3K pathway. Diabetes Metab Syndr Obes Targets Ther. 2021;14:1183–93.
Wang L, Liu WX, Huang XG. MicroRNA-199a-3p inhibits angiogenesis by targeting the VEGF/PI3K/AKT signalling pathway in an in vitro model of diabetic retinopathy. Exp Mol Pathol. 2020;116:104488.
Cao YL, Liu DJ, Zhang HG. MiR-7 regulates the PI3K/AKT/VEGF pathway of retinal capillary endothelial cell and retinal pericytes in diabetic rat model through IRS-1 and inhibits cell proliferation. Eur Rev Med Pharmacol Sci. 2018;22:4427–30.
Qin B, Liu J, Liu S, Li B, Ren J. MiR-20b targets AKT3 and modulates vascular endothelial growth factor-mediated changes in diabetic retinopathy. Acta Biochim Biophys Sin. 2016;48:732–40.
Yang WZ, Yang J, Xue LP, Xiao LB, Li Y. MiR-126 overexpression inhibits high glucose-induced migration and tube formation of rhesus macaque choroid-retinal endothelial cells by obstructing VEGFA and PIK3R2. J Diabetes Complications. 2017;31:653–63.
Chen X, Yu X, Li X, Li L, Li F, Guo T, et al. MiR-126 targets IL-17A to enhance proliferation and inhibit apoptosis in high-glucose-induced human retinal endothelial cells. Biochem Cell Biol. 2020;98:277–83.
Zhao C, Fei X, Xu B, Lu Y, Zhang Q. Long non-coding RNA HEIH contributes to diabetic retinopathy by regulating miR-939/VEGF axis. Int J Clin Exp Pathol. 2019;12:2022–33.
Okikawa S, Morine Y, Saito Y, Yamada S, Tokuda K, Teraoku H, et al. Inhibition of the VEGF signaling pathway attenuates tumor‑associated macrophage activity in liver cancer. Oncol Rep. 2022;47:71.
Stewart MW, Stewart MLJCO. Progression to macula-off tractional retinal detachment after a contralateral intraoperative intravitreal bevacizumab injection for proliferative diabetic retinopathy. Clin Ophthalmol. 2015;9:409–11.
Torres-Soriano ME, Reyna-Castelán E, Hernández-Rojas M, García-Aguirre G, Kon-Jara V, Diaz-Rubio JL, et al. Tractional retinal detachment after intravitreal injection of bevacizumab in proliferative diabetic retinopathy. Retin Cases Brief Rep. 2009;3:70–3.
Sinawat S, Rattanapakorn T, Sanguansak T, Yospaiboon YJE. Intravitreal bevacizumab for proliferative diabetic retinopathy with new dense vitreous hemorrhage after full panretinal photocoagulation. Eye. 2013;27:1391–6.
Medina RJ, O’Neill CL, Devine AB, Gardiner TA, Stitt AW. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PLoS ONE. 2008;3:e2584.
Krohne TU, Müller A, Larsen PP, Holz FG. [Long-term effects of anti-VEGF therapy for retinopathy of prematurity]. Ophthalmologe. 2018;115:464–8.
Le YZ, Xu B, Chucair-Elliott AJ, Zhang H, Zhu M. VEGF mediates retinal Müller cell viability and neuroprotection through BDNF in diabetes. Biomolecules. 2021;11:712.
Park HY, Kim JH, Park CK. Neuronal cell death in the inner retina and the influence of vascular endothelial growth factor inhibition in a diabetic rat model. Am J Pathol. 2014;184:1752–62.
Foxton RH, Finkelstein A, Vijay S, Dahlmann-Noor A, Khaw PT, Morgan JE, et al. VEGF-A is necessary and sufficient for retinal neuroprotection in models of experimental glaucoma. Am J Pathol. 2013;182:1379–90.
Huang D, Zhao C, Ju R, Kumar A, Tian G, Huang L, et al. VEGF-B inhibits hyperglycemia- and macugen-induced retinal apoptosis. Sci Rep. 2016;6:26059.
Mei W, Zhu B, Shu Y, Liang Y, Lin M, He M, et al. GDF11 protects against glucotoxicity-induced mice retinal microvascular endothelial cell dysfunction and diabetic retinopathy disease. Mol Cell Endocrinol. 2021;537:111422.
Yun JH. Hepatocyte growth factor prevents pericyte loss in diabetic retinopathy. Microvas Res. 2021;133:104103.
You M, Xia X, Li H, Wu J, Rong R, Zeng Z, et al. Normal vitreous promotes angiogenesi via the epidermal growth factor receptor. FASEB J. 2020;34:14799–809.
Shao J, Pan X, Yin X, Fan G, Tan C, Yao Y, et al. KCNQ1OT1 affects the progression of diabetic retinopathy by regulating miR-1470 and epidermal growth factor receptor. J Cell Physiol. 2019;234:17269–79.
Yan PS, Tang S, Zhang HF, Guo YY, Zeng ZW, Wen Q. Nerve growth factor protects against palmitic acid-induced injury in retinal ganglion cells. Neural Regen Res. 2016;11:1851–6.
Kim JY, Choi JS, Song SH, Im JE, Kim JM, Kim K, et al. Stem cell factor is a potent endothelial permeability factor. Arterioscler Thromb Vasc Biol. 2014;34:1459–67.
Fort PE, Losiewicz MK, Pennathur S, Jefferson LS, Kimball SR, Abcouwer SF, et al. mTORC1-independent reduction of retinal protein synthesis in type 1 diabetes. Diabetes. 2014;63:3077–90.
Fox TE, Young MM, Pedersen MM, Han X, Gardner TW, Kester M. Diabetes diminishes phosphatidic acid in the retina: a putative mediator for reduced mTOR signaling and increased neuronal cell death. Invest Ophthalmol Vis Sci. 2012;53:7257–67.
Chen Q, Tang L, Xin G, Li S, Ma L, Xu Y, et al. Oxidative stress mediated by lipid metabolism contributes to high glucose-induced senescence in retinal pigment epithelium. Free Radic Biol Med. 2019;130:48–58.
Qin YJ, Xiao K, Zhong Z, Zhao Y, Yu T, Sun XF. LECT2 ameliorates blood-retinal barrier impairment secondary to diabetes via activation of the Tie2/Akt/mTOR signaling pathway. Invest Ophthalmol Vis Sci. 2022;63:7.
Ran Z, Zhang Y, Wen X, Ma J. Curcumin inhibits high glucose‑induced inflammatory injury in human retinal pigment epithelial cells through the ROS‑PI3K/AKT/mTOR signaling pathway. Mol Med Rep. 2019;19:1024–31.
Shi J, Lv H, Tang C, Li Y, Huang J, Zhang H. Mangiferin inhibits cell migration and angiogenesis via PI3K/AKT/mTOR signaling in high glucose‑ and hypoxia‑induced RRCECs. Mol Med Rep. 2021;23:473.
Li R, Du J, Yao Y, Yao G, Wang X. Adiponectin inhibits high glucose-induced angiogenesis via inhibiting autophagy in RF/6A cells. J Cell Physiol. 2019;234:20566–76.
Jacot JL, Sherris D. Potential therapeutic roles for inhibition of the PI3K/Akt/mTOR pathway in the pathophysiology of diabetic retinopathy. J Ophthalmol. 2011;2011:589813.
Calton MA, Vollrath D. The mTOR kinase inhibitor INK128 blunts migration of cultured retinal pigment epithelial cells. Adv Exp Med Biol. 2016;854:709–15.
Dennis MD, Kimball SR, Fort PE, Jefferson LS. Regulated in development and DNA damage 1 is necessary for hyperglycemia-induced vascular endothelial growth factor expression in the retina of diabetic rodents. J Biol Chem. 2015;290:3865–74.
Yang Q, Li S, Zhou Z, Yang X, Liu Y, Hao K, et al. Trimetazidine mitigates high glucose-induced retinal endothelial dysfunction by inhibiting PI3K/Akt/mTOR pathway-mediated autophagy. Bioengineered. 2022;13:7515–27.
He Y, Dan Y, Gao X, Huang L, Lv H, Chen J. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am J Physiol Endocrinol Metab. 2021;320:E598–608.
Yang Z, Hu H, Zou Y, Luo W, Xie L, You Z. miR-7 reduces high glucose induced-damage via HoxB3 and PI3K/AKT/mTOR signaling pathways in retinal pigment epithelial cells. Curr Mol Med. 2020;20:372–8.
Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–86.
Alikhani M, Roy S, Graves DT. FOXO1 plays an essential role in apoptosis of retinal pericytes. Mol Vis. 2010;16:408–15.
Behl Y, Krothapalli P, Desta T, Roy S, Graves DT. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58:917–25.
Zhou Z, Liu J, Bi C, Chen L, Jiao Y, Cui L. Knockdown of FOXO6 inhibits high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells. J Cell Biochem. 2019;120:9716–23.
Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev. 2004;18:1060–71.
Shankar S, Chen Q, Srivastava RK. Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to enhance antiangiogenic effects of EGCG through activation of FOXO transcription factor. J Mol Signal. 2008;3:7.
Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther. 2010;332:125–34.
Chan CM, Huang JH, Chiang HS, Wu WB, Lin HH, Hong JY, et al. Effects of (-)-epigallocatechin gallate on RPE cell migration and adhesion. Mol Vis. 2010;16:586–95.
Zheng T, Yang X, Wu D, Xing S, Bian F, Li W, et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3β pathway. Br J Pharmacol. 2015;172:3284–301.
Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31:1121–33.
Shaw P, Chattopadhyay A. Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J Cell Physiol. 2020;235:3119–30.
Zhu H, Zhang W, Zhao Y, Shu X, Wang W, Wang D, et al. GSK3β-mediated tau hyperphosphorylation triggers diabetic retinal neurodegeneration by disrupting synaptic and mitochondrial functions. Mol Neurodegener. 2018;13:62.
Li Z, Ma L, Chen X, Li Y, Li S, Zhang J, et al. Glycogen synthase kinase-3: a key kinase in retinal neuron apoptosis in early diabetic retinopathy. Chin Med J. 2014;127:3464–70.
Devi TS, Singh LP, Hosoya K, Terasaki T. GSK-3β/CREB axis mediates IGF-1-induced ECM/adhesion molecule expression, cell cycle progression and monolayer permeability in retinal capillary endothelial cells: implications for diabetic retinopathy. Biochim Biophys Acta. 2011;1812:1080–8.
Zhu F, Shao J, Tian Y, Xu Z. Sulfiredoxin-1 protects retinal ganglion cells from high glucose-induced oxidative stress and inflammatory injury by potentiating Nrf2 signaling via the Akt/GSK-3β pathway. Int Immunopharmacol. 2021;101:108221.
Zhang L, Zhu T, He F, Li X. Senescence marker protein 30 (SMP30) protects against high glucose-induced apoptosis, oxidative stress and inflammatory response in retinal ganglion cells by enhancing Nrf2 activation via regulation of Akt/GSK-3β pathway. Int Immunopharmacol. 2021;101:108238.
Zhou Z, Li H, Bai S, Xu Z, Jiao Y. Loss of serine/threonine protein kinase 25 in retinal ganglion cells ameliorates high glucose-elicited damage through regulation of the AKT-GSK-3β/Nrf2 pathway. Biochem Biophys Res Commun. 2022;600:87–93.
Millán I, Desco MDC, Torres-Cuevas I, Pérez S, Pulido I, Mena-Mollá S, et al. Pterostilbene prevents early diabetic retinopathy alterations in a rabbit experimental model. Nutrients. 2019;12:82.
Ying Y, Zhang YL, Ma CJ, Li MQ, Tang CY, Yang YF, et al. Neuroprotective effects of ginsenoside Rg1 against hyperphosphorylated tau-induced diabetic retinal neurodegeneration via activation of IRS-1/Akt/GSK3β signaling. J Agric Food Chem. 2019;67:8348–60.
Shi Y, Zhang Y, Li Y, Tong C. Retracted Article: Sauchinone inhibits high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells. RSC Adv. 2019;9:17065–71.
Shivarudrappa AH, Ponesakki G. Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. J Cell Commun Signal. 2020;14:207–21.
Lai TT, Yang CM, Yang CH. Astaxanthin protects retinal photoreceptor cells against high glucose-induced oxidative stress by induction of antioxidant enzymes via the PI3K/Akt/Nrf2 pathway. Antioxidants. 2020;9:729.
Qin X, Li N, Zhang M, Lin S, Zhu J, Xiao D, et al. Tetrahedral framework nucleic acids prevent retina ischemia-reperfusion injury from oxidative stress via activating the Akt/Nrf2 pathway. Nanoscale. 2019;11:20667–75.
Yang J, Cheng M, Gu B, Wang J, Yan S, Xu D. CircRNA_09505 aggravates inflammation and joint damage in collagen-induced arthritis mice via miR-6089/AKT1/NF-κB axis. Cell Death Dis. 2020;11:833.
Zhou W, Fu XQ, Zhang LL, Zhang J, Huang X, Lu XH, et al. The AKT1/NF-kappaB/Notch1/PTEN axis has an important role in chemoresistance of gastric cancer cells. Cell Death Dis. 2013;4:e847.
Meng F, Liu L, Chin PC, D’Mello SR. Akt is a downstream target of NF-kappa B. J Biol Chem. 2002;277:29674–80.
Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–8.
Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–7.
Jiang T, Chang Q, Zhao Z, Yan S, Wang L, Cai J, et al. Melatonin-mediated cytoprotection against hyperglycemic injury in Müller cells. PLoS ONE. 2012;7:e50661.
Jiang T, Chang Q, Cai J, Fan J, Zhang X, Xu G. Protective effects of melatonin on retinal inflammation and oxidative stress in experimental diabetic retinopathy. Oxid Med Cell Longev. 2016;2016:3528274.
Tang L, Zhang C, Lu L, Tian H, Liu K, Luo D, et al. Melatonin maintains inner blood-retinal barrier by regulating microglia via inhibition of PI3K/Akt/Stat3/NF-κB signaling pathways in experimental diabetic retinopathy. Front Immunol. 2022;13:831660.
Xue B, Wang P, Yu W, Feng J, Li J, Zhao R, et al. CD146 as a promising therapeutic target for retinal and choroidal neovascularization diseases. Sci China Life Sci. 2022;65:1157–70.
Heydari M, Zare M, Badie MR, Watson RR, Talebnejad MR, Afarid M. Crocin as a vision supplement. Clin Exp Optom. 2023;106:249–56.
Yang X, Huo F, Liu B, Liu J, Chen T, Li J, et al. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J Mol Neurosci. 2017;61:581–9.
Cai F, Jiang H, Li Y, Li Q, Yang C. Upregulation of long non-coding RNA SNHG16 promotes diabetes-related RMEC dysfunction via activating NF-κB and PI3K/AKT pathways. Mol Ther Nucleic Acids. 2021;24:512–27.
Zhu H, Wang X, Wang X, Liu B, Yuan Y, Zuo X. Curcumin attenuates inflammation and cell apoptosis through regulating NF-κB and JAK2/STAT3 signaling pathway against acute kidney injury. Cell Cycle. 2020;19:1941–51.
Cho CH, Roh KH, Lim NY, Park SJ, Park S, Kim HW. Role of the JAK/STAT pathway in a streptozotocin-induced diabetic retinopathy mouse model. Graefes Arch Clin Exp Ophthalmol. 2022;260:3553–63.
Yun JH, Lee DH, Jeong HS, Kim SH, Ye SK, Cho CH. STAT3 activation in microglia increases pericyte apoptosis in diabetic retinas through TNF-ɑ/AKT/p70S6 kinase signaling. Biochem Biophys Res Commun. 2022;613:133–9.
Xu W, Chen Q, Zhang X, Zhao Y, Wu S, Yang C, et al. Genipin protects against mitochondrial damage of the retinal pigment epithelium under hyperglycemia through the AKT pathway mediated by the miR-4429/JAK2 signaling axis. Ann Transl Med. 2022;10:587.
Wang Q, Pfister F, Dorn-Beineke A, vom Hagen F, Lin J, Feng Y, et al. Low-dose erythropoietin inhibits oxidative stress and early vascular changes in the experimental diabetic retina. Diabetologia. 2010;53:1227–38.
Garcia-Ramírez M, Hernández C, Ruiz-Meana M, Villarroel M, Corraliza L, García-Dorado D, et al. Erythropoietin protects retinal pigment epithelial cells against the increase of permeability induced by diabetic conditions: essential role of JAK2/ PI3K signaling. Cell Signal. 2011;23:1596–602.
Hu LM, Luo Y, Zhang J, Lei X, Shen J, Wu Y, et al. EPO reduces reactive gliosis and stimulates neurotrophin expression in Muller cells. Front Biosci. 2011;3:1541–55.
Xu G, Kang D, Zhang C, Lou H, Sun C, Yang Q, et al. Erythropoietin protects retinal cells in diabetic rats through upregulating ZnT8 via activating ERK pathway and inhibiting HIF-1α expression. Invest Ophthalmol Vis Sci. 2015;56:8166–78.
Ma Y, Zhao Q, Shao Y, Cao MZ, Zhao M, Wang D. Melatonin inhibits the inflammation and apoptosis in rats with diabetic retinopathy via MAPK pathway. Eur Rev Med Pharmacol Sci. 2020;24:7545.
Qin D, Zheng XX, Jiang YR. Apelin-13 induces proliferation, migration, and collagen I mRNA expression in human RPE cells via PI3K/Akt and MEK/Erk signaling pathways. Mol Vis. 2013;19:2227–36.
Li Y, Bai YJ, Jiang YR. Apelin induces the proliferation, migration and expression of cytoskeleton and tight junction proteins in human RPE cells via PI-3K/Akt and MAPK/Erk signaling pathways. Int J Clin Exp Pathol. 2017;10:10711–29.
Li Y, Bai YJ, Jiang YR, Yu WZ, Shi X, Chen L, et al. Apelin-13 is an early promoter of cytoskeleton and tight junction in diabetic macular edema via PI-3K/Akt and MAPK/Erk signaling pathways. BioMed Res Int. 2018;2018:3242574.
Shibagaki F, Ishimaru Y, Sumino A, Yamamuro A, Yoshioka Y, Maeda S. Systemic administration of an apelin receptor agonist prevents NMDA-induced loss of retinal neuronal cells in mice. Neurochem Res. 2020;45:752–9.
Maugeri G, D’Amico AG, Gagliano C, Saccone S, Federico C, Cavallaro S, et al. VIP family members prevent outer blood retinal barrier damage in a model of diabetic macular edema. J Cell Physiol. 2017;232:1079–85.
Fabian E, Reglodi D, Mester L, Szabo A, Szabadfi K, Tamas A, et al. Effects of PACAP on intracellular signaling pathways in human retinal pigment epithelial cells exposed to oxidative stress. J Mol Neurosci. 2012;48:493–500.
Szabadfi K, Szabo A, Kiss P, Reglodi D, Setalo G Jr., Kovacs K, et al. PACAP promotes neuron survival in early experimental diabetic retinopathy. Neurochem Int. 2014;64:84–91.
Qiu F, Tong H, Wang Y, Tao J, Wang H, Chen L. Recombinant human maspin inhibits high glucose-induced oxidative stress and angiogenesis of human retinal microvascular endothelial cells via PI3K/AKT pathway. Mol Cell Biochem. 2018;446:127–36.
Qiu F, Tong H, Wang Y, Tao J, Wang H, Chen L. Inhibition of miR-21-5p suppresses high glucose-induced proliferation and angiogenesis of human retinal microvascular endothelial cells by the regulation of AKT and ERK pathways via maspin. Biosci Biotechnol Biochem. 2018;82:1366–76.
Liu Y, Leo LF, McGregor C, Grivitishvili A, Barnstable CJ, Tombran-Tink J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol Med. 2012;18:1387–401.
Sheikpranbabu S, Haribalaganesh R, Lee KJ, Gurunathan S. Pigment epithelium-derived factor inhibits advanced glycation end products-induced retinal vascular permeability. Biochimie. 2010;92:1040–51.
Haribalaganesh R, Sheikpranbabu S, Elayappan B, Venkataraman D, Gurunathan S. Pigment-epithelium-derived factor down regulates hyperglycemia-induced apoptosis via PI3K/Akt activation in goat retinal pericytes. Angiogenesis. 2009;12:381–9.
Cai X, Li J, Wang M, She M, Tang Y, Li J, et al. GLP-1 treatment improves diabetic retinopathy by alleviating autophagy through GLP-1R-ERK1/2-HDAC6 signaling pathway. Int J Med Sci. 2017;14:1203–12.
Sampedro J, Bogdanov P, Ramos H, Solà-Adell C, Turch M, Valeri M, et al. New insights into the mechanisms of action of topical administration of GLP-1 in an experimental model of diabetic retinopathy. J Clin Med. 2019;8:339.
Lin WJ, Ma XF, Hao M, Zhou HR, Yu XY, Shao N, et al. Liraglutide attenuates the migration of retinal pericytes induced by advanced glycation end products. Peptides. 2018;105:7–13.
Shu X, Zhang Y, Li M, Huang X, Yang Y, Zeng J, et al. Topical ocular administration of the GLP-1 receptor agonist liraglutide arrests hyperphosphorylated tau-triggered diabetic retinal neurodegeneration via activation of GLP-1R/Akt/GSK3β signaling. Neuropharmacology. 2019;153:1–12.
Klaassen I, de Vries EW, Vogels IMC, van Kampen AHC, Bosscha MI, Steel DHW, et al. Identification of proteins associated with clinical and pathological features of proliferative diabetic retinopathy in vitreous and fibrovascular membranes. PLoS ONE. 2017;12:e0187304.
Yoshimasa T, Sibley DR, Bouvier M, Lefkowitz RJ, Caron MG. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987;327:67–70.
Acknowledgements
Due to space constraints, we apologize to those whose work we could not cite.
Funding
This work was supported by the Natural Science Foundation of China (Grant number 82371084), the Strategic Priority Research Program of Chinese Academy of Sciences (Grant number XDA16040200), the Natural Science Foundation of Zhejiang Province (Grant number LZ19H120001), the Major Science and Technology Project of Zhejiang Province (Grant number 2018C03017), the Nature Science Foundation of China (Grant number 82201194), the Zhejiang Medical Health Science and technology program (Grant number WKJ-ZJ-1820), the Traditional Chinese Medicine innovative talent support program (Grant number 2023ZR106).
Author information
Authors and Affiliations
Contributions
JL and KC wrote the manuscript, XL, XZ, LZ, QY, YX, CX, XW, YS and JT prepared the references and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Chen, K., Li, X. et al. Mechanistic insights into the alterations and regulation of the AKT signaling pathway in diabetic retinopathy. Cell Death Discov. 9, 418 (2023). https://doi.org/10.1038/s41420-023-01717-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-023-01717-2
This article is cited by
-
Modelling neurodegeneration and inflammation in early diabetic retinopathy using 3D human retinal organoids
In vitro models (2024)
-
Transcriptional patterns of human retinal pigment epithelial cells under protracted high glucose
Molecular Biology Reports (2024)